Abstract
The OGG1 gene of Saccharomyces cerevisiae encodes a DNA glycosylase activity that is a functional analog of the Fpg protein from Escherichia coli and excises 7,8-dihydro-8-oxoguanine (8-oxoG) from damaged DNA. The repair of this ubiquitous kind of oxidative damage is essential to prevent mutations both in bacteria and in yeast. A human cDNA clone carrying an ORF displaying homology to the yeast protein was identified. The predicted protein has 345 amino acids and a molecular mass of 39 kDa. This protein shares a 38% sequence identity with the yeast Ogg1 protein, adding this novel human gene product to the growing family of enzymes that the repair of oxidatively damaged bases and are related to the E. coli endonuclease III. Northern blot analysis indicates that this gene, localized to chromosome 3p25, is ubiquitously expressed in human tissues. The cloned coding sequence was expressed in an E. coli strain that carried a disrupted fpg gene, the bacterial functional analog of OGG1. Cell-free extracts from these cultures displayed a specific lyase activity on duplex DNA that carried an 8-oxoG/C base pair. The products of the reaction are consistent with an enzymatic activity like the one displayed by the yeast Ogg1. Analysis of the substrate specificity reveals a very strong preference for DNA fragments harboring 8-oxoG/C base pairs. The pattern of specificity correlates well with the one found for the yeast enzyme. Moreover, when the human coding sequence was expressed in a yeast strain mutant in OGG1 it was able to complement the spontaneous mutator phenotype. These results make this novel gene (hOGG1) a strong candidate for the human homolog of the yeast OGG1 and suggest an important role of its product in the protection of the genome from the mutagenic effects of the oxidatively damaged purines.
Reactive oxygen species (ROS) formed in cells either as by-products of aerobic metabolism or as a consequence of exposure to environmental mutagens can attack DNA or its precursors, yielding oxidatively damaged bases and strand breakage (1, 2). Unrepaired oxidative damage to DNA has been suggested to play a role in carcinogenesis and aging through mutations in genes controlling these biological processes (3–5). Several lines of evidence suggest that an oxidatively damaged form of guanine, 7,8-dihydro-8-oxoguanine (8-oxoG), is critical in terms of mutagenesis (6, 7). In Escherichia coli, two DNA glycosylases cooperate to prevent mutagenesis by 8-oxoG: the Fpg protein, which excises 8-oxoG in damaged DNA (8–10) and the MutY protein, which excises the adenine residues incorporated by DNA polymerases opposite 8-oxoG (11–13). Inactivation of both the fpg (mutM) and mutY (micA) genes of E. coli results in a strong G⋅C → T⋅A mutator phenotype (14–17).
In Saccharomyces cerevisiae, the OGG1 gene encodes an 8-oxoG DNA glycosylase activity that reduces the mutator phenotype of the fpg mutY mutant of E. coli (18). The Ogg1 protein contains 376 amino acids, and, although it was cloned by functional complementation of the E. coli fpg mutY mutant, it does not show significant sequence homology with bacterial Fpg proteins (18). The Ogg1 protein is a DNA glycosylase/apurinic (AP) lyase that catalyzes both the release of 8-oxoG and the cleavage of DNA at the resulting AP site via a β-elimination reaction (18–20). Recently, it has been demonstrated that Ogg1-deficient strains of S. cerevisiae exhibit a mutator phenotype and specifically accumulate G⋅C → T⋅A transversions (21). These results indicate that, despite the absence of sequence homology, the yeast Ogg1 protein is the functional analog of the bacterial Fpg protein (22). The current hypothesis proposes that endogenous ROS attack DNA yielding 8-oxoG which causes mutations if not repaired by proteins such as Fpg, MutY, or Ogg1, which are part of the base excision repair pathway (23).
The study of repair genes involved in preventing mutagenesis has facilitated the understanding of cancer susceptibility in man. An example of this is the demonstration that inherited susceptibility to heriditary nonpolyposis colon cancer (HNPCC) is due to deficiencies in the human homologs of bacterial mutator genes mutS and mutL (24). Therefore, the identification of homologs of bacterial or yeast mutator genes such as fpg, mutY, or OGG1 may also provide valuable information concerning the biological impact of oxidative stress on the etiologies of degenerative diseases in higher organisms. Amino acid sequence of DNA repair proteins is often well conserved from S. cerevisiae to humans (2, 25, 26). We have used sequence homology as a criterion to identify potential human homologs of the yeast OGG1 gene. We report the isolation of a human cDNA (hOGG1) encoding a 345-aa protein with 38% identity to the yeast Ogg1. The hOGG1 gene was localized to chromosome 3p25 and Northern blot analysis suggests a ubiquitous expression in human tissues. Cell-free extracts of E. coli cultures carrying a disrupted fpg gene and expressing the hOgg1 protein catalyze the cleavage of DNA containing an 8-oxoG/C base pair. The expression of the human protein in E. coli fpg mutY complements the strong mutator phenotype of this strain. Furthermore, the hOgg1 protein suppresses the mutator phenotype of ogg1 strains of S. cerevisiae. These results suggest that hOGG1 is a mammalian homolog of the yeast OGG1.
MATERIALS AND METHODS
Strains, Enzymes, and Microbiological Methods.
E. coli strains used are derived from CC104 (27) (ara, Δ(gpt-lac), rpsL/F′ (lacI, lacZ, proA+ B+)); PR180 (CC104 mutY::Kanr); PR221 (CC104 fpg::Kanr); and PR195 (CC104 fpg::Kanr mutY::Kanr). Strains were grown on either Luria–Bertani (LB) or minimal A media (28). Saccharomyces cerevisiae strains used were FF18733 (MATa, his7, leu2, lys1, ura3, trp1) and CD138 (MATa, his7, leu2, lys1, ura3, ogg1::TRP1) (21). Yeast strains were grown in standard medium at 30°C with agitation. YNBD minimal medium (0.7% yeast nitrogen base without amino acids and 2% glucose) was prepared as described (21). According to the auxotrophic requirement of yeast strains, histidine (100 μg/ml), leucine (100 μg/ml), lysine (40 μg/ml), uracil, or tryptophane (20 μg/ml) were added to minimal medium. Transformation of S. cerevisiae was performed using the acetate lithium treatment (29). The homogeneous Fpg protein of E. coli and Ogg1 protein of S. cerevisiae were prepared as described (18, 30).
Isolation of a hOgg1 cDNA Clone.
cDNA clones showing homology with the OGG1 gene of S. cerevisiae were identified in the database of human expressed sequence tag sequences (31). The two longest clones (N55394 from a multiple sclerosis lesions library and W04935 from a fetal lung library) were obtained from the IMAGE Consortium and sequenced. The 3′ halves of W04935 and N55394 are identical. As for the 5′ regions, W04935 has a 440-bp deletion and extends 285 bp beyond the N55394 5′ end (Fig. 1A). To isolate the 5′ region of the cDNA the rapid amplification of cDNA ends (RACE) protocol was used on an anchor-ligated cDNA (human prostate Marathon Ready, CLONTECH). The conditions for the PCRs were as recommended by the manufacturer. The gene-specific primers used were as follows: 5′-CCACTCCAGGCAGGATGCAGAG-3′ for the initial amplification and 5′-TTCTTCCAGGATGGCTCGGGCAC-3′ for the nested reaction. The amplification products were purified from an agarose gel, digested with NotI and PstI, and cloned into pBluescript II KS (Stratagene). For Northern blot analysis the cDNA fragment from clone N55394 was radioactively labeled and used on a CLONTECH human multiple-tissue blot. After stripping the blot, a human β-actin probe was used as a control for the amount of mRNA loaded in the different lanes.
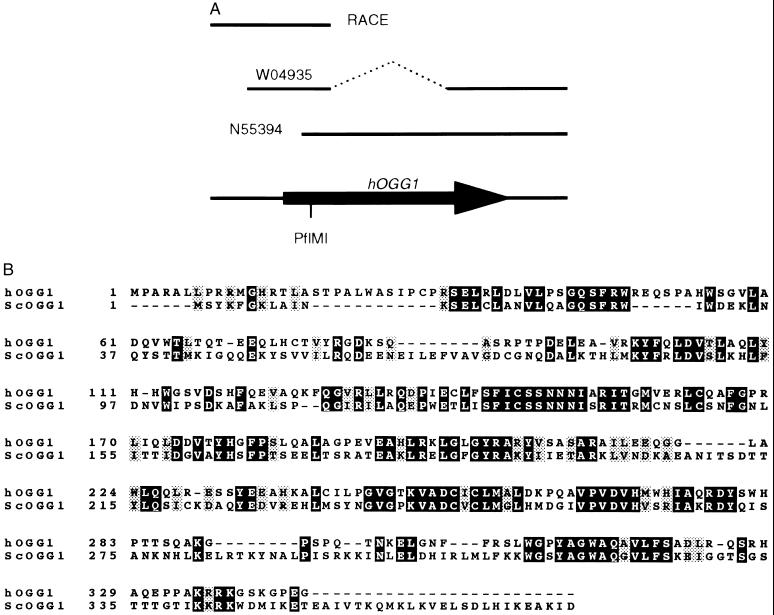
Figure 1.
Cloning of a human homolog of OGG1. (A) Schematic representation of the sources of cDNA used to assemble the complete clone. The open reading frame is indicated by an arrow. (B) Alignment of the encoded protein with the S. cerevisiae Ogg1 protein. Positions with identical residues are enclosed in black boxes.
Expression of hOGG1 in E. coli, Determination of Mutation Frequencies, and Preparation of Bacterial Extracts.
A cDNA carrying the complete ORF was assembled using a unique PflMI restriction site and cloned into pTrc99A (Pharmacia) to create plasmid pPR55. Plasmid pPR59 was obtained by PCR amplification of the hOGG1 ORF sequence and subcloning into pKK223 (Pharmacia). PR195 cells harboring either this plasmid or the vector pKK223 were grown in LB broth containing 100 μg/ml ampicillin and 1 mM isopropyl-β-d-thiogalactoside (IPTG). To determine the spontaneous frequencies of rifampicin-resistant mutants and of lactose revertants, appropriate dilutions of at least 10 overnight cultures, originally inoculated with 103 cells, were plated on either LB agar, LB agar with rifampicin (100 μg/ml), or minimal lactose (0.2%) agar. Colonies were counted the following day in the case of the LB plates and 48 hr after plating for the minimal lactose plates.
For analysis of the enzymatic activity of the human gene, cultures of PR221 cells harboring pPR55 were grown at 37°C in 20 ml of LB-broth medium containing 100 μg/ml of ampicillin until OD600 = 1.0 and supplemented with 1 mM IPTG. Cultures were then incubated at 37°C for 2.5 hr under vigorous agitation. The cells were harvested by centrifugation, washed, and stored at −80°C. Cell-free extracts were prepared as previously described (32), and protein concentration was measured according to Bradford (33).
Assay for the Repair of 8-oxoG.
The 34-mer oligonucleotides containing a single 8-oxoG or a G residue at position 16 were synthesized as previously described (34, 35). The sequences used in this study were the following: oligo 1, 5′-GGCTTCATCGTTATT(8-oxoG)ATGACCTGGTGGATACCG-5′*; oligo 2, 5′-GGCTTCATCGTTATT(G)ATGACCTGGTGGATACCG-5′*. Complementary sequences with a cytosine, thymine, guanine, or adenine opposite 8-oxoG were also synthesized. To protect oligonucleotides from degradation in cell-free extracts, the nucleotide at the 3′ end was inverted, yielding a 5′-(N)n-3′-P-3′-N-5′* sequence with two 5′ ends. The 34-mer oligonucleotides were labeled at both ends using [γ32P]ATP and T4 polynucleotide kinase. The 32P-labeled strand was hybridized with a complementary sequence by heating the mixture at 90°C for 10 min followed by slow cooling to room temperature. The assay mixtures (final volume, 25 μl) contained 25 mM Tris⋅HCl (pH 7.6), 2 mM Na2⋅EDTA, 50 mM KCl, 50 fmol of 32P-labeled DNA duplex, and bacterial cell-free extracts, Fpg protein, or Ogg1 protein. The reactions were performed at 37°C for 30 min, and the products were separated by 20% denaturing PAGE containing 7 M urea (18, 35, 36).
Fluorescent in Situ Hybridization.
Fluorescent in situ hybridization was performed as previously described (37). Plasmid pPR55 was labeled with biotin-11-dUTP (Sigma) using the nick translation mix (Boehringer Mannheim) according to the supplier. The labeled product was hybridized at a final concentration of 20 ng/ml at 37°C. Posthybridization washes were performed at 39°C for periods of 3 min each.
Expression of the hOgg1 Protein in S. cerevisiae and Determination of the Spontaneous Mutation Frequencies.
The cDNA fragment from pPR55 containing the ORF was isolated and subcloned into the yeast expression vector pYX212 (R & D Systems), yielding pPRY2, in which the hOGG1 coding sequence is expressed from the constitutive TPI promoter. To determine the spontaneous mutation frequencies, yeast cultures were grown to saturation in 2 ml of YNBD medium at 30°C for 2 days. Each culture was started from an initial inoculum of less than 104 cells. The final cell densities of the cultures were determined by plating dilutions on YNBD agar plates and counting the colonies after 3 days at 30°C. The quantification of canavanine-resistant mutants (CanR) was done by plating 0.1 ml of the undiluted cultures on YNBD plates containing 60 μg/ml of canavanine sulfate (Sigma). The plates were incubated for 3–4 days at 30°C before counting the colonies. As in the case of the bacterial experiments, the mutation rates were calculated using the median number of mutants per culture (38).
RESULTS
Isolation of a Human Homolog of Yeast OGG1.
Several human expressed tag clones with homology to the S. cerevisiae OGG1 gene were detected by a search in a database of expressed sequence tag sequences. Of those, the longest two (1 kb) were chosen. Sequencing of these clones revealed that they corresponded to the same gene but clone W04935 carried a 440-bp deletion with respect to N55394. This deletion seems to be the product of a recombination event between 8-bp direct repeats. The 5′ end of the human transcript was identified by the cloning and sequencing of RACE PCR products. The compiled sequence (1637 bp) of the assembled cDNA (shown schematically in Fig. 1A) contains an open reading frame that encodes 345 amino acids (Fig. 1B) and starts 338 bp from the 5′ end. A second ATG codon is present 30 bp downstream from the first one. From these two putative initiation codons the first one has a sequence context closer to the consensus for vertebrate mRNA ATG initiation codons (39). The predicted protein has a molecular mass of 38,782 Da and a theoretical isoelectric point of 8.89. A consensus polyadenylylation site (AATAAA) is present 22 bp before the poly(A) start at the 3′ end.
Fig. 1B also shows the comparison of the protein sequence with the Ogg1 protein of S. cerevisiae. Overall, the two proteins display a 38% identity that spans throughout the whole sequence. The helix–hairpin–helix motif, characteristic of the DNA-binding site from the E. coli endonuclease III (38), is conserved (positions 245–270) as is a putative nuclear localization sequence (amino acids 335–338). Aside from these motifs, other regions of high identity but unknown function are observed, notably residues 31–47 and 128–159. The novel human protein is therefore designated hOgg1.
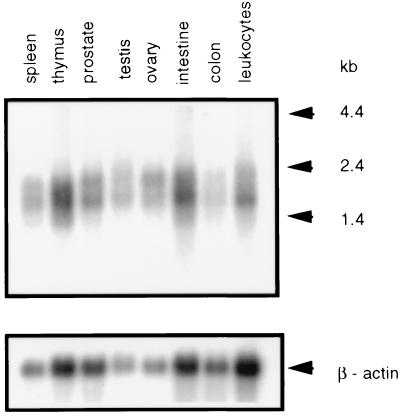
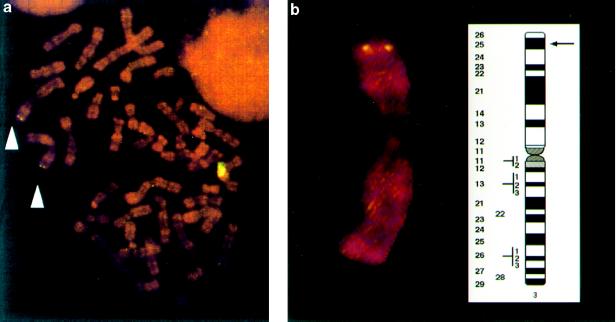
Northern blot analysis identified a mRNA of approximately 1.7 kb as the main transcription product encoded by hOGG1. The gene is expressed at about the same level in all the human tissues analyzed, with the possible exception of the colon where the hOGG1 message is less abundant (Fig. 2). Using in situ hybridization, the hOGG1 gene was localized to human chromosome 3p25 (Fig. 3).
Figure 2.
Northern blot analysis of human mRNA from various tissues. The same blot was hybridized with a hOGG1 probe (Upper) or a β-actin probe (Lower).
Figure 3.
Chromosome localization of the hOGG1 gene determined by FISH. (a) Example of hybridization signals of twin dots on human chromosome 3. (b) Ideogram of human chromosome 3 showing localization of the hOGG1 gene on 3p25 based on analysis of metaphases with twin dots.
hOgg1 Has an 8-oxoG DNA Nicking Activity.
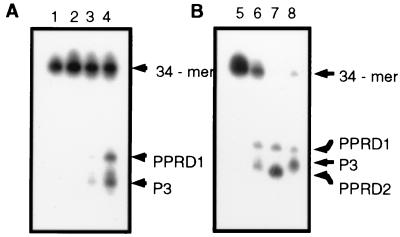
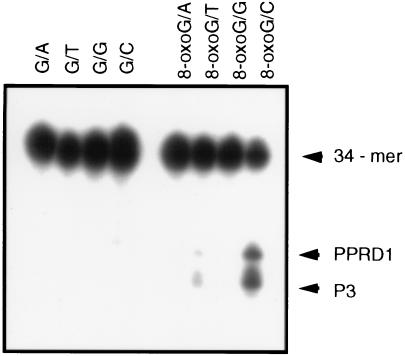
The hOGG1 cDNA was subcloned into the bacterial expression vector pTrc 99A, creating plasmid pPR55, in which the expression of the cDNA can be induced with IPTG. Cell-free extracts of E. coli fpg− (PR221) cultures harboring either the vector or pPR55 were tested for their capacity to cleave a 34-mer double-stranded substrate containing an 8-oxoG/C base pair at a defined position. Fig. 4A shows that crude extracts from cultures of PR221 cells harboring the vector plasmid pTrc 99A and treated with IPTG do not incise the 8-oxoG/C-containing duplex (lane 2). In contrast, crude extracts of parallel cultures of PR221 cells hosting plasmid pPR55 display a nicking activity that is induced by treatment of the cultures with IPTG (lanes 3 and 4). The products of the cleavage reactions in these extracts were compared with the products of the purified Fpg and Ogg1 proteins when confronted with the same substrate (Fig. 4B). The crude extracts yield 3′ and 5′ oligonucleotides [bands PPRD1 (5′-P-(N17)-3′-P-3′-N-32P-5′)] and P3 [5′-32P-(N15)-3′-P-dR)] like the ones obtained with the purified yeast protein (lanes 6 and 8). In the case of the Fpg protein, the 5′ oligonucleotide generates a lower band (PPRD2) in the autoradiogram (lane 7).
Figure 4.
Cleavage of the 8-oxoG/C duplex by crude extracts of E. coli fpg. The sequences of the 34-mer oligonucleotides containing a single 8-oxoG are reported in Materials and Methods. The 8-oxoG/C duplex was incubated either with cell-free extracts or purified Fpg or Ogg1 protein. The two extremities of the 8-oxoG-containing strand were 32P-labeled, and, consequently, two oligonucleotides were generated after cleavage. The products of the reaction were analyzed by denaturing 20% PAGE containing 7 M urea. PPRD1 is the 3′ oligonucleotide [5′P-(N17)-3′-N-32P-5′], and PPRD2 is the 5′ oligonucleotide [5′-32P-(N15)-3′-P], which are generated by chemical cleavage of the 34-mer oligonucleotide at 8-oxoG by 1 M piperidine at 90°C for 60 min or by the Fpg protein. The product P3 corresponds to PPRD2 with a deoxyribose residue attached at its 3′ end. (A) Cell-free extracts (30 μg of protein) obtained from cultures of pPR221 (fpg) hosting the vector pTrc99A (lane 2) or pPR55 (lanes 3 and 4). For lane 4, the culture was treated with 1 mM IPTG before lysis. (B) Comparison of the products of the 8-oxoG/C incision by crude extracts from PR221 cells expressing hOgg1 (lane 6), purified Fpg protein (2 ng) (lane 7), or purified Ogg1 (5 ng) (lane 8). Lanes 1 and 5 correspond to the untreated 34-mer oligonucleotide.
Substrate Specificity of the hOgg1 Protein.
The capacity of extracts from hOgg1-expressing cells to initiate the repair of various mismatches was analyzed using 34-mer DNA duplexes containing an 8-oxoG or a G residue placed opposite of each of the four normal DNA bases. Fig. 5 shows that the extract-nicking activity has a marked preference for the duplex containing the 8-oxoG/C base pair followed by the 8-oxoG/T. In contrast, the 8-oxoG/G duplex is poorly recognized by the nicking activity and the 8-oxoG/A is not incised at a detectable rate. The same extract does not display a nicking activity on duplexes carrying the three G/N mismatches or the normal G/C base pair (Fig. 5). This substrate specificity of the crude extracts from cells expressing the human Ogg1 protein parallels the specificity of the yeast enzyme. These results, together with the cleavage pattern described above and the sequence similarities, are consistent with the hypothesis that the hOgg1 protein is a functional homolog of the yeast 8-oxoG DNA glycosylase Ogg1.
Figure 5.
Cleavage of DNA duplexes containing a single 8-oxoG mismatched with one of the four DNA bases by cell-free extracts of E. coli fpg cells expressing hOgg1. The sequences of the 34-mer oligonucleotides containing 8-oxoG or G at position 16 are reported in Materials and Methods. The 8-oxoG or G16 containing strands were labeled and annealed with one of the four complementary sequences carrying A, T, C, or G opposite to the 8-oxoG or G16. These substrates were incubated with cell-free extracts (30 μg of protein) of PR221 hosting pPR55 induced with 1 mM IPTG. The analysis of the products of the reaction was as described in the legend of Fig. 4.
Expression of hOGG1 in E. coli fpg mutY or in S. cerevisiae ogg1 Complements the Mutator Phenotypes.
The hOGG1 coding sequence was cloned into the expression vector pKK223 and transformed into an E. coli strain PR195, in which the mutY and fpg genes are disrupted. This strain displays a strong, spontaneous mutator phenotype due to its incapacity to eliminate errors induced by the presence of 8-oxoG in its DNA (6, 7). The rates of either mutation to rifampicin resistance or reversion to lac+ were determined. The results shown in Table 1 demonstrate that the expression of the human protein in this strain strongly reduces the mutation frequencies for both markers analyzed, partially complementing the mutator phenotype. It is particularly interesting to note that the high frequency of lac reversion in strain PR195 is due exclusively to the occurrence of G/C to T/A transversions (27), therefore suggesting that the antimutator effect of the human enzyme is indeed correlated with its capacity to remove endogenously generated 8-oxoG from DNA.
Table 1.
Spontaneous mutagenesis of the mutant strain E. coli fpg mutY expressing the human hOGG1 gene
| Bacterial strain | Plasmid | Rifr/108 | Lac+/108 |
|---|---|---|---|
| PR180 (mutY) | none | 23 ± 2 | 6.4 ± 1.1 |
| PR195 (fpg mutY) | pKK223 | 131 ± 17 | 1,160 ± 185 |
| PR195 (fpg mutY) | pPR59 | 36 ± 5 | 26 ± 7 |
pPR59 expresses the human hOgg1. pKK223 is the expression vector used for bacteria.
The hOGG1 cDNA was also subcloned in the yeast expression vector pYX212 and introduced into strain CD138 (ogg1). The mutation rate to canavanine resistance was determined for the wild-type strain as well as for CD138 harboring either the vector alone or the vector expressing the human cDNA (pPRY2). Table 2 shows the results of this experiment. The expression of hOgg1 in the ogg1 yeast mutant strain suppresses the mutator phenotype, suggesting that, consistent with the sequence similarity and its enzymatic activity, the human gene is a functional homolog of the yeast OGG1 that is implicated in the repair of 8-oxoG generated endogenously.
Table 2.
Spontaneous mutagenesis of the mutant strain S. cerevisiae ogg1 expressing the human hOGG1 gene
| Yeast strain | Plasmid | Canr/107 |
|---|---|---|
| FF18733 (wild type) | None | 14 ± 3 |
| CD138 (ogg1) | pYX212 | 96 ± 13 |
| CD138 (ogg1) | pPRY2 | 19 ± 3 |
pYX212 is the expression vector used for yeast. pPRY2 expresses the human hOgg1.
DISCUSSION
The removal of 8-oxoG from DNA by the Fpg protein of E. coli or the Ogg1 protein of S. cerevisiae has been implicated in the prevention of mutations arising from the attack of DNA by endogenous ROS and therefore contributes to the stability of the genome (6, 7, 10, 21, 22). Here we reported the cloning and characterization of a cDNA coding for the hOgg1 protein, a human homolog of the Ogg1 protein of S. cerevisiae. The hOgg1 protein has 345 amino acids and displays a 38% identity with the yeast protein. The overall conservation of the sequence and, in particular, of the consensus sequence for the HhH structural motif as well as that of two blocks of homology between residues 247 and 279 containing the lysine (K250) and the aspartic acid (D268) strongly suggests that hOgg1 is a member of the family of DNA glycosylases/AP lyases sharing a common ancestor gene with the endonuclease III of E. coli (19, 20, 26, 40). The presence of a putative nuclear localization sequence suggests that the substrate of this enzyme is the chromosomal DNA. Another form of cDNA from this same gene, which lacks the nuclear localization sequence, has been identified (T. Roldan-Arjona and T. Lindahl, personal communication), raising the possibility of a mitochondrial function derived from an alternative splicing of the hOGG1 mRNA. The hOGG1 gene encodes a 1.7-kb mRNA that is expressed at similar levels in the various tissues tested. The ubiquitous expression of hOGG1 is expected for a function whose biological role is to remove a DNA lesion generated by endogenous by-products of aerobic metabolism.
The sequence homology suggests that the hOgg1 protein is a structural and functional homolog of the Ogg1 protein from S. cerevisiae. The results reported in this study support this hypothesis. First, the expression of the hOgg1 protein in yeast cells carrying a disrupted ogg1 gene suppresses their spontaneous mutator phenotype. Second, the capacity of hOgg1 to repair 8-oxoG residues in DNA was analyzed in cell-free extracts of E. coli (fpg−) strain transformed with a vector expressing hOgg1. These experiments show that this human protein can indeed cleave the strand carrying the oxidized base. The pattern of the products is indistinguishable from those generated by the yeast Ogg1 enzyme. Third, the rank order in the cleavage efficiency for different substrates parallels the specificity of the yeast OGG1 gene product (18–20). The analysis of the reaction products suggests that the incision reflects the excision of the 8-oxoG and subsequent cleavage at the resulting AP site.
In bacteria (7) as well as in yeast (21), the removal of 8-oxoG from DNA is essential for the accurate maintenance of the genetic information. In human cells, 8-oxoG has been shown to be released by a DNA glycosylase activity, but the gene coding for this function has not been identified (41). Furthermore, an activity that incises DNA containing 8-oxoG/A mismatches has been partially purified from HeLa cells (42). A human homolog of the mutY gene of E. coli has been cloned, but no functional studies have been reported (43). Finally, a human homolog of the bacterial endonuclease III has been recently identified (26). These data, together with the cloning of hOGG1, presented in this paper, strongly suggest that human cells possess repair systems similar to those of bacteria or yeast to protect the genome from the mutagenic lesions induced by endogenous and environmental ROS.
The localization of the hOGG1 gene to chromosome 3p25 opens the interesting possibility that defects in this gene could play a role in determining some of the phenotypes of Fanconi’s anemia cells, since complementation group D has been mapped to the same large region (44). Recent evidence shows that sensitivity to mitomycin C of cells from patients with the disease could be due to the generation by this mutagen of oxygen radicals rather than its crosslinking capacity (45). Moreover, it has been reported that 8-oxoG concentration is increased in the DNA from lymphoblasts from Fanconi’s anemia patients (46).
To conclude, the cloning of a human cDNA coding for an 8-oxoG repair enzyme is an important step toward the understanding of the biological impact of endogenous ROS on the etiologies of degenerative diseases in humans. By analogy with studies showing that mutator genes correlate with cancer predisposition, a defect in the human hOGG1 gene could provoke susceptibility to spontaneous or oxidative stress-induced cancer in humans.
Acknowledgments
We thank Drs. F. Fabre and D. Thomas for yeast strains and plasmids, Dr. P. Nehls for the oligonucleotides with 8-oxoG, F. Reille for his technical assistance, and Drs. B. Dutrillaux, S. Murli, R. Polakiewicz, and L. Samson for their helpful advice and discussions. The authors gratefully acknowledge the Commissariat à l’Energie Atomique, the Centre National de la Recherche Scientifique (ACC-SV8), and National Institutes of Health (Grant 9R01 GM54186-35 to M.S.F.) for their support.
ABBREVIATIONS
- ROS
reactive oxygen species
- 8-oxoG
7,8-dihydro-8-oxoguanine
- AP
apurinic/apyrimidinic
- IPTG
isopropyl β-d-thiogalactopyranoside
- RACE
rapid amplification of cDNA ends
- IPTG
isopropyl-β-d-thiogalactoside
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the EMBL database (accession no. Y11731).
References
- 1.Dizdaroglu M. Free Radical Biol Med. 1991;10:225–242. doi: 10.1016/0891-5849(91)90080-m. [DOI] [PubMed] [Google Scholar]
- 2.Demple B, Harrisson L. Annu Rev Biochem. 1994;63:915–948. doi: 10.1146/annurev.bi.63.070194.004411. [DOI] [PubMed] [Google Scholar]
- 3.Breimer L H. Mol Carcinogen. 1990;3:188–197. doi: 10.1002/mc.2940030405. [DOI] [PubMed] [Google Scholar]
- 4.Ames B N, Shinegawa M K, Hagen T M. Proc Natl Acad Sci USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feig, D. I., Reid, T. M. & Loeb, L. A. (1994) Cancer Res. Suppl 54, 1890–1894. [PubMed]
- 6.Michaels M L, Miller J H. J Bacteriol. 1992;174:6321–6325. doi: 10.1128/jb.174.20.6321-6325.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grollman A P, Moriya M. Trends Genet. 1993;9:246–249. doi: 10.1016/0168-9525(93)90089-z. [DOI] [PubMed] [Google Scholar]
- 8.Tchou J, Kasai H, Shibutani S, Chung M H, Laval J, Grollman A P, Nishimura S. Proc Natl Acad Sci USA. 1991;88:4690–4694. doi: 10.1073/pnas.88.11.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boiteux S, Gajewski E, Laval J, Dizdaroglu M. Biochemistry. 1992;31:106–110. doi: 10.1021/bi00116a016. [DOI] [PubMed] [Google Scholar]
- 10.Boiteux S. Photochem Photobiol. 1993;19:87–96. doi: 10.1016/1011-1344(93)87101-r. [DOI] [PubMed] [Google Scholar]
- 11.Au K G, Clark S, Miller J H, Modrich P. Proc Natl Acad Sci USA. 1989;86:8777–8781. doi: 10.1073/pnas.86.22.8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai-Wu J J, Liu H F, Lu A L. Proc Natl Acad Sci USA. 1992;89:8779–8783. doi: 10.1073/pnas.89.18.8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michaels L M, Tchou J, Grollman A P, Miller J H. Biochemistry. 1992;31:10964–10968. doi: 10.1021/bi00160a004. [DOI] [PubMed] [Google Scholar]
- 14.Radicella J P, Clark E A, Fox M S. Proc Natl Acad Sci USA. 1988;85:9674–9678. doi: 10.1073/pnas.85.24.9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michaels M L, Cruz C, Grollman A P, Miller J H. Proc Natl Acad Sci USA. 1992;89:7022–7025. doi: 10.1073/pnas.89.15.7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duwat P, De Oliveira R, Ehrlich D S, Boiteux S. Microbiology. 1995;141:411–417. doi: 10.1099/13500872-141-2-411. [DOI] [PubMed] [Google Scholar]
- 17.Tajiri T, Maki H, Sekiguchi M. Mutat Res. 1995;336:257–267. doi: 10.1016/0921-8777(94)00062-b. [DOI] [PubMed] [Google Scholar]
- 18.Auffret van der Kemp P, Thomas D, Barbey R, De Oliveira R, Boiteux S. Proc Natl Acad Sci USA. 1996;93:5197–5202. doi: 10.1073/pnas.93.11.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nash H M, Bruner S D, Scharer O D, Kawate T, Addona T A, Spooner E, Lane W S, Verdine G L. Curr Biol. 1996;6:968–980. doi: 10.1016/s0960-9822(02)00641-3. [DOI] [PubMed] [Google Scholar]
- 20.Boiteux S, Laval J. In: Base Excision Repair. Hickson I, editor. Austin: R. G. Landes Company; 1997. , in press. [Google Scholar]
- 21.Thomas D, Scott A D, Barbey R, Padula M, Boiteux S. Mol Gen Genet. 1996;254:171–178. doi: 10.1007/s004380050405. [DOI] [PubMed] [Google Scholar]
- 22.Boiteux S. In: Oxidative Stress, Cancer, AIDS and Degenerative Diseases. Montagnier L, Pasquier C, Olivier R, editors. New York: Dekker; 1997. pp. 351–358. [Google Scholar]
- 23.Kubota Y, Nash R A, Klungland A, Schar P, Barnes D E, Lindahl T. EMBO J. 1997;15:6662–6670. [PMC free article] [PubMed] [Google Scholar]
- 24.Kolodner R D. Trends Biochem Sci. 1995;237:397–401. doi: 10.1016/s0968-0004(00)89087-8. [DOI] [PubMed] [Google Scholar]
- 25.Hoiejmakers J H J. Trends Genet. 1993;9:211–217. doi: 10.1016/0168-9525(93)90121-w. [DOI] [PubMed] [Google Scholar]
- 26.Aspinwall R, Rothwell D G, Roldan-Arjona T, Anselmino C, Ward C J, Cheadles J P, Sampson J R, Lindahl T, Harris P C, Hickson I. Proc Natl Acad Sci USA. 1997;94:109–114. doi: 10.1073/pnas.94.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cupples C G, Miller J H. Proc Natl Acad Sci USA. 1989;86:5345–5349. doi: 10.1073/pnas.86.14.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller J H. Experiments in Molecular Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Lab. Press; 1972. pp. 431–434. [Google Scholar]
- 29.Gietz D A, St. Jean R A, Schiestl R H. Nucleic Acids Res. 1992;20:1425–1426. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boiteux S, O’Connor T R, Lederer F, Gouyette A, Laval J. J Biol Chem. 1990;265:3916–3922. [PubMed] [Google Scholar]
- 31.Adams M D, Kelley J M, Gocayne J D, Dubnick M, Polymeropoulos M H, Xiao H, Merril C R, Wu A, Olde B, Moreno R F, Kerlavage A R, McCombie W R, Venter J C. Science. 1991;252:1651–1656. doi: 10.1126/science.2047873. [DOI] [PubMed] [Google Scholar]
- 32.Boiteux S, O’Connor T R, Laval J. EMBO J. 1987;6:3177–3183. doi: 10.1002/j.1460-2075.1987.tb02629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 34.Ramalho-Ordigao J F, Rosch H, Selter H, Frohlich A, Lorenz A, Montenach M, Seliger H. Antisense Res Dev. 1992;2:129–146. doi: 10.1089/ard.1992.2.129. [DOI] [PubMed] [Google Scholar]
- 35.Castaing B, Geiger A, Seliger H, Nehls P, Laval J, Boiteux S. Nucleic Acids Res. 1993;21:2889–2905. doi: 10.1093/nar/21.12.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Oliveira R, Auffret van der Kemp P, Thomas D, Geiger A, Nehls P, Boiteux S. Nucleic Acids Res. 1994;22:3760–3764. doi: 10.1093/nar/22.18.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desmaze C, Scambler P, Prieur M, Halford S, Sidi D, Le Deist F, Aurias A. Hum Genet. 1993;90:663–665. doi: 10.1007/BF00202489. [DOI] [PubMed] [Google Scholar]
- 38.Lea D E, Coulson C A. J Genet. 1949;49:264–285. doi: 10.1007/BF02986080. [DOI] [PubMed] [Google Scholar]
- 39.Kozak M. Nucleic Acids Res. 1987;15:8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thayer M M, Ahern H, Xing D, Cunningham R P, Tainer J A. EMBO J. 1995;16:4108–4120. doi: 10.1002/j.1460-2075.1995.tb00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bessho T, Tano K, Kasai H, Ohtsuka E, Nishimura S. J Biol Chem. 1993;268:19416–19421. [PubMed] [Google Scholar]
- 42.McGoldrick J P, Yeng-Chen Yeh, Solomon M, Essigman J, Lu A L. Mol Cell Biol. 1995;15:989–996. doi: 10.1128/mcb.15.2.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slupska M M, Baikolov C, Luther W M, Chiang J-H, Wei Y-F, Miller J H. J Bacteriol. 1996;178:3885–3892. doi: 10.1128/jb.178.13.3885-3892.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whitney M, Thayer M, Reifsteck C, Olson S, Smith L, Jakobs P M, Leach R, Naylor S, Joenje H, Grompe M. Nat Genet. 1995;11:341–343. doi: 10.1038/ng1195-341. [DOI] [PubMed] [Google Scholar]
- 45.Clarke A A, Philpott N J, Gordon-Smith E C, Rutherford T R. Br J Haematol. 1997;96:240–247. doi: 10.1046/j.1365-2141.1997.d01-2023.x. [DOI] [PubMed] [Google Scholar]
- 46.Takeuchi T, Morimoto K. Carcinogenesis. 1993;14:1115–1120. doi: 10.1093/carcin/14.6.1115. [DOI] [PubMed] [Google Scholar]