Abstract
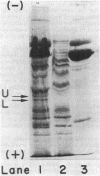
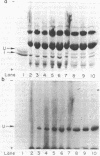
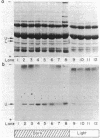
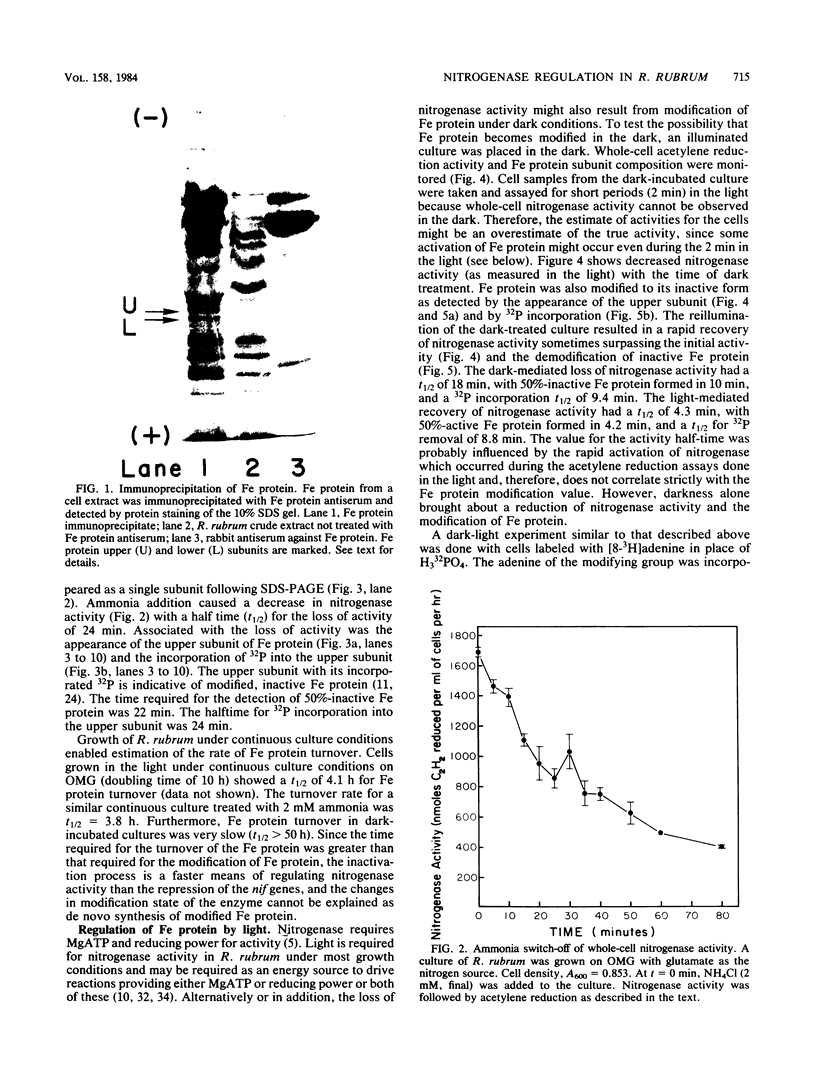
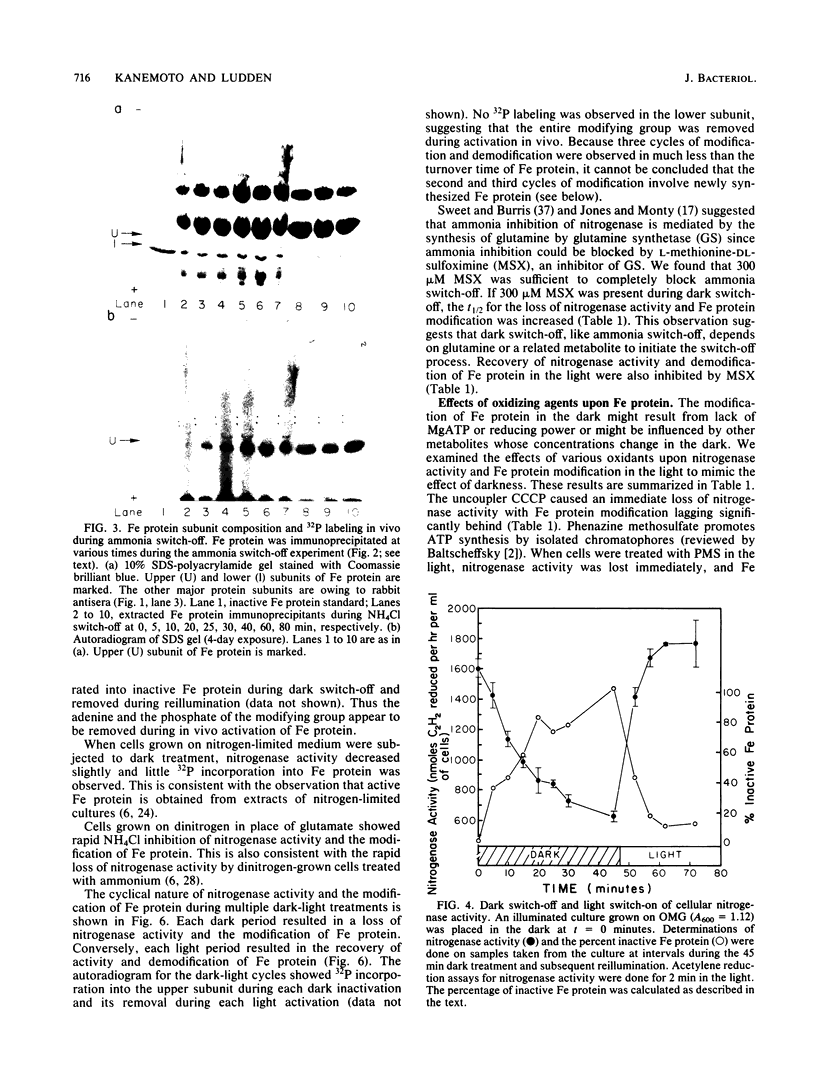
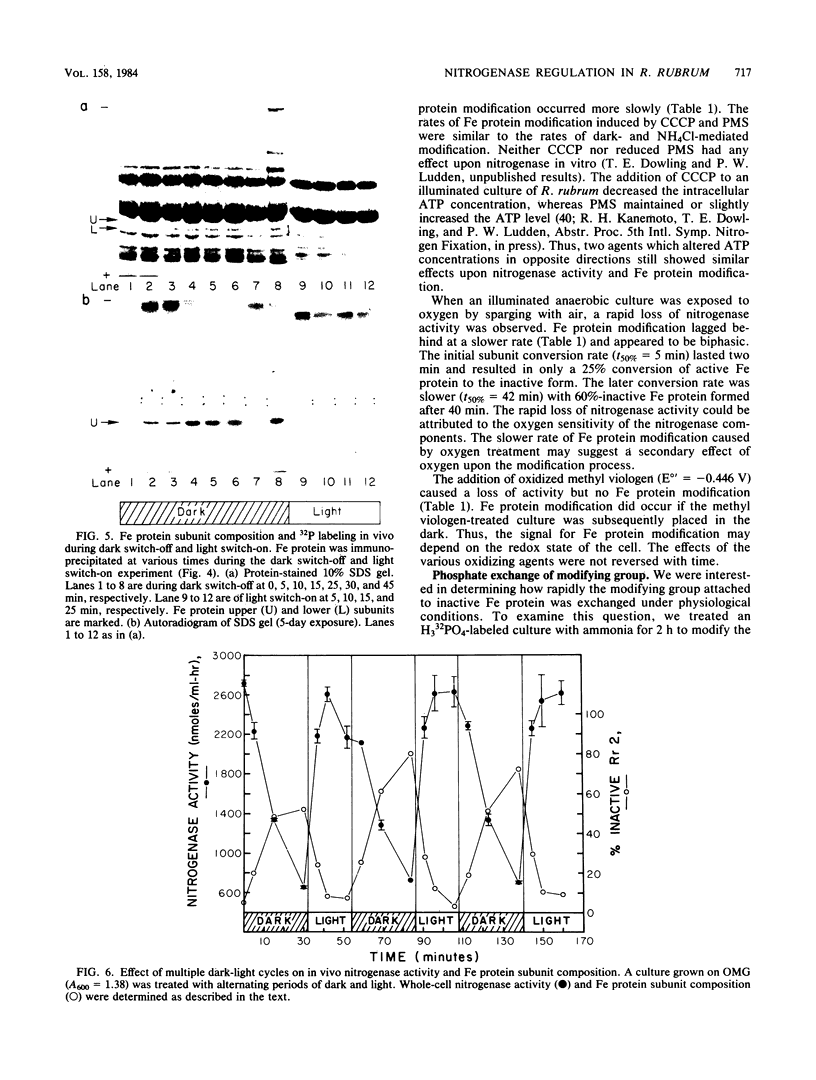
A procedure for the immunoprecipitation of Fe protein from cell extracts was developed and used to monitor the modification of Fe protein in vivo. The subunit pattern of the isolated Fe protein after sodium dodecyl sulfate-polyacrylamide gel electrophoresis was assayed by Coomassie brilliant blue protein staining and autoradiographic 32P detection of the modifying group. Whole-cell nitrogenase activity was also monitored during Fe protein modification. The addition of ammonia, darkness, oxygen, carbonyl cyanide m-chlorophenylhydrazone, and phenazine methosulfate each resulted in a loss of whole-cell nitrogenase activity and the in vivo modification of Fe protein. For ammonia and darkness, the rate of loss of nitrogenase activity was similar to that for Fe protein modification. The reillumination of a culture incubated in the dark brought about a rapid recovery of nitrogenase activity and the demodification of Fe protein. Cyclic dark-light treatments resulted in matching cycles of nitrogenase activity and Fe protein modification. Carbonyl cyanide m-chlorophenylhydrazone and phenazine methosulfate treatments caused an immediate loss of nitrogenase activity, whereas Fe protein modification occurred at a slower rate. Oxygen treatment resulted in a rapid loss of activity but only an incomplete modification of the Fe protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bognar A., Desrosiers L., Libman M., Newman E. B. Control of nitrogenase in a photosynthetic autotrophic bacterium, Ectothiorhodospira sp. J Bacteriol. 1982 Nov;152(2):706–713. doi: 10.1128/jb.152.2.706-713.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Burns R. C., Bulen W. A. A procedure for the preparation of extracts from Rhodospirillum rubrum catalyzing N2 reduction and ATP-dependent H2 evolution. Arch Biochem Biophys. 1966 Feb;113(2):461–463. doi: 10.1016/0003-9861(66)90215-3. [DOI] [PubMed] [Google Scholar]
- Carithers R. P., Yoch D. C., Arnon D. I. Two forms of nitrogenase from the photosynthetic bacterium Rhodospirillum rubrum. J Bacteriol. 1979 Feb;137(2):779–789. doi: 10.1128/jb.137.2.779-789.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling T. E., Preston G. G., Ludden P. W. Heat activation of the Fe protein of nitrogenase from Rhodospirillum rubrum. J Biol Chem. 1982 Dec 10;257(23):13987–13992. [PubMed] [Google Scholar]
- Gest H., Kamen M. D. Photoproduction of Molecular Hydrogen by Rhodospirillum rubrum. Science. 1949 Jun 3;109(2840):558–559. doi: 10.1126/science.109.2840.558. [DOI] [PubMed] [Google Scholar]
- Gotto J. W., Yoch D. C. Regulation of Rhodospirillum rubrum nitrogenase activity. Properties and interconversion of active and inactive Fe protein. J Biol Chem. 1982 Mar 25;257(6):2868–2873. [PubMed] [Google Scholar]
- Haaker H., Laane C., Hellingwerf K., Houwer B., Konings W. N., Veeger C. Short-term regulation of the nitrogenase activity in Rhodopseudomonas sphaeroides. Eur J Biochem. 1982 Oct;127(3):639–645. doi: 10.1111/j.1432-1033.1982.tb06920.x. [DOI] [PubMed] [Google Scholar]
- Hallenbeck P. C., Meyer C. M., Vignais P. M. Nitrogenase from the photosynthetic bacterium Rhodopseudomonas capsulata: purification and molecular properties. J Bacteriol. 1982 Feb;149(2):708–717. doi: 10.1128/jb.149.2.708-717.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard K. S., Hales B. J., Socolofsky M. D. Nitrogen fixation and ammonia switch-off in the photosynthetic bacterium Rhodopseudomonas viridis. J Bacteriol. 1983 Jul;155(1):107–112. doi: 10.1128/jb.155.1.107-112.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson B. C., Gest H. Adenylylation/deadenylylation control of the glutamine synthetase of Rhodopseudomonas capsulata. Eur J Biochem. 1977 Dec 1;81(2):365–371. doi: 10.1111/j.1432-1033.1977.tb11960.x. [DOI] [PubMed] [Google Scholar]
- Jones B. L., Monty K. J. Glutamine as a feedback inhibitor of the Rhodopseudomonas sphaeroides nitrogenase system. J Bacteriol. 1979 Sep;139(3):1007–1013. doi: 10.1128/jb.139.3.1007-1013.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch B., Evans H. J. Reduction of acetylene to ethylene by soybean root nodules. Plant Physiol. 1966 Dec;41(10):1748–1750. doi: 10.1104/pp.41.10.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ludden P. W., Burris R. H. Activating factor for the iron protein of nitrogenase from Rhodospirillum rubrum. Science. 1976 Oct 22;194(4263):424–426. doi: 10.1126/science.824729. [DOI] [PubMed] [Google Scholar]
- Ludden P. W., Burris R. H. Purification and properties of nitrogenase from Rhodospirillum rubrum, and evidence for phosphate, ribose and an adenine-like unit covalently bound to the iron protein. Biochem J. 1978 Oct 1;175(1):251–259. doi: 10.1042/bj1750251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludden P. W., Burris R. H. Removal of an adenine-like molecule during activation of dinitrogenase reductase from Rhodospirillum rubrum. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6201–6205. doi: 10.1073/pnas.76.12.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludden P. W., Preston G. G., Dowling T. E. Comparison of active and inactive forms of iron protein from Rhodospirillum rubrum. Biochem J. 1982 Jun 1;203(3):663–668. doi: 10.1042/bj2030663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan M. T., Wall J. D., Gest H. Dark anaerobic dinitrogen fixation by a photosynthetic microorganism. Science. 1979 Jun 29;204(4400):1429–1430. doi: 10.1126/science.204.4400.1429. [DOI] [PubMed] [Google Scholar]
- Munson T. O., Burris R. H. Nitrogen fixation by Rhodospirillum rubrum grown in nitrogen-limited continuous culture. J Bacteriol. 1969 Mar;97(3):1093–1098. doi: 10.1128/jb.97.3.1093-1098.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson A. H., Nordlund S. Regulation of nitrogenase synthesis in intact cells of Rhodospirillum rubrum: inactivation of nitrogen fixation by ammonia, L-glutamine and L-asparagine. J Gen Microbiol. 1975 Nov;91(1):53–62. doi: 10.1099/00221287-91-1-53. [DOI] [PubMed] [Google Scholar]
- Nordlund S., Eriksson U., Baltscheffsky H. Necessity of a membrane component for nitrogenase activity in Rhodospirillum rubrum. Biochim Biophys Acta. 1977 Oct 12;462(1):187–195. doi: 10.1016/0005-2728(77)90201-8. [DOI] [PubMed] [Google Scholar]
- Nordlund S., Ludden P. W. Incorporation of adenine into the modifying group of inactive iron protein of nitrogenase from Rhodospirillum rubrum. Biochem J. 1983 Mar 1;209(3):881–884. doi: 10.1042/bj2090881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORMEROD J. G., ORMEROD K. S., GEST H. Light-dependent utilization of organic compounds and photoproduction of molecular hydrogen by photosynthetic bacteria; relationships with nitrogen metabolism. Arch Biochem Biophys. 1961 Sep;94:449–463. doi: 10.1016/0003-9861(61)90073-x. [DOI] [PubMed] [Google Scholar]
- Pratt D. C., Frenkel A. W. Studies on Nitrogen Fixation and Photosynthesis of Rhodospirillum Rubrum. Plant Physiol. 1959 May;34(3):333–337. doi: 10.1104/pp.34.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston G. G., Ludden P. W. Change in subunit composition of the iron protein of nitrogenase from Rhodospirillum rubrum during activation and inactivation of iron protein. Biochem J. 1982 Sep 1;205(3):489–494. doi: 10.1042/bj2050489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schick H. J. Regulation of photoreduction in Rhodospirillum rubrum by ammonia. Arch Mikrobiol. 1971;75(2):110–120. doi: 10.1007/BF00407999. [DOI] [PubMed] [Google Scholar]
- Schick H. J. Substrate and light dependent fixation of molecular nitrogen in Rhodospirillum rubrum. Arch Mikrobiol. 1971;75(2):89–101. doi: 10.1007/BF00407997. [DOI] [PubMed] [Google Scholar]
- Stewart W. D., Fitzgerald G. P., Burris R. H. In situ studies on N2 fixation using the acetylene reduction technique. Proc Natl Acad Sci U S A. 1967 Nov;58(5):2071–2078. doi: 10.1073/pnas.58.5.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet W. J., Burris R. H. Inhibition of nitrogenase activity by NH+4 in Rhodospirillum rubrum. J Bacteriol. 1981 Feb;145(2):824–831. doi: 10.1128/jb.145.2.824-831.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoch D. C., Cantu M. Changes in the regulatory form of Rhodospirillum rubrum nitrogenase as influenced by nutritional and environmental factors. J Bacteriol. 1980 Jun;142(3):899–907. doi: 10.1128/jb.142.3.899-907.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoch D. C., Gotto J. W. Effect of light intensity and inhibitors of nitrogen assimilation on NH4+ inhibition of nitrogenase activity in Rhodospirillum rubrum and Anabaena sp. J Bacteriol. 1982 Aug;151(2):800–806. doi: 10.1128/jb.151.2.800-806.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoch D. C. Regulation of nitrogenase A and R concentrations in Rhodopseudomonas capsulata by glutamine synthetase. Biochem J. 1980 Apr 1;187(1):273–276. doi: 10.1042/bj1870273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumft W. G., Castillo F. Regulatory properties of the nitrogenase from Rhodopseudomonas palustris. Arch Microbiol. 1978 Apr 27;117(1):53–60. doi: 10.1007/BF00689351. [DOI] [PubMed] [Google Scholar]