Abstract
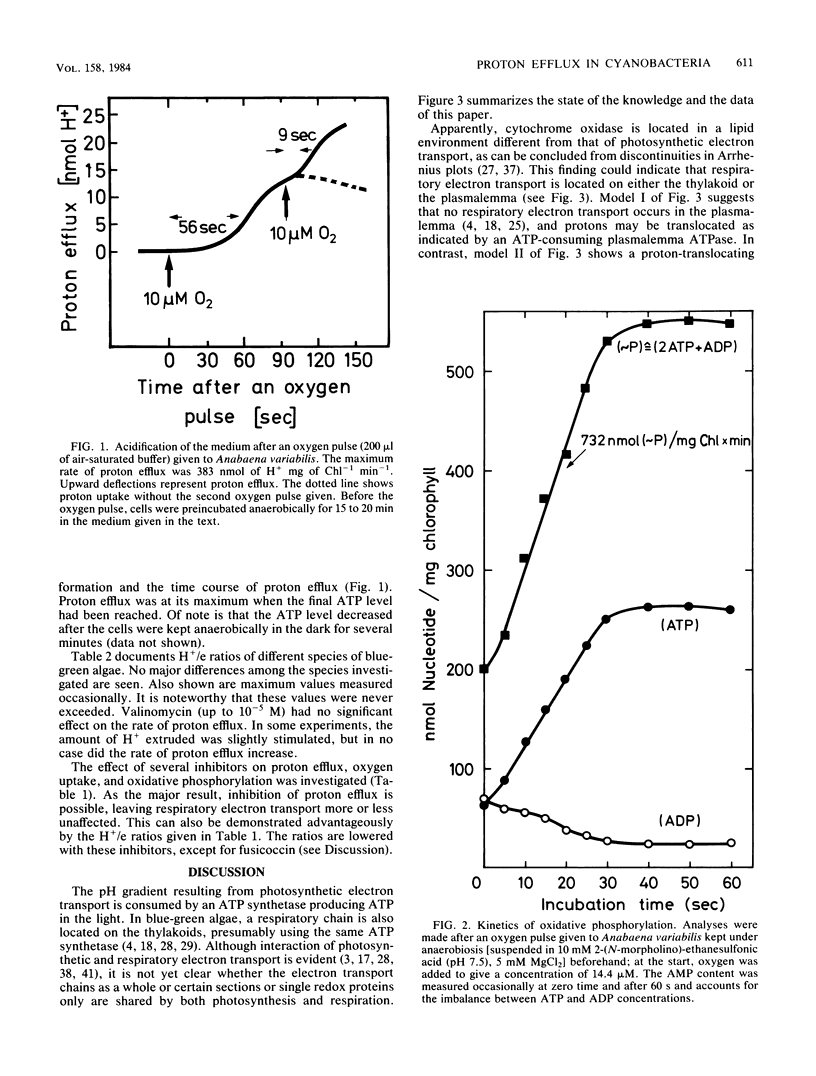
The oxygen-dependent proton efflux (in the dark) of intact cells of Anabaena variabilis and four other cyanobacteria (blue-green algae) was investigated. In contrast to bacteria and isolated mitochondria, an H+/e ratio (= protons translocated per electron transported) of only 0.23 to 0.35 and a P/e ratio of 0.8 to 1.5 were observed, indicative of respiratory electron transport being localized essentially on the thylakoids, not on the cytoplasmic membrane. Oxygen-induced acidification of the medium was sensitive to cyanide and the uncoupler carbonyl cyanide m-chlorophenylhydrazone. Inhibitors such as 2,6-dinitrophenol and vanadate exhibited a significant decrease in the H+/e ratio. After the oxygen pulse, electron transport started immediately, but proton efflux lagged 40 to 60 s behind, a period also needed before maximum ATP pool levels were attained. We suggest that proton efflux in A. variabilis is due to a proton-translocating ATP hydrolase (ATP-consuming ATPase) rather than to respiratory electron transport located on the cytoplasmic membrane.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandre A., Reynafarje B., Lehninger A. L. Stoichiometry of vectorial H+ movements coupled to electron transport and to ATP synthesis in mitochondria. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5296–5300. doi: 10.1073/pnas.75.11.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder A. Respiration and photosynthesis in energy-transducing membranes of cyanobacteria. J Bioenerg Biomembr. 1982 Dec;14(5-6):271–286. doi: 10.1007/BF00743057. [DOI] [PubMed] [Google Scholar]
- Bisalputra T., Brown D. L., Weier T. E. Possible respiratory sites in a blue-green alga Nostoc sphaericum as demonstrated by potassium tellurite and tetranitro-blue tetrazolium reduction. J Ultrastruct Res. 1969 Apr;27(2):182–197. [PubMed] [Google Scholar]
- Fillingame R. H. The proton-translocating pumps of oxidative phosphorylation. Annu Rev Biochem. 1980;49:1079–1113. doi: 10.1146/annurev.bi.49.070180.005243. [DOI] [PubMed] [Google Scholar]
- Jones C. W., Brice J. M., Downs A. J., Drozd J. W. Bacterial respiration-linked proton translocation and its relationship to respiratory-chain composition. Eur J Biochem. 1975 Mar 17;52(2):265–271. doi: 10.1111/j.1432-1033.1975.tb03994.x. [DOI] [PubMed] [Google Scholar]
- Lee-Kaden J., Simonis W. Amino acid uptake and energy coupling dependent on photosynthesis in Anacystis nidulans. J Bacteriol. 1982 Jul;151(1):229–236. doi: 10.1128/jb.151.1.229-236.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D. J., Jones C. W. Oxidative phosphorylation in bacteria which contain different cytochrome oxidases. Eur J Biochem. 1973 Jul 2;36(1):144–151. doi: 10.1111/j.1432-1033.1973.tb02894.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Acid-base titration across the membrane system of rat-liver mitochondria. Catalysis by uncouplers. Biochem J. 1967 Aug;104(2):588–600. doi: 10.1042/bj1040588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschinger H. DCCD induced sodium uptake by Anacystis nidulans. Arch Microbiol. 1977 Jun 20;113(3):285–291. doi: 10.1007/BF00492037. [DOI] [PubMed] [Google Scholar]
- Peschek G. A. Proton pump coupled to cytochrome c oxidase in the cyanobacterium Anacystis nidulans. J Bacteriol. 1983 Jan;153(1):539–542. doi: 10.1128/jb.153.1.539-542.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschek G. A., Schmetterer G. Evidence for plastoquinol-cytochrome f/b-563 reductase as a common electron donor to P700 and cytochrome oxidase in cyanobacteria. Biochem Biophys Res Commun. 1982 Oct 15;108(3):1188–1195. doi: 10.1016/0006-291x(82)92126-x. [DOI] [PubMed] [Google Scholar]
- Prochaska L. J., Bisson R., Capaldi R. A., Steffens G. C., Buse G. Inhibition of cytochrome c oxidase function by dicyclohexylcarbodiimide. Biochim Biophys Acta. 1981 Sep 14;637(2):360–373. doi: 10.1016/0005-2728(81)90175-4. [DOI] [PubMed] [Google Scholar]
- Raboy B., Padan E. Active transport of glucose and alpha-methylglucoside in the cyanobacterium Plectonema boryanum. J Biol Chem. 1978 May 10;253(9):3287–3291. [PubMed] [Google Scholar]
- Reed R. H., Rowell P., Stewart W. D. Characterization of the transport of potassium ions in the cyanobacterium Anabaena variabilis Kütz. Eur J Biochem. 1981 May 15;116(2):323–330. doi: 10.1111/j.1432-1033.1981.tb05337.x. [DOI] [PubMed] [Google Scholar]
- Scholes P., Mitchell P., Moyle J. The polarity of proton translocation in some photosynthetic microorganisms. Eur J Biochem. 1969 Apr;8(3):450–454. doi: 10.1111/j.1432-1033.1969.tb00548.x. [DOI] [PubMed] [Google Scholar]



