Abstract
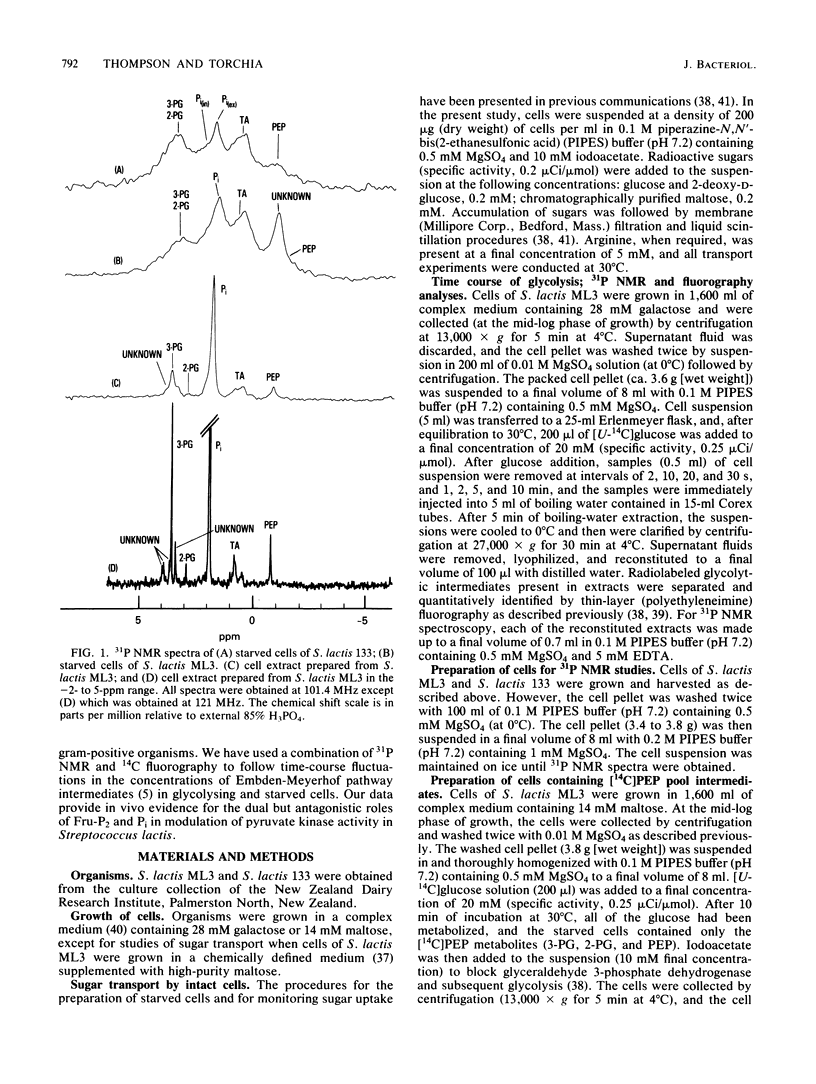
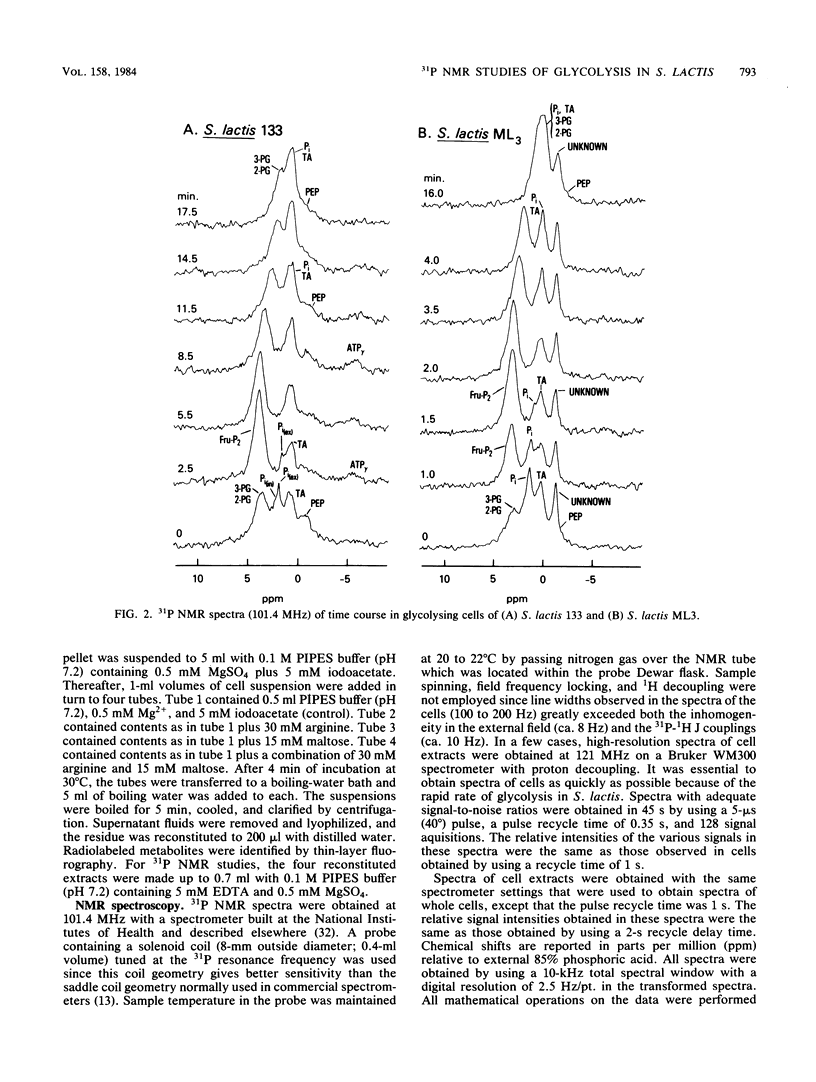
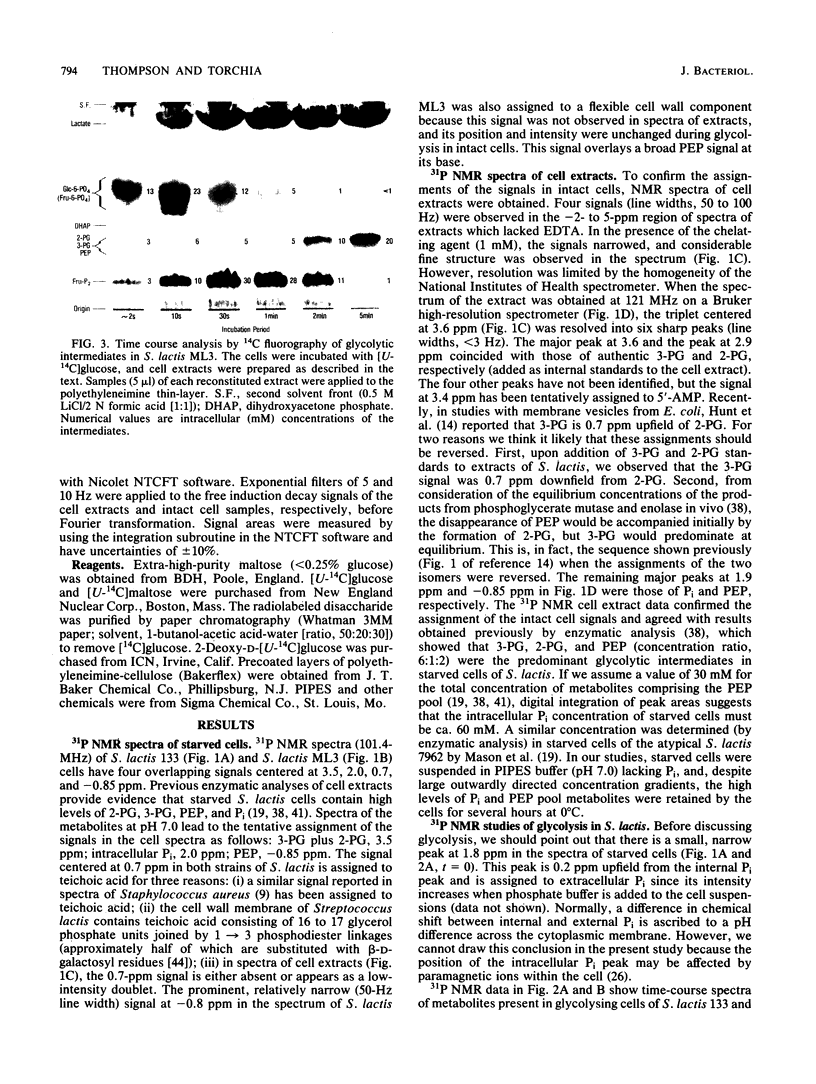
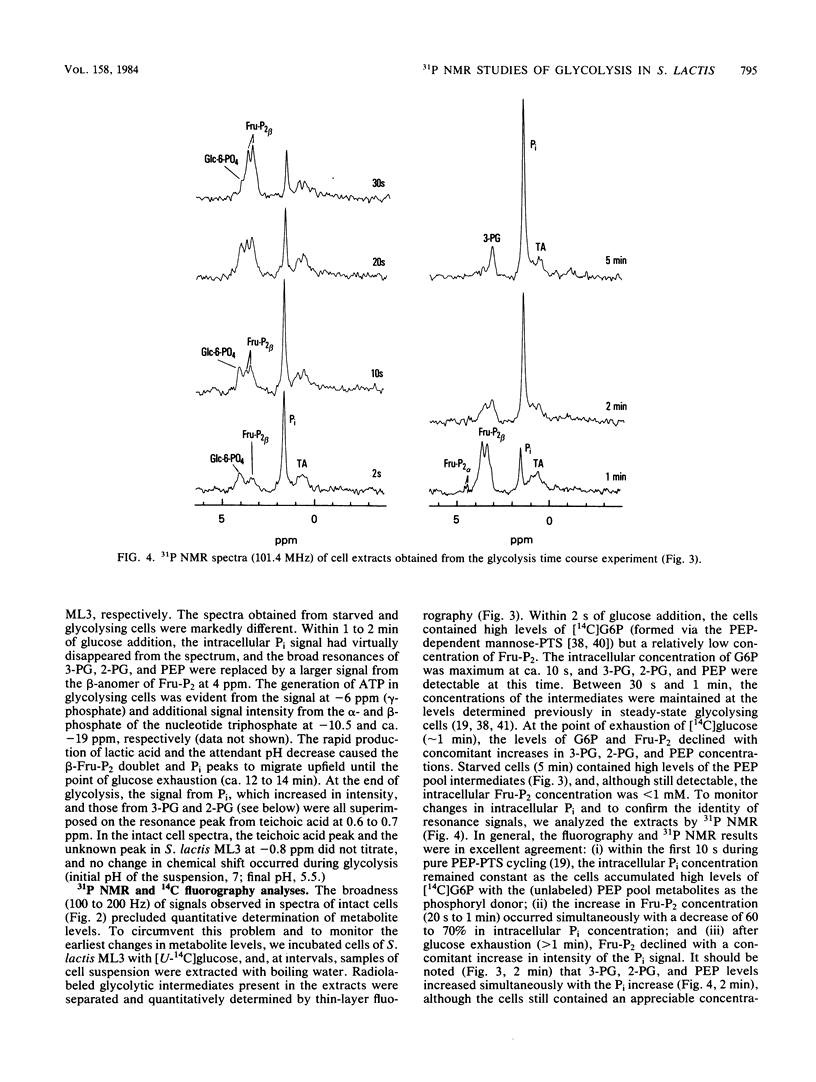
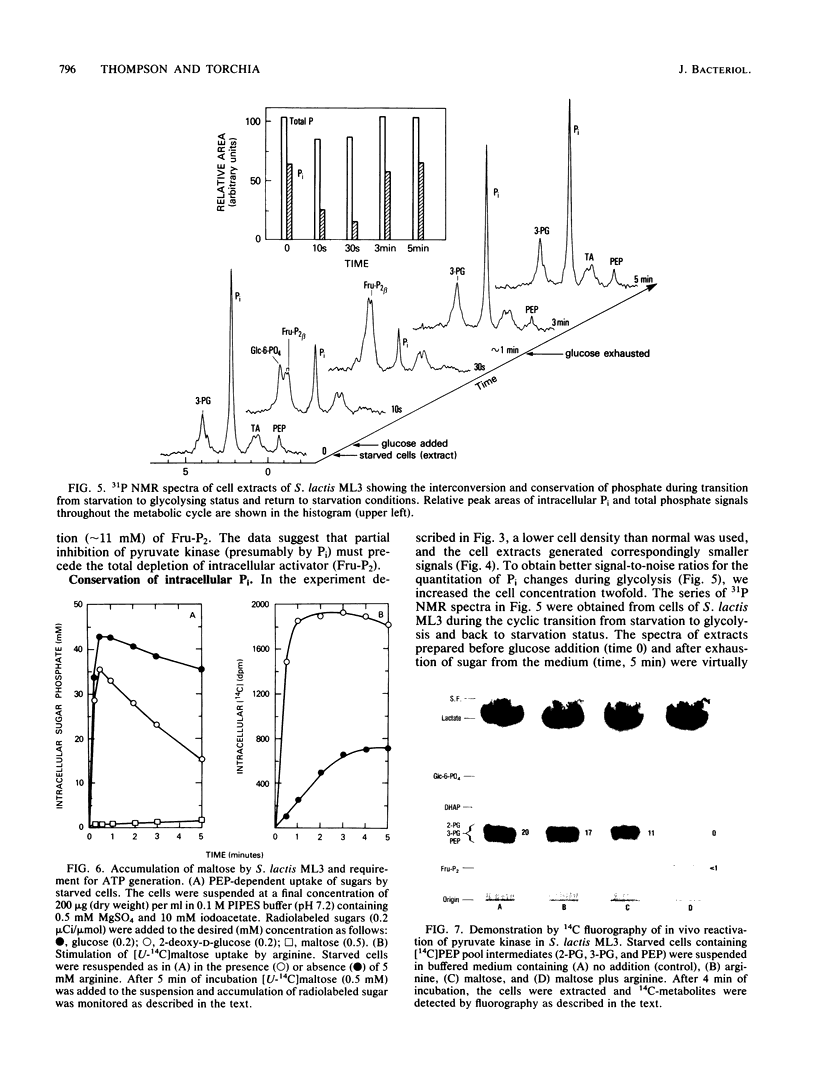
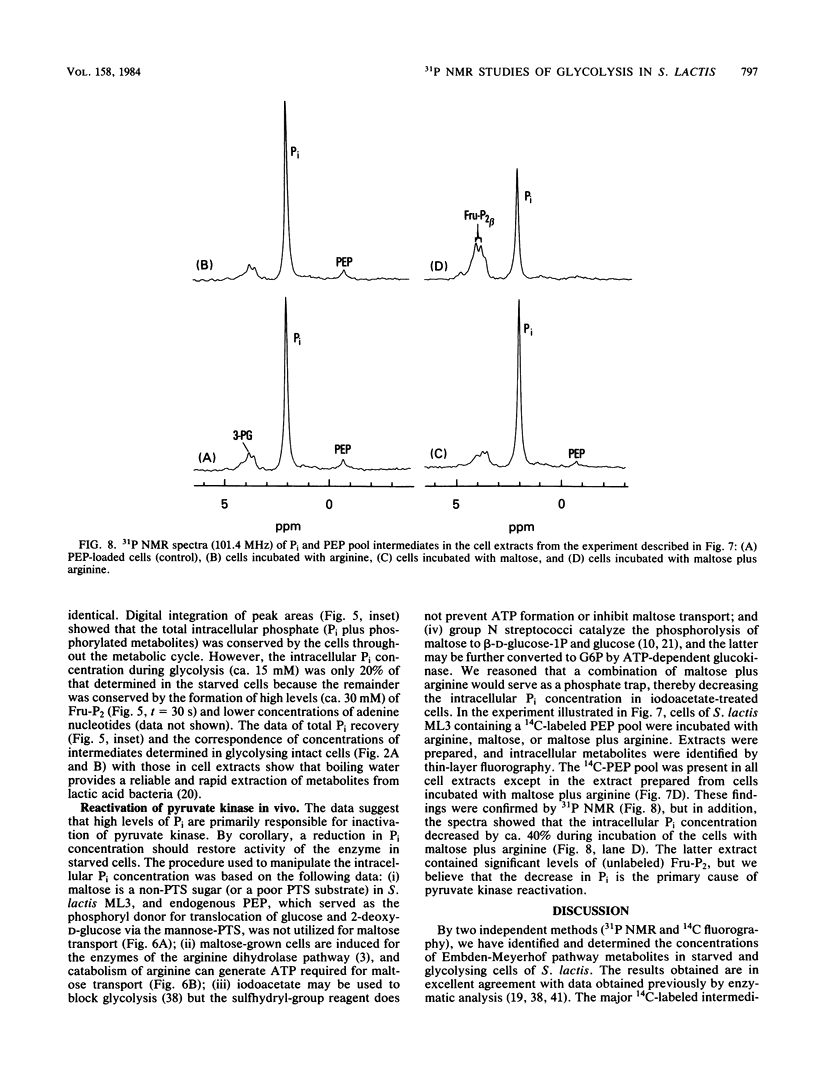
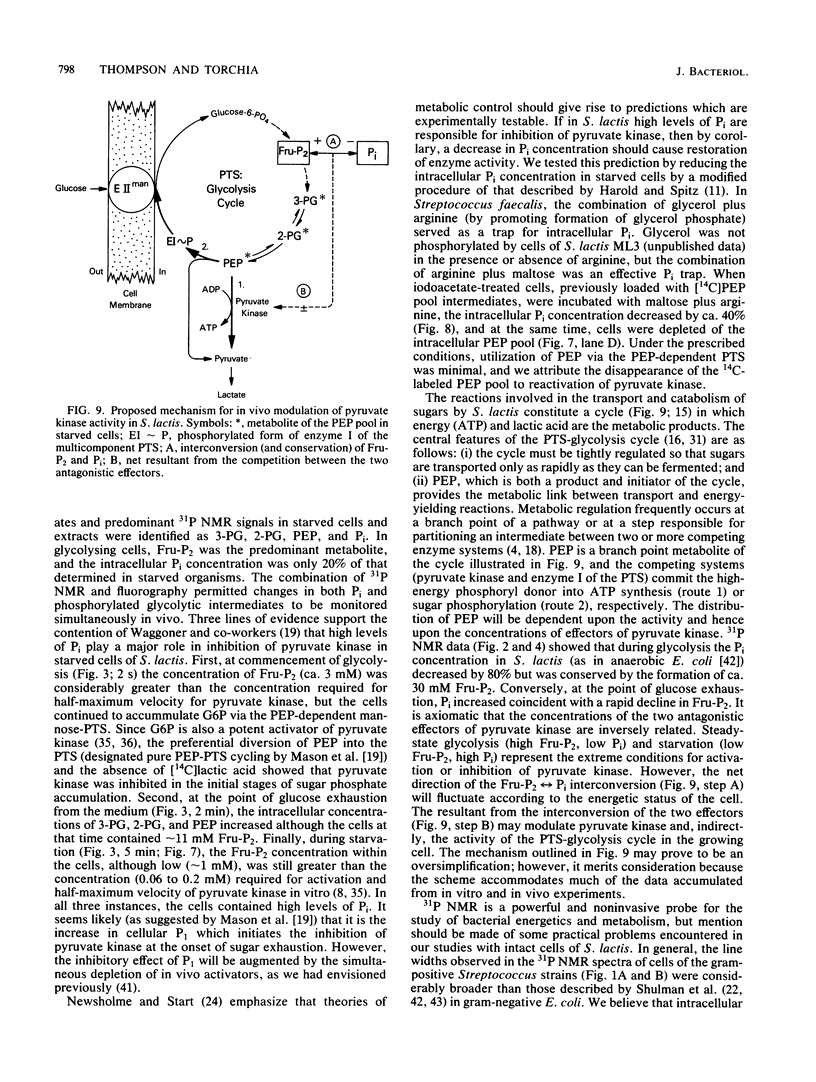
High-resolution 31P nuclear magnetic resonance spectroscopy and 14C fluorography have been used to identify and quantitate intermediates of the Embden-Meyerhof pathway in intact cells and cell extracts of Streptococcus lactis. Glycolysing cells contained high levels of fructose 1,6-bisphosphate (a positive effector of pyruvate kinase) but comparatively low concentrations of other glycolytic metabolites. By contrast, starved organisms contained only high levels of 3-phosphoglycerate, 2-phosphoglycerate, and phosphoenolpyruvate. The concentration of Pi (a negative effector of pyruvate kinase) in starved cells was fourfold greater than that maintained by glycolysing cells. The following result suggest that retention of the phosphoenolpyruvate pool by starved cells is a consequence of Pi-mediated inhibition of pyruvate kinase: the increase in the phosphoenolpyruvate pool (and Pi) preceded depletion of fructose 1,6-bisphosphate, and reduction in intracellular Pi (by a maltose-plus-arginine phosphate trap) caused the restoration of pyruvate kinase activity in starved cells. Time course studies showed that Pi was conserved by formation of fructose 1,6-bisphosphate during glycolysis. Conversely, during starvation high levels of Pi were generated concomitant with depletion of intracellular fructose 1,6-bisphosphate. The concentrations of Pi and fructose 1,6-bisphosphate present in starved and glycolysing cells of S. lactis varied inversely. The activity of pyruvate kinase in the growing cell may be modulated by the relative concentrations of the two antagonistic effectors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbe K., Takahashi S., Yamada T. Purification and properties of pyruvate kinase from Streptococcus sanguis and activator specificity of pyruvate kinase from oral streptococci. Infect Immun. 1983 Mar;39(3):1007–1014. doi: 10.1128/iai.39.3.1007-1014.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbe K., Yamada T. Purification and properties of pyruvate kinase from Streptococcus mutans. J Bacteriol. 1982 Jan;149(1):299–305. doi: 10.1128/jb.149.1.299-305.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelal A. T. Arginine catabolism by microorganisms. Annu Rev Microbiol. 1979;33:139–168. doi: 10.1146/annurev.mi.33.100179.001035. [DOI] [PubMed] [Google Scholar]
- Chassy B. M., Thompson J. Regulation of lactose-phosphoenolpyruvate-dependent phosphotransferase system and beta-D-phosphogalactoside galactohydrolase activities in Lactobacillus casei. J Bacteriol. 1983 Jun;154(3):1195–1203. doi: 10.1128/jb.154.3.1195-1203.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins L. B., Thomas T. D. Pyruvate kinase of Streptococcus lactis. J Bacteriol. 1974 Oct;120(1):52–58. doi: 10.1128/jb.120.1.52-58.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow V. L., Pritchard G. G. Purification and properties of pyruvate kinase from Streptococcus lactis. Biochim Biophys Acta. 1976 Jun 7;438(1):90–101. doi: 10.1016/0005-2744(76)90225-4. [DOI] [PubMed] [Google Scholar]
- Ezra F. S., Lucas D. S., Mustacich R. V., Russell A. F. Phosphorus-31 and carbon-13 nuclear magnetic resonance studies of anaerobic glucose metabolism and lactate transport in Staphylococcus aureus cells. Biochemistry. 1983 Aug 2;22(16):3841–3849. doi: 10.1021/bi00285a020. [DOI] [PubMed] [Google Scholar]
- FITTING C., DOUDOROFF M. Phosphorolysis of maltose by enzyme preparations from Neisseria meningitidis. J Biol Chem. 1952 Nov;199(1):153–163. [PubMed] [Google Scholar]
- Harold F. M., Spitz E. Accumulation of arsenate, phosphate, and aspartate by Sreptococcus faecalis. J Bacteriol. 1975 Apr;122(1):266–277. doi: 10.1128/jb.122.1.266-277.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt A. G., Simplaceanu V., Hong J. S., Ho C. Phosphorus-31 nuclear magnetic resonance investigation of membrane vesicles from Escherichia coli. Biochemistry. 1983 Dec 20;22(26):6130–6134. doi: 10.1021/bi00295a014. [DOI] [PubMed] [Google Scholar]
- Kornberg H. L. Formation and utilization of PEP in microbial carbohydrate transport. Curr Top Cell Regul. 1981;18:313–327. doi: 10.1016/b978-0-12-152818-8.50024-4. [DOI] [PubMed] [Google Scholar]
- Liao C. L., Atkinson D. E. Regulation at the phosphoenolpyruvate branchpoint in Azotobacter vinelandii: pyruvate kinase. J Bacteriol. 1971 Apr;106(1):37–44. doi: 10.1128/jb.106.1.37-44.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIZUSHIMA S., KITAHARA K. QUANTITATIVE STUDIES ON GLYCOLYTIC ENZYMES IN LACTOBACILLUS PLANTARUM. II. INTRACELLULAR CONCENTRATIONS OF GLYCOLYTIC INTERMEDIATES IN GLUCOSE-METABOLIZING WASHED CELLS. J Bacteriol. 1964 Jun;87:1429–1435. doi: 10.1128/jb.87.6.1429-1435.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason P. W., Carbone D. P., Cushman R. A., Waggoner A. S. The importance of inorganic phosphate in regulation of energy metabolism of Streptococcus lactis. J Biol Chem. 1981 Feb 25;256(4):1861–1866. [PubMed] [Google Scholar]
- Moustafa H. H., Collins E. B. Role of galactose or glucose-1-phosphate in preventing the lysis of Streptococcus diacetilactis. J Bacteriol. 1968 Feb;95(2):592–602. doi: 10.1128/jb.95.2.592-602.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navon G., Ogawa S., Shulman R. G., Yamane T. High-resolution 31P nuclear magnetic resonance studies of metabolism in aerobic Escherichia coli cells. Proc Natl Acad Sci U S A. 1977 Mar;74(3):888–891. doi: 10.1073/pnas.74.3.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navon G., Shulman R. G., Yamane T., Eccleshall T. R., Lam K. B., Baronofsky J. J., Marmur J. Phosphorus-31 nuclear magnetic resonance studies of wild-type and glycolytic pathway mutants of Saccharomyces cerevisiae. Biochemistry. 1979 Oct 16;18(21):4487–4499. doi: 10.1021/bi00588a006. [DOI] [PubMed] [Google Scholar]
- Nicolay K., Kaptein R., Hellingwerf K. J., Konings W. N. 31P nuclear magnetic resonance studies of energy transduction in Rhodopseudomonas sphaeroides. Eur J Biochem. 1981 May;116(1):191–197. doi: 10.1111/j.1432-1033.1981.tb05318.x. [DOI] [PubMed] [Google Scholar]
- Nicolay K., van Gemerden H., Hellingwerf K. J., Konings W. N., Kaptein R. In vivo 31P and 13C nuclear magnetic resonance studies of acetate metabolism in Chromatium vinosum. J Bacteriol. 1983 Aug;155(2):634–642. doi: 10.1128/jb.155.2.634-642.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano A. H., Trifone J. D., Brustolon M. Distribution of the phosphoenolpyruvate:glucose phosphotransferase system in fermentative bacteria. J Bacteriol. 1979 Jul;139(1):93–97. doi: 10.1128/jb.139.1.93-97.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S. K., Sullivan C. E., Torchia D. A. Solid state 13C NMR study of collagen molecular dynamics in hard and soft tissues. J Biol Chem. 1983 Aug 25;258(16):9762–9767. [PubMed] [Google Scholar]
- Simpson S. J., Bendall M. R., Egan A. F., Vink R., Rogers P. J. High-field phosphorus NMR studies of the stoichiometry of the lactate/proton carrier in Streptococcus faecalis. Eur J Biochem. 1983 Oct 17;136(1):63–69. doi: 10.1111/j.1432-1033.1983.tb07705.x. [DOI] [PubMed] [Google Scholar]
- Smart J. B., Pritchard G. G. Control of pyruvate kinase activity during glycolysis and gluconeogenesis in Propionibacterium shermanii. J Gen Microbiol. 1982 Jan;128(1):167–176. doi: 10.1099/00221287-128-1-167. [DOI] [PubMed] [Google Scholar]
- Thomas T. D. Activator specificity of pyruvate kinase from lactic streptococci. J Bacteriol. 1976 Mar;125(3):1240–1242. doi: 10.1128/jb.125.3.1240-1242.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. D. Regulation of lactose fermentation in group N streptococci. Appl Environ Microbiol. 1976 Oct;32(4):474–478. doi: 10.1128/aem.32.4.474-478.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. Characteristics and energy requirements of an alpha-aminoisobutyric acid transport system in Streptococcus lactis. J Bacteriol. 1976 Aug;127(2):719–730. doi: 10.1128/jb.127.2.719-730.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Chassy B. M. Novel phosphoenolpyruvate-dependent futile cycle in Streptococcus lactis: 2-deoxy-D-glucose uncouples energy production from growth. J Bacteriol. 1982 Sep;151(3):1454–1465. doi: 10.1128/jb.151.3.1454-1465.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. In vivo regulation of glycolysis and characterization of sugar: phosphotransferase systems in Streptococcus lactis. J Bacteriol. 1978 Nov;136(2):465–476. doi: 10.1128/jb.136.2.465-476.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Saier M. H., Jr Regulation of methyl-beta-d-thiogalactopyranoside-6-phosphate accumulation in Streptococcus lactis by exclusion and expulsion mechanisms. J Bacteriol. 1981 Jun;146(3):885–894. doi: 10.1128/jb.146.3.885-894.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Thomas T. D. Phosphoenolpyruvate and 2-phosphoglycerate: endogenous energy source(s) for sugar accumulation by starved cells of Streptococcus lactis. J Bacteriol. 1977 May;130(2):583–595. doi: 10.1128/jb.130.2.583-595.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurbil K., Rottenberg H., Glynn P., Shulman R. G. 31P nuclear magnetic resonance studies of bioenergetics and glycolysis in anaerobic Escherichia coli cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2244–2248. doi: 10.1073/pnas.75.5.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Characterization of group N streptococcus lipoteichoic acid. Infect Immun. 1975 May;11(5):973–981. doi: 10.1128/iai.11.5.973-981.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]