Abstract
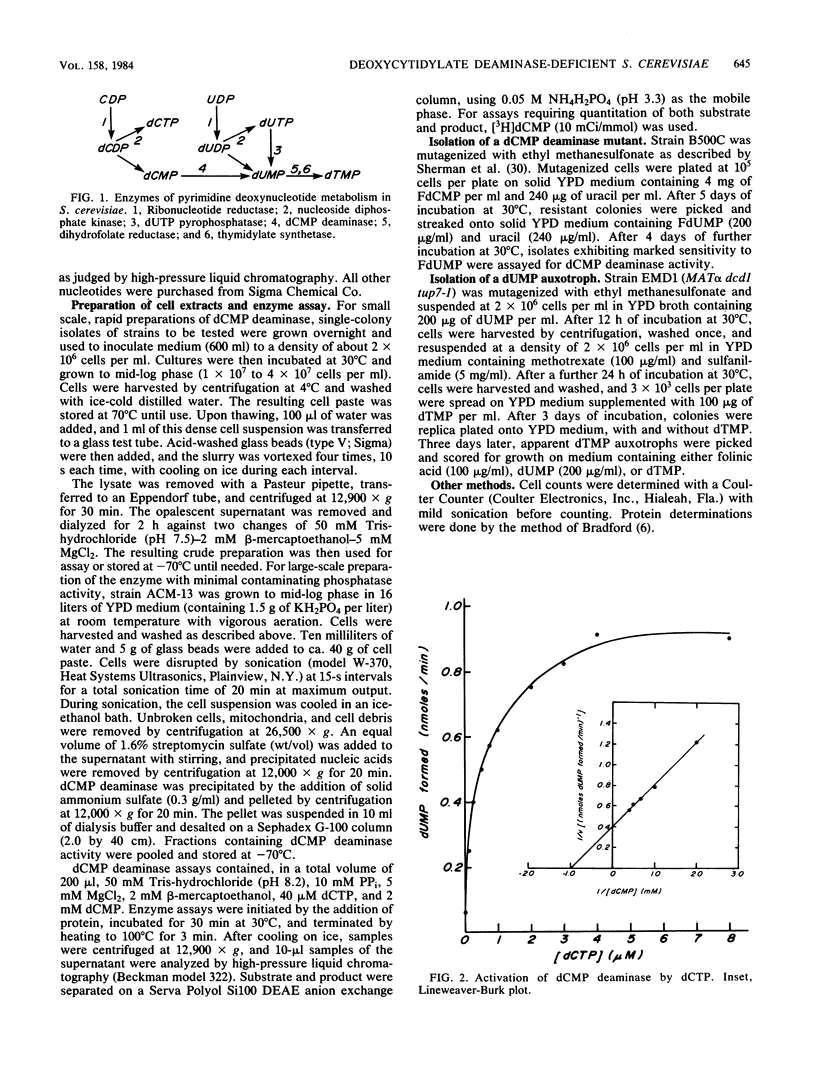
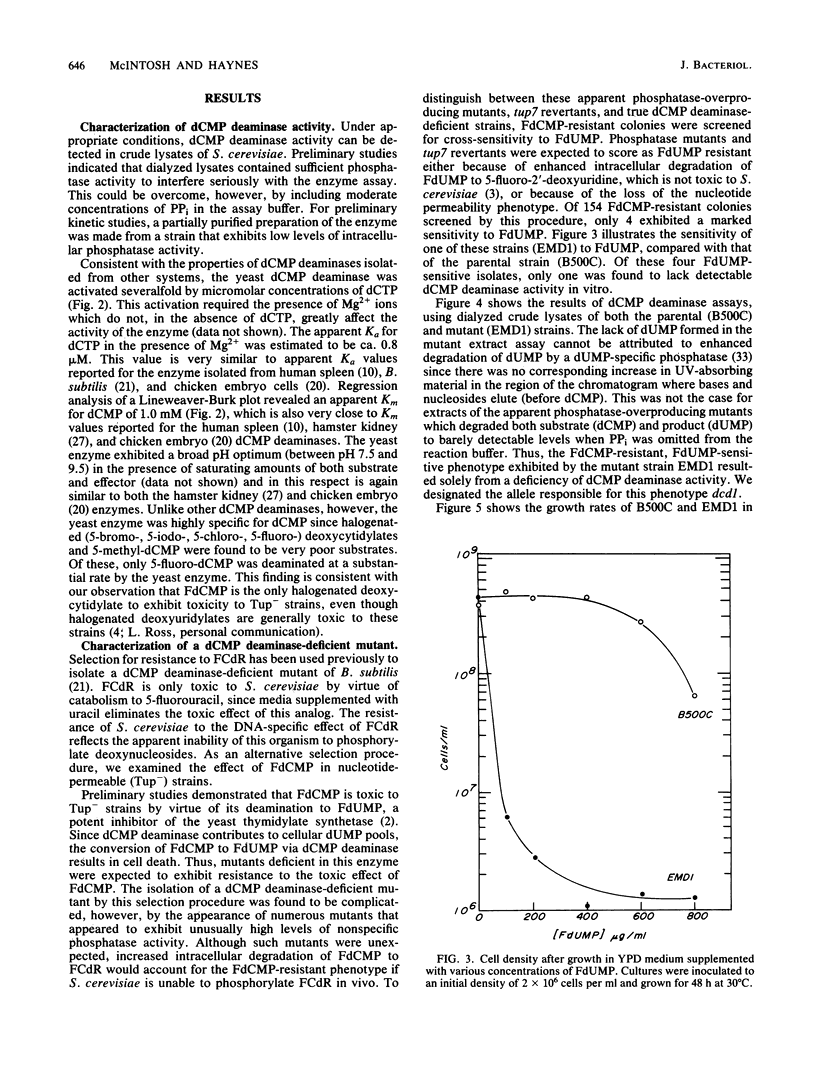
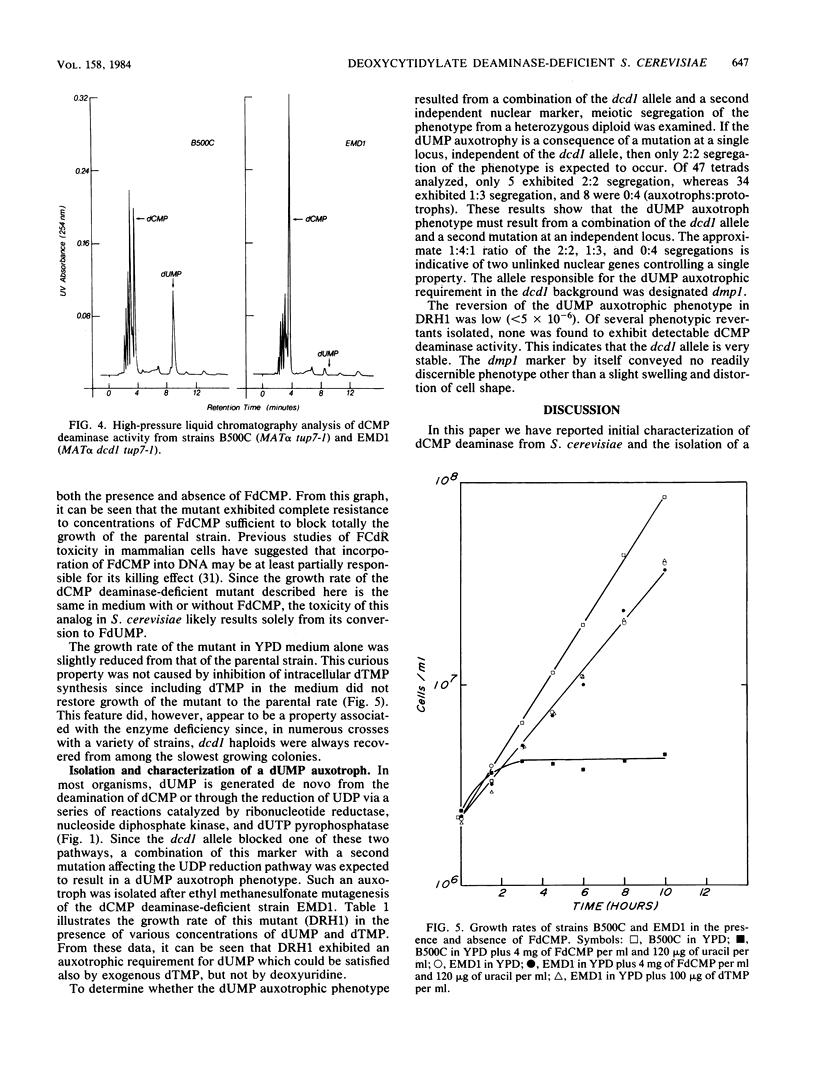
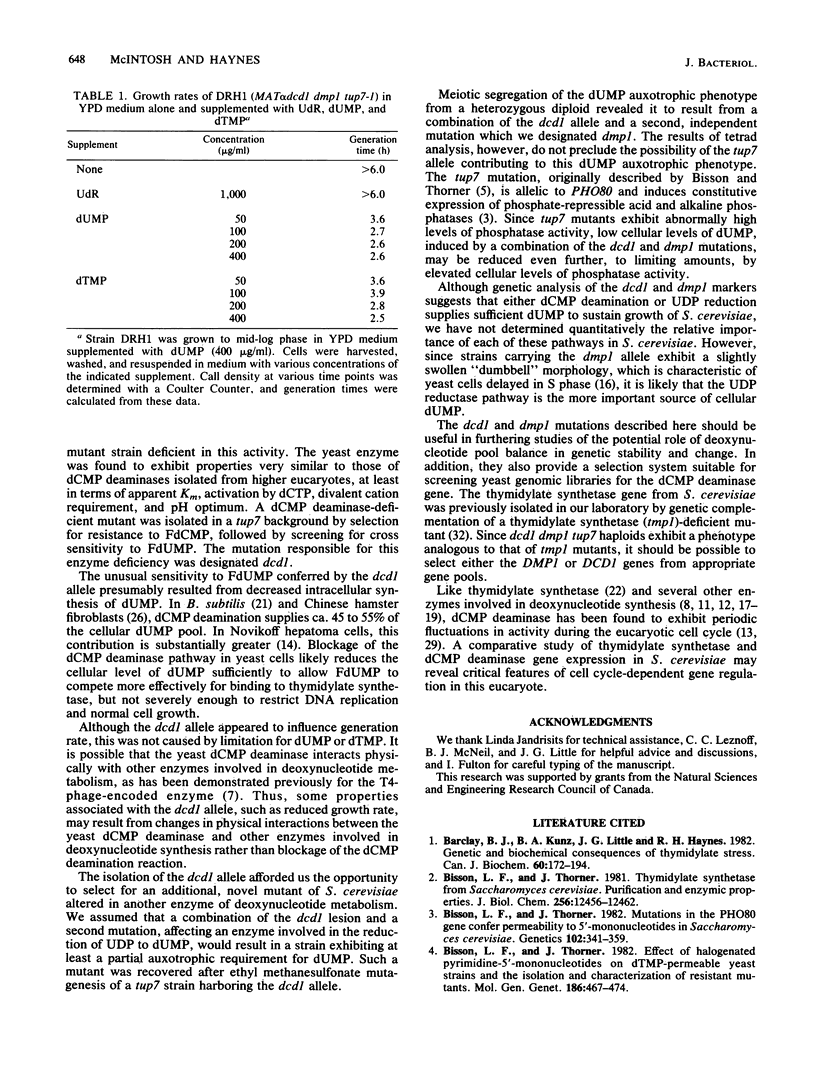
Deoxycytidylate deaminase activity in Saccharomyces cerevisiae has been partially characterized. The yeast enzyme was found to exhibit properties similar to those of dCMP deaminases isolated from higher eucaryotes. A mutant strain completely deficient in dCMP deaminase activity was isolated by selection for resistance to 5-fluoro-2'-deoxycytidylate followed by screening for cross sensitivity to 5-fluoro-2'-deoxyuridylate, a potent inhibitor of the yeast thymidylate synthetase. We have designated this new allele dcd1 . A strain exhibiting an auxotrophic requirement for dUMP was isolated after mutagenesis of a dcd1 tup7 haploid. Genetic analysis revealed that this auxotrophic phenotype resulted from a combination of the dcd1 allele and a second, unlinked, nuclear mutation that we designated dmp1 . This allele, which by itself conveys no readily discernible phenotype, presumably impairs efficient synthesis of dUMP from UDP. The auxotrophic requirement of dcd1 dmp1 tup7 strains also can be satisfied by exogenous dTMP but not deoxyuridine.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barclay B. J., Kunz B. A., Little J. G., Haynes R. H. Genetic and biochemical consequences of thymidylate stress. Can J Biochem. 1982 Mar;60(3):172–184. doi: 10.1139/o82-023. [DOI] [PubMed] [Google Scholar]
- Bisson L. F., Thorner J. Effect of halogenated pyrimidine 5'-mononucleotides on dTMP-permeable yeast strains and the isolation and characterization of resistant mutants. Mol Gen Genet. 1982;186(4):467–474. doi: 10.1007/BF00337949. [DOI] [PubMed] [Google Scholar]
- Bisson L. F., Thorner J. Exogenous dTMP utilization by a novel tup mutant of Saccharomyces cerevisiae. J Bacteriol. 1982 Oct;152(1):111–119. doi: 10.1128/jb.152.1.111-119.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson L. F., Thorner J. Mutations in the pho80 gene confer permeability to 5'-mononucleotides in Saccharomyces cerevisiae. Genetics. 1982 Nov;102(3):341–359. doi: 10.1093/genetics/102.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson L. F., Thorner J. Thymidylate synthetase from Saccharomyces cerevisiae. Purification and enzymic properties. J Biol Chem. 1981 Dec 10;256(23):12456–12462. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chiu C. S., Cook K. S., Greenberg G. R. Characteristics of a bacteriophage T4-induced complex synthesizing deoxyribonucleotides. J Biol Chem. 1982 Dec 25;257(24):15087–15097. [PubMed] [Google Scholar]
- Duker N. J., Grant C. L. Alterations in the levels of deoxyuridine triphosphatase, uracil-DNA glycosylase and AP endonuclease during the cell cycle. Exp Cell Res. 1980 Feb;125(2):493–497. doi: 10.1016/0014-4827(80)90145-7. [DOI] [PubMed] [Google Scholar]
- Duncan B. K., Diamond G. R., Bessman M. J. Regulation of enzymatic activity through subunit interaction. A possible example. J Biol Chem. 1972 Dec 25;247(24):8136–8138. [PubMed] [Google Scholar]
- Ellims P. H., Kao A. Y., Chabner B. A. Deoxycytidylate deaminase. Purification and some properties of the enzyme isolated from human spleen. J Biol Chem. 1981 Jun 25;256(12):6335–6340. [PubMed] [Google Scholar]
- Eriksson S., Martin D. W., Jr Ribonucleotide reductase in cultured mouse lymphoma cells. Cell cycle-dependent variation in the activity of subunit protein M2. J Biol Chem. 1981 Sep 25;256(18):9436–9440. [PubMed] [Google Scholar]
- Fink K. Thymidine phosphorylation in synchronous cultures of Tetrahymena pyriformis GL. Exp Cell Res. 1980 Jun;127(2):438–441. doi: 10.1016/0014-4827(80)90449-8. [DOI] [PubMed] [Google Scholar]
- Gelbard A. S., Kim J. H., Perez A. G. Fluctuations in deoxycytidine monophosphate deaminase activity during the cell cycle in synchronous populations of HeLa cells. Biochim Biophys Acta. 1969 Jun 17;182(2):564–566. doi: 10.1016/0005-2787(69)90209-3. [DOI] [PubMed] [Google Scholar]
- Hendrickson S. L., Wu J. S., Johnson L. F. Cell cycle regulation of dihydrofolate reductase mRNA metabolism in mouse fibroblasts. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5140–5144. doi: 10.1073/pnas.77.9.5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. C. The regulation of thymidylate biosynthesis in Novikoff hepatoma cells and the effects of amethopterin, 5-fluorodeoxyuridine, and 3-deazauridine. J Biol Chem. 1978 Oct 25;253(20):7440–7446. [PubMed] [Google Scholar]
- Kunz B. A., Barclay B. J., Game J. C., Little J. G., Haynes R. H. Induction of mitotic recombination in yeast by starvation for thymine nucleotides. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6057–6061. doi: 10.1073/pnas.77.10.6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz B. A. Genetic effects of deoxyribonucleotide pool imbalances. Environ Mutagen. 1982;4(6):695–725. doi: 10.1002/em.2860040609. [DOI] [PubMed] [Google Scholar]
- Lowdon M., Vitols E. Ribonucleotide reductase activity during the cell cycle of Saccharomyces cerevisiae. Arch Biochem Biophys. 1973 Sep;158(1):177–184. doi: 10.1016/0003-9861(73)90611-5. [DOI] [PubMed] [Google Scholar]
- Mahagaokar S., Orengo A., Rao P. N. The turnover of deoxyuridine triphosphate during the HeLa cell cycle. Exp Cell Res. 1980 Jan;125(1):86–94. doi: 10.1016/0014-4827(80)90192-5. [DOI] [PubMed] [Google Scholar]
- Møllgaard H., Neuhard J. Deoxycytidylate deaminase from Bacillus subtilis. Purification, characterization, and physiological function. J Biol Chem. 1978 May 25;253(10):3536–3542. [PubMed] [Google Scholar]
- Navalgund L. G., Rossana C., Muench A. J., Johnson L. F. Cell cycle regulation of thymidylate synthetase gene expression in cultured mouse fibroblasts. J Biol Chem. 1980 Aug 10;255(15):7386–7390. [PubMed] [Google Scholar]
- Neuhard J., Thomassen E. Deoxycytidine triphosphate deaminase: identification and function in Salmonella typhimurium. J Bacteriol. 1971 Feb;105(2):657–665. doi: 10.1128/jb.105.2.657-665.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan G. A., Edlin G., Fuchs J. A., Neuhard J., Thomassen E. Deoxycytidine triphosphate deaminase: characterization of an Escherichia coli mutant deficient in the enzyme. J Bacteriol. 1971 Feb;105(2):666–672. doi: 10.1128/jb.105.2.666-672.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan G. A., Neuhard J. Pyrimidine metabolism in microorganisms. Bacteriol Rev. 1970 Sep;34(3):278–343. doi: 10.1128/br.34.3.278-343.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolton H. A., Keir H. M. Deoxycytidylate deaminase. Properties of the enzyme from cultured kidney cells of baby hamster. Biochem J. 1974 Jul;141(1):211–217. doi: 10.1042/bj1410211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergott R. C., Debeer L. J., Bessman M. J. On the regulation of a bacterial deoxycytidylate deaminase. J Biol Chem. 1971 Dec 25;246(24):7755–7758. [PubMed] [Google Scholar]
- Shen S. R., Schmidt R. R. Enzymic control of nucleic acid synthesis during synchronous growth of Chlorella pyrenoidosa. II. Deoxycytidine monophosphate deaminase. Arch Biochem Biophys. 1966 Jul;115(1):13–20. doi: 10.1016/s0003-9861(66)81031-7. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Yoshida S., Saneyoshi M., Yamaguchi T. Utilization of 5-fluoro-2'-deoxyuridine triphosphate and 5-fluoro-2'-deoxycytidine triphosphate in DNA synthesis by DNA polymerases alpha and beta from calf thymus. Cancer Res. 1981 Oct;41(10):4132–4135. [PubMed] [Google Scholar]
- Taylor G. R., Barclay B. J., Storms R. K., Friesen J. D., Haynes R. H. Isolation of the thymidylate synthetase gene (TMP1) by complementation in Saccharomyces cerevisiae. Mol Cell Biol. 1982 Apr;2(4):437–442. doi: 10.1128/mcb.2.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uerkvitz W., Karlström O., Munch-Petersen A. A deoxyuridine monophosphate phosphatase detected in mutants of Escherichia coli lacking alkaline phosphatase and 5'-nucleotidase. Mol Gen Genet. 1973 Mar 19;121(4):337–346. doi: 10.1007/BF00433232. [DOI] [PubMed] [Google Scholar]
- Vitols E., Bauer V. A., Stanbrough E. C. Ribonucleotide reductase from Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1970 Oct 9;41(1):71–77. doi: 10.1016/0006-291x(70)90470-5. [DOI] [PubMed] [Google Scholar]
- de Saint Vincent B. R., Déchamps M., Buttin G. The modulation of the thymidine triphosphate pool of Chinese hamster cells by dCMP deaminase and UDP reductase. Thymidine auxotrophy induced by CTP in dCMP deaminase-deficient lines. J Biol Chem. 1980 Jan 10;255(1):162–167. [PubMed] [Google Scholar]



