Abstract
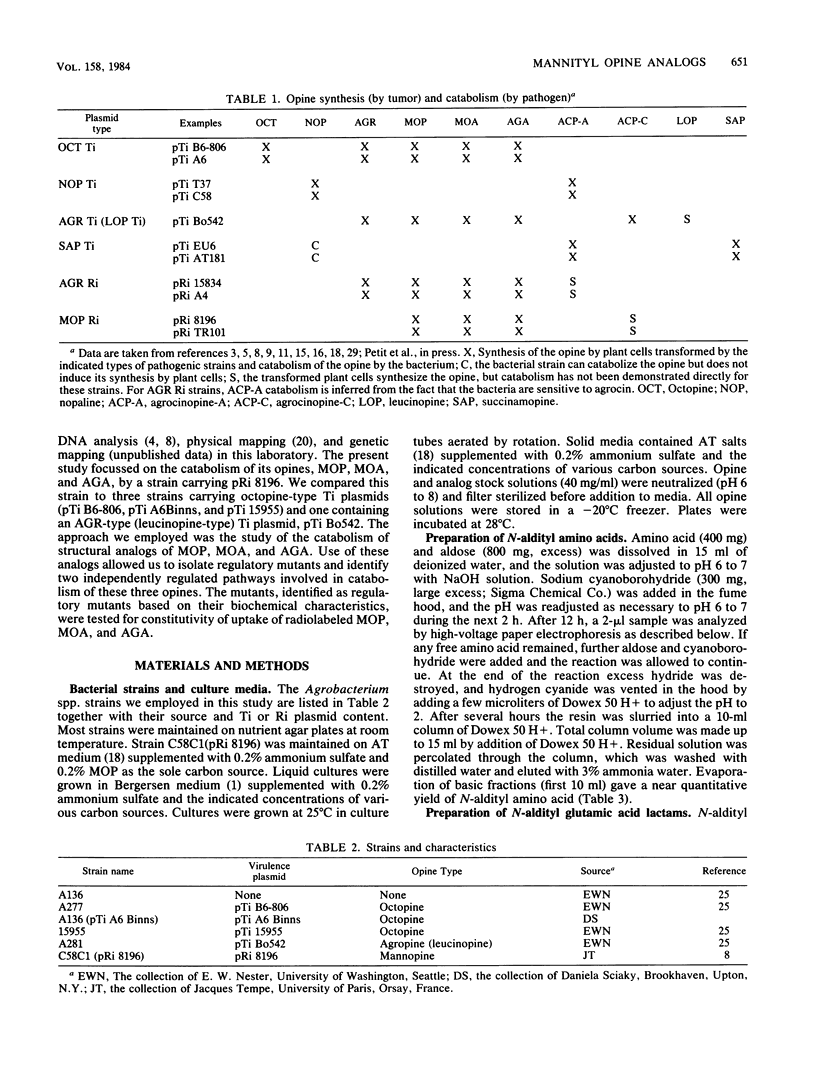
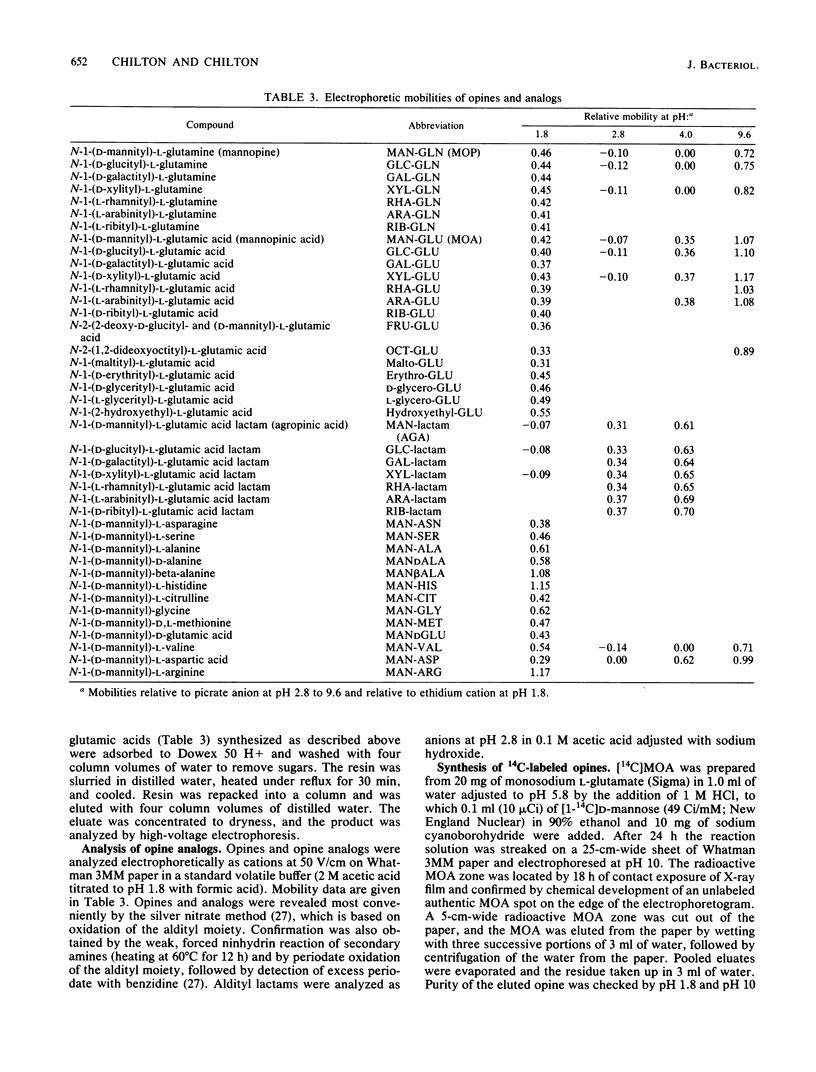
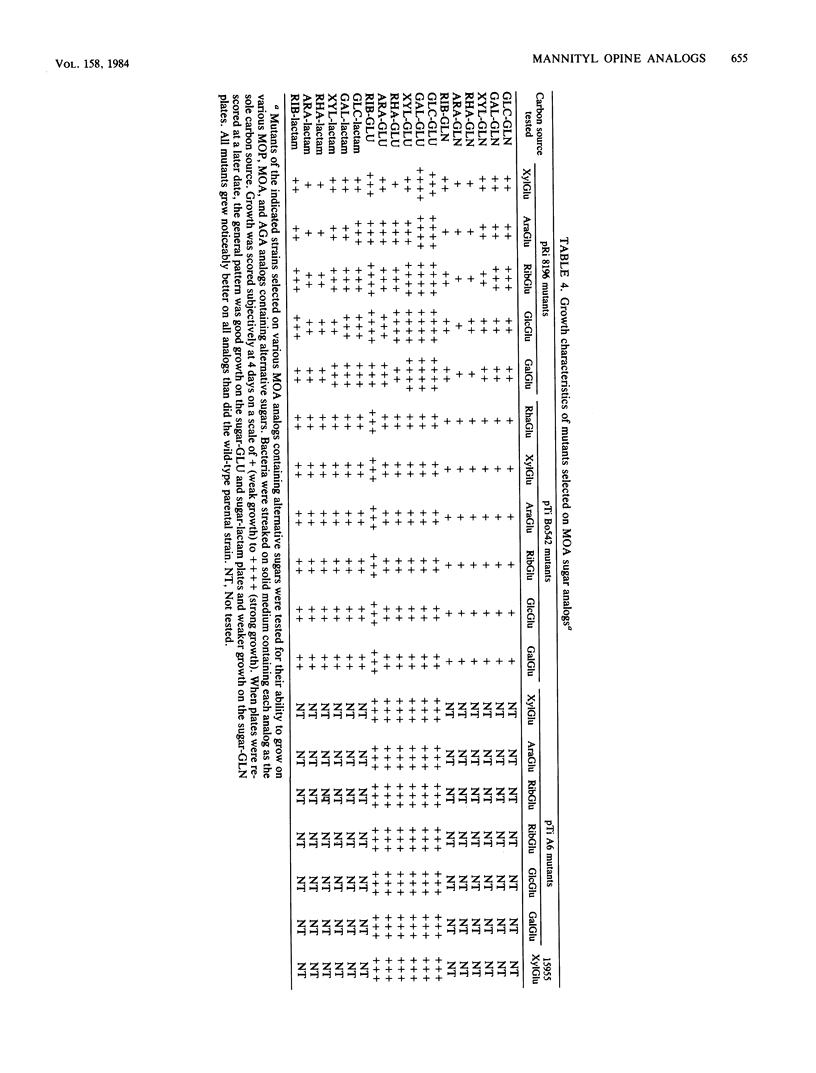
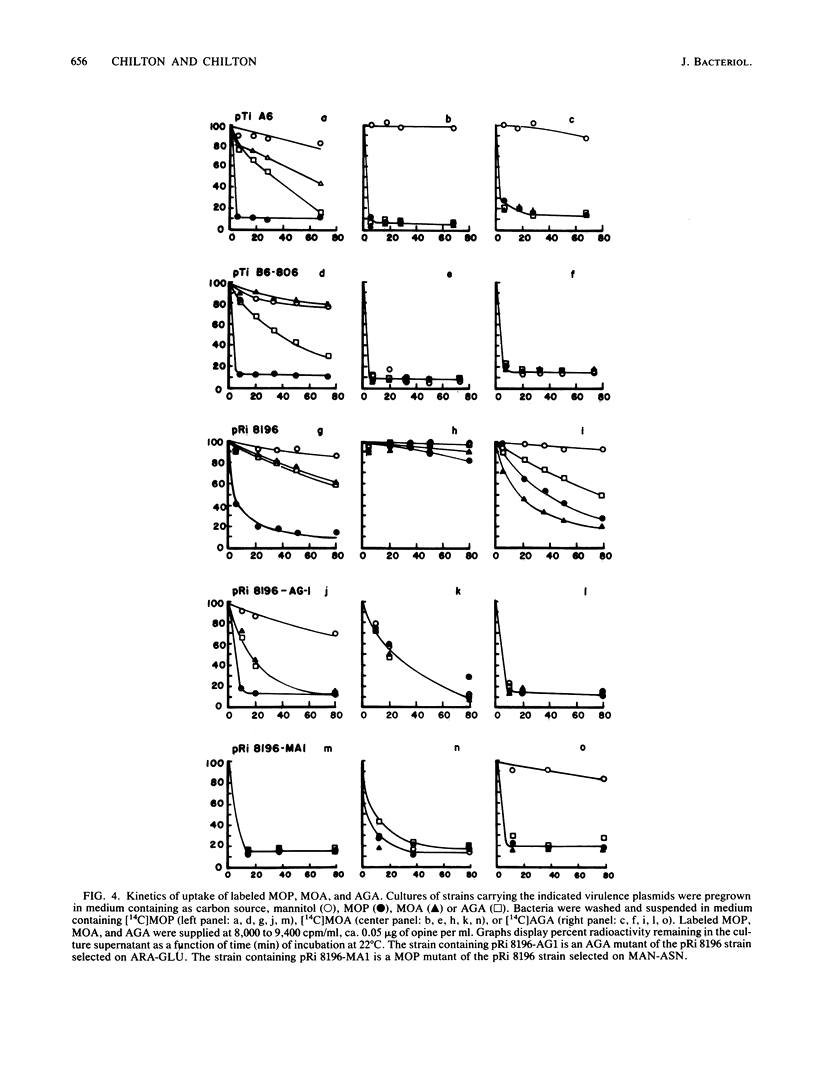
Five virulent Agrobacterium spp. strains that can catabolize the mannityl opines mannopine (MOP), mannopinic acid ( MOA ), and agropinic acid (AGA) were tested for their ability to grow on analogs of these compounds. Analogs containing alternative amino acids replacing glutamic acid or glutamine were generally refused by these bacteria, but mutants were obtained that catabolized the entire family of analogs. In the case of strain C58C1 (pRi 8196), we demonstrated that typical mutants were constitutive for MOP uptake, whereas the wild-type parent was inducible by MOP. Analogs of MOA prepared from a variety of sugars instead of mannose were generally refused, except for a strain carrying pTi B6-806, which grew well on all such analogs. The analogs allowed selection of mutants of all strains. Although most wild-type strains were inducible for AGA uptake, typical mutants selected from strain C58C1 (pRi 8196) were found to be constitutive for uptake of AGA, as was the wild-type strain carrying pTi B6-806. Such constitutive mutants grew on all sugar analogs of MOP, MOA , and AGA tested. The pTi B6-806-containing strain was tested for growth on a more extended series of analogs, including tetrose , triose, diose , and disaccharide analogs, all of which were accepted. Only ketose analogs were refused. Selection of promiscuous regulatory mutants by the two types of opine analogs suggests that the repressor proteins of MOP and AGA permease/ catabolase systems are chiefly responsible for the specificity of the pathways.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevan M., Barnes W. M., Chilton M. D. Structure and transcription of the nopaline synthase gene region of T-DNA. Nucleic Acids Res. 1983 Jan 25;11(2):369–385. doi: 10.1093/nar/11.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomhoff G., Klapwijk P. M., Kester H. C., Schilperoort R. A., Hernalsteens J. P., Schell J. Octopine and nopaline synthesis and breakdown genetically controlled by a plasmid of Agrobacterium tumefaciens. Mol Gen Genet. 1976 May 7;145(2):177–181. doi: 10.1007/BF00269591. [DOI] [PubMed] [Google Scholar]
- Byrne M. C., Koplow J., David C., Tempé J., Chilton M. D. Structure of T-DNA in roots transformed by Agrobacterium rhizogenes. J Mol Appl Genet. 1983;2(2):201–209. [PubMed] [Google Scholar]
- Chang C. C., Chen C. M., Adams B. R., Trost B. M. Leucinopine, a characteristic compound of some crown-gall tumors. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3573–3576. doi: 10.1073/pnas.80.12.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton M. D., Farrand S. K., Levin R., Nester E. W. RP4 promotion of transfer of a large Agrobacterium plasmid which confers virulence. Genetics. 1976 Aug;83(4):609–618. doi: 10.1093/genetics/83.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton W. S., Tempé J., Matzke M., Chilton M. D. Succinamopine: a new crown gall opine. J Bacteriol. 1984 Feb;157(2):357–362. doi: 10.1128/jb.157.2.357-362.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Evidence for diverse types of large plasmids in tumor-inducing strains of Agrobacterium. J Bacteriol. 1976 Apr;126(1):157–165. doi: 10.1128/jb.126.1.157-165.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Greve H., Decraemer H., Seurinck J., Van Montagu M., Schell J. The functional organization of the octopine Agrobacterium tumefaciens plasmid pTiB6s3. Plasmid. 1981 Sep;6(2):235–248. doi: 10.1016/0147-619x(81)90069-x. [DOI] [PubMed] [Google Scholar]
- De Greve H., Dhaese P., Seurinck J., Lemmers M., Van Montagu M., Schell J. Nucleotide sequence and transcript map of the Agrobacterium tumefaciens Ti plasmid-encoded octopine synthase gene. J Mol Appl Genet. 1982;1(6):499–511. [PubMed] [Google Scholar]
- Depicker A., Stachel S., Dhaese P., Zambryski P., Goodman H. M. Nopaline synthase: transcript mapping and DNA sequence. J Mol Appl Genet. 1982;1(6):561–573. [PubMed] [Google Scholar]
- Garfinkel D. J., Simpson R. B., Ream L. W., White F. F., Gordon M. P., Nester E. W. Genetic analysis of crown gall: fine structure map of the T-DNA by site-directed mutagenesis. Cell. 1981 Nov;27(1 Pt 2):143–153. doi: 10.1016/0092-8674(81)90368-8. [DOI] [PubMed] [Google Scholar]
- Guyon P., Chilton M. D., Petit A., Tempé J. Agropine in "null-type" crown gall tumors: Evidence for generality of the opine concept. Proc Natl Acad Sci U S A. 1980 May;77(5):2693–2697. doi: 10.1073/pnas.77.5.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsters M., Silva B., Van Vliet F., Genetello C., De Block M., Dhaese P., Depicker A., Inzé D., Engler G., Villarroel R. The functional organization of the nopaline A. tumefaciens plasmid pTiC58. Plasmid. 1980 Mar;3(2):212–230. doi: 10.1016/0147-619x(80)90110-9. [DOI] [PubMed] [Google Scholar]
- Koplow J., Byrne M. C., Jen G., Tempé J., Chilton M. D. Physical map of the Agrobacterium rhizogenes strain 8196 virulence plasmid. Plasmid. 1984 Jan;11(1):17–27. doi: 10.1016/0147-619x(84)90003-9. [DOI] [PubMed] [Google Scholar]
- Murai N., Kemp J. D. Octopine synthase mRNA isolated from sunflower crown gall callus is homologous to the Ti plasmid of Agrobacterium tumefaciens. Proc Natl Acad Sci U S A. 1982 Jan;79(1):86–90. doi: 10.1073/pnas.79.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciaky D., Montoya A. L., Chilton M. D. Fingerprints of Agrobacterium Ti plasmids. Plasmid. 1978 Feb;1(2):238–253. doi: 10.1016/0147-619x(78)90042-2. [DOI] [PubMed] [Google Scholar]
- Smith E. F., Townsend C. O. A PLANT-TUMOR OF BACTERIAL ORIGIN. Science. 1907 Apr 26;25(643):671–673. doi: 10.1126/science.25.643.671. [DOI] [PubMed] [Google Scholar]
- Valdes F., Dasheiff R. M., Birmingham F., Crutcher K. A., McNamara J. O. Benzodiazepine receptor increases after repeated seizures: evidence for localization to dentate granule cells. Proc Natl Acad Sci U S A. 1982 Jan;79(1):193–197. doi: 10.1073/pnas.79.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Larebeke N., Engler G., Holsters M., Van den Elsacker S., Zaenen I., Schilperoort R. A., Schell J. Large plasmid in Agrobacterium tumefaciens essential for crown gall-inducing ability. Nature. 1974 Nov 8;252(5479):169–170. doi: 10.1038/252169a0. [DOI] [PubMed] [Google Scholar]
- Van Larebeke N., Genetello C., Schell J., Schilperoort R. A., Hermans A. K., Van Montagu M., Hernalsteens J. P. Acquisition of tumour-inducing ability by non-oncogenic agrobacteria as a result of plasmid transfer. Nature. 1975 Jun 26;255(5511):742–743. doi: 10.1038/255742a0. [DOI] [PubMed] [Google Scholar]
- Watson B., Currier T. C., Gordon M. P., Chilton M. D., Nester E. W. Plasmid required for virulence of Agrobacterium tumefaciens. J Bacteriol. 1975 Jul;123(1):255–264. doi: 10.1128/jb.123.1.255-264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. R., Braun A. C. CROWN GALL PRODUCTION BY BACTERIA-FREE TUMOR TISSUES. Science. 1941 Sep 5;94(2436):239–241. doi: 10.1126/science.94.2436.239. [DOI] [PubMed] [Google Scholar]
- Willmitzer L., Simons G., Schell J. The TL-DNA in octopine crown-gall tumours codes for seven well-defined polyadenylated transcripts. EMBO J. 1982;1(1):139–146. doi: 10.1002/j.1460-2075.1982.tb01137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaenen I., Van Larebeke N., Van Montagu M., Schell J. Supercoiled circular DNA in crown-gall inducing Agrobacterium strains. J Mol Biol. 1974 Jun 15;86(1):109–127. doi: 10.1016/s0022-2836(74)80011-2. [DOI] [PubMed] [Google Scholar]
- Zambryski P., Holsters M., Kruger K., Depicker A., Schell J., Van Montagu M., Goodman H. M. Tumor DNA structure in plant cells transformed by A. tumefaciens. Science. 1980 Sep 19;209(4463):1385–1391. doi: 10.1126/science.6251546. [DOI] [PubMed] [Google Scholar]


