Abstract
A cysteine-dependent strain of Saccharomyces cerevisiae and its prototrophic revertants accumulated cystathionine in cells. The cystathionine accumulation was caused by a single mutation having a high incidence of gene conversion. The mutation was designated cys3 and was shown to cause loss of gamma-cystathionase activity. Cysteine dependence of the initial strain was determined by two linked and interacting mutations, cys3 and cys1 . Since cys1 mutations cause a loss of serine acetyltransferase activity, our observation led to the conclusion that S. cerevisiae synthesizes cysteine by sulfhydrylation of serine with hydrogen sulfide and by cleavage of cystathionine which is synthesized from serine and homocysteine.
Full text
PDF
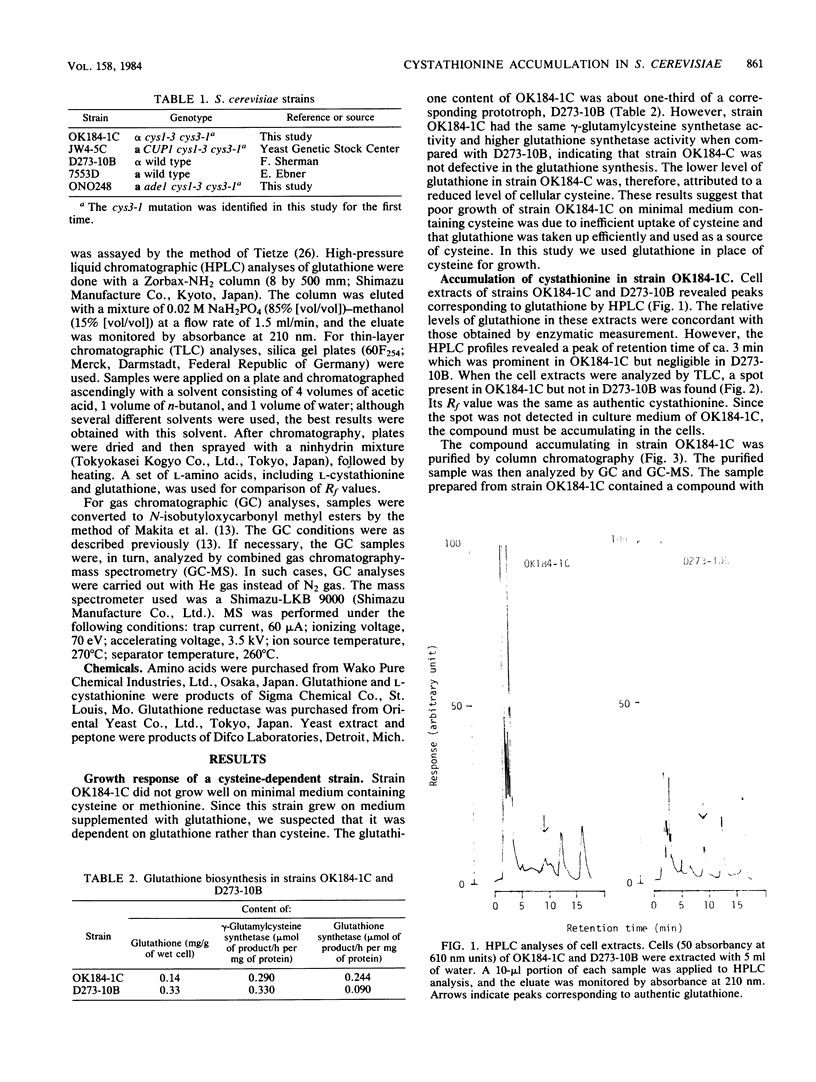
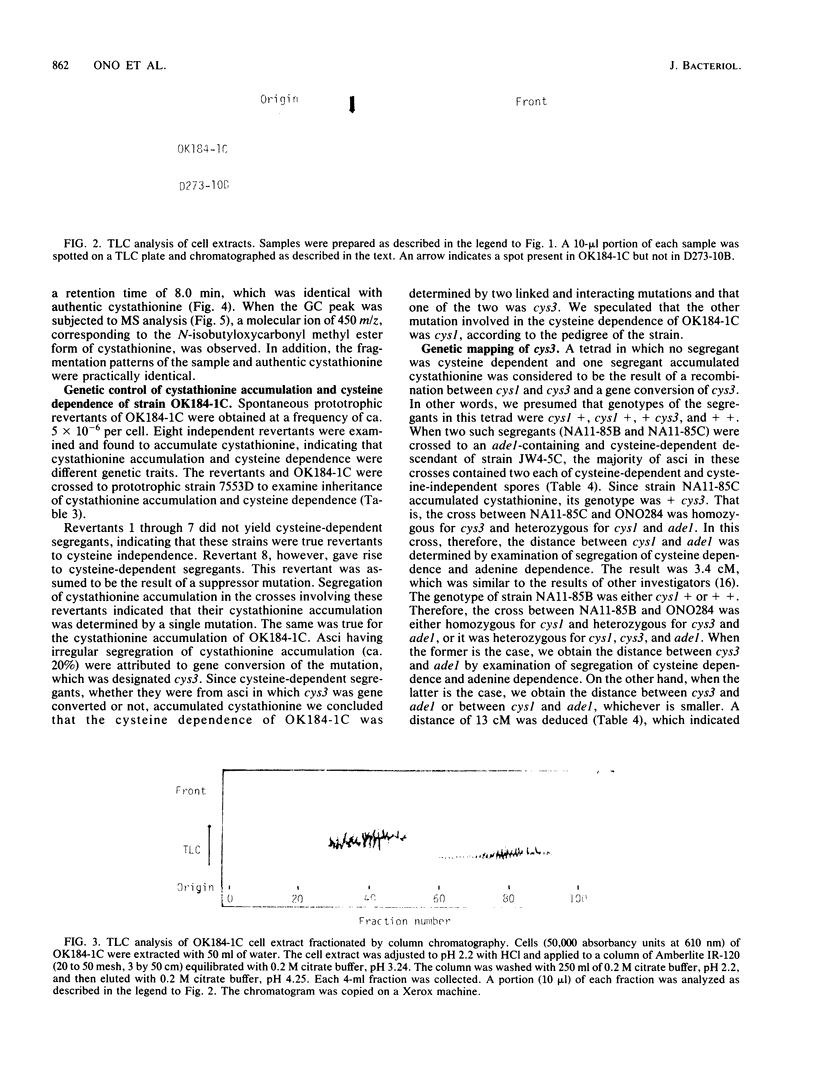
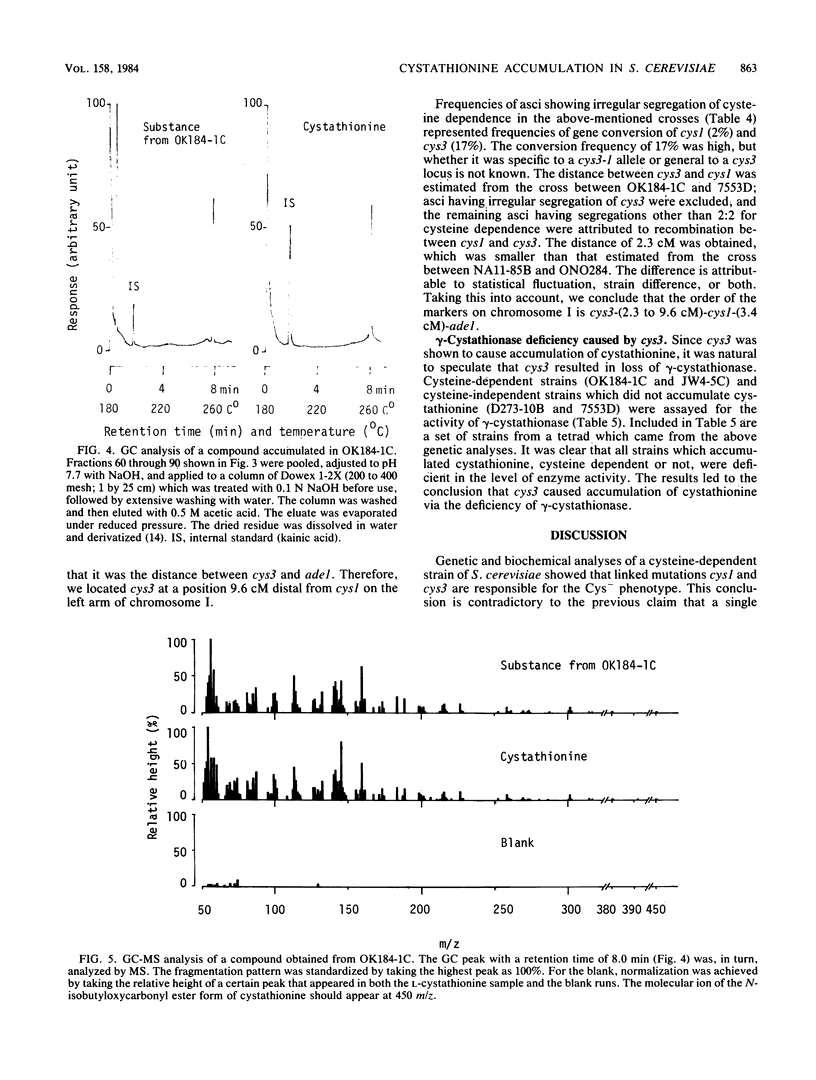
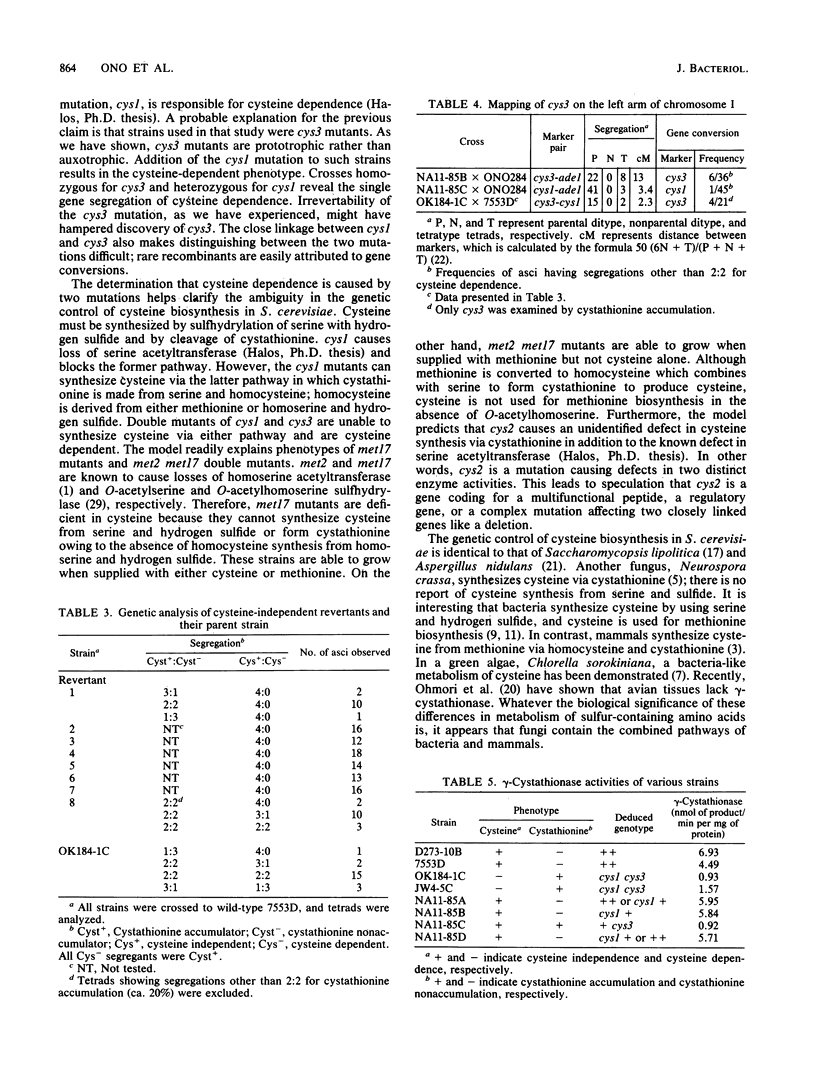

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ebner E., Mason T. L., Schatz G. Mitochondrial assembly in respiration-deficient mutants of Saccharomyces cerevisiae. II. Effect of nuclear and extrachromosomal mutations on the formation of cytochrome c oxidase. J Biol Chem. 1973 Aug 10;248(15):5369–5378. [PubMed] [Google Scholar]
- FLAVIN M., SLAUGHTER C. CYSTATHIONINE CLEAVAGE ENZYMES OF NEUROSPORA. J Biol Chem. 1964 Jul;239:2212–2219. [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H., Datko A. H. Homocysteine biosynthesis in green plants. Physiological importance of the transsulfuration pathway in Chlorella sorokiniana growing under steady state conditions with limiting sulfate. J Biol Chem. 1978 Aug 25;253(16):5665–5677. [PubMed] [Google Scholar]
- Kaplan M. M., Flavin M. Cystathionine gamma-synthetase of Salmonella. Catalytic properties of a new enzyme in bacterial methionine biosynthesis. J Biol Chem. 1966 Oct 10;241(19):4463–4471. [PubMed] [Google Scholar]
- Kerr D., Flavin M. Inhibition of cystathionine gamma-synthase by S-adenosylmethionine: A control mechanism for methionine synthesis in Neurospora. Biochim Biophys Acta. 1969 Feb 18;177(1):177–179. doi: 10.1016/0304-4165(69)90086-5. [DOI] [PubMed] [Google Scholar]
- Kredich N. M., Tomkins G. M. The enzymic synthesis of L-cysteine in Escherichia coli and Salmonella typhimurium. J Biol Chem. 1966 Nov 10;241(21):4955–4965. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Masselot M., De Robichon-Szulmajster H. Methionine biosynthesis in Saccharomyces cerevisiae. I. Genetical analysis of auxotrophic mutants. Mol Gen Genet. 1975 Aug 5;139(2):121–132. doi: 10.1007/BF00264692. [DOI] [PubMed] [Google Scholar]
- Masselot M., Surdin-Kerjan Y. Methionine biosynthesis in Saccharomyces cerevisiae. II. Gene-enzyme relationships in the sulfate assimilation pathway. Mol Gen Genet. 1977 Jul 7;154(1):23–30. doi: 10.1007/BF00265572. [DOI] [PubMed] [Google Scholar]
- Mortimer R. K., Schild D. Genetic map of Saccharomyces cerevisiae. Microbiol Rev. 1980 Dec;44(4):519–571. doi: 10.1128/mr.44.4.519-571.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morzycka E., Paszewski A. Two pathways of cysteine biosynthesis in Saccharomycopsis lipolytica. FEBS Lett. 1979 May 1;101(1):97–100. doi: 10.1016/0014-5793(79)81302-2. [DOI] [PubMed] [Google Scholar]
- Murata K., Tani K., Kato J., Chibata I. Excretion of glutathione by methylglyoxal-resistant Escherichia coli. J Gen Microbiol. 1980 Oct;120(2):545–547. doi: 10.1099/00221287-120-2-545. [DOI] [PubMed] [Google Scholar]
- Ohmori S., Mizuno S., Ikeda M., Yao K. A sensitive gas chromatographic assay for cystathionase and cystathionine: application of this assay to biological samples and kinetic studies. Gas chromatographic assay for cystathionase and cystathionine. Chem Pharm Bull (Tokyo) 1982 Jun;30(6):2099–2104. doi: 10.1248/cpb.30.2099. [DOI] [PubMed] [Google Scholar]
- Paszewski A., Grabski J. Enzymatic lesions in methionine mutants of Aspergillus nidulans: role and regulation of an alternative pathway for cysteine and methionine synthesis. J Bacteriol. 1975 Nov;124(2):893–904. doi: 10.1128/jb.124.2.893-904.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins D. D. Biochemical Mutants in the Smut Fungus Ustilago Maydis. Genetics. 1949 Sep;34(5):607–626. doi: 10.1093/genetics/34.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robichon-Szulmajster H., Cherest H. Regulation of homoserine O-transacetylase, first step in methionine biosyntheis in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1967 Jul 21;28(2):256–262. doi: 10.1016/0006-291x(67)90438-x. [DOI] [PubMed] [Google Scholar]
- SCHLOSSMANN K., BRUEGGEMANN J., LYNEN F. [Biosynthesis of cysteine. I. Detection and isolation of serine sulfhydrase from baker's yeast]. Biochem Z. 1962;336:258–273. [PubMed] [Google Scholar]
- Savin M. A., Flavin M. Cystationine synthesis in yeast: an alternative pathway for homocysteine biosynthesis. J Bacteriol. 1972 Oct;112(1):299–303. doi: 10.1128/jb.112.1.299-303.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Wickerham L. J. A Critical Evaluation of the Nitrogen Assimilation Tests Commonly Used in the Classification of Yeasts. J Bacteriol. 1946 Sep;52(3):293–301. [PMC free article] [PubMed] [Google Scholar]
- Yamagata S., Takeshima K., Naiki N. O-acetylserine and O-acetylhomoserine sulfhydrylase of yeast; studies with methionine auxotrophs. J Biochem. 1975 May;77(5):1029–1036. doi: 10.1093/oxfordjournals.jbchem.a130803. [DOI] [PubMed] [Google Scholar]