Abstract
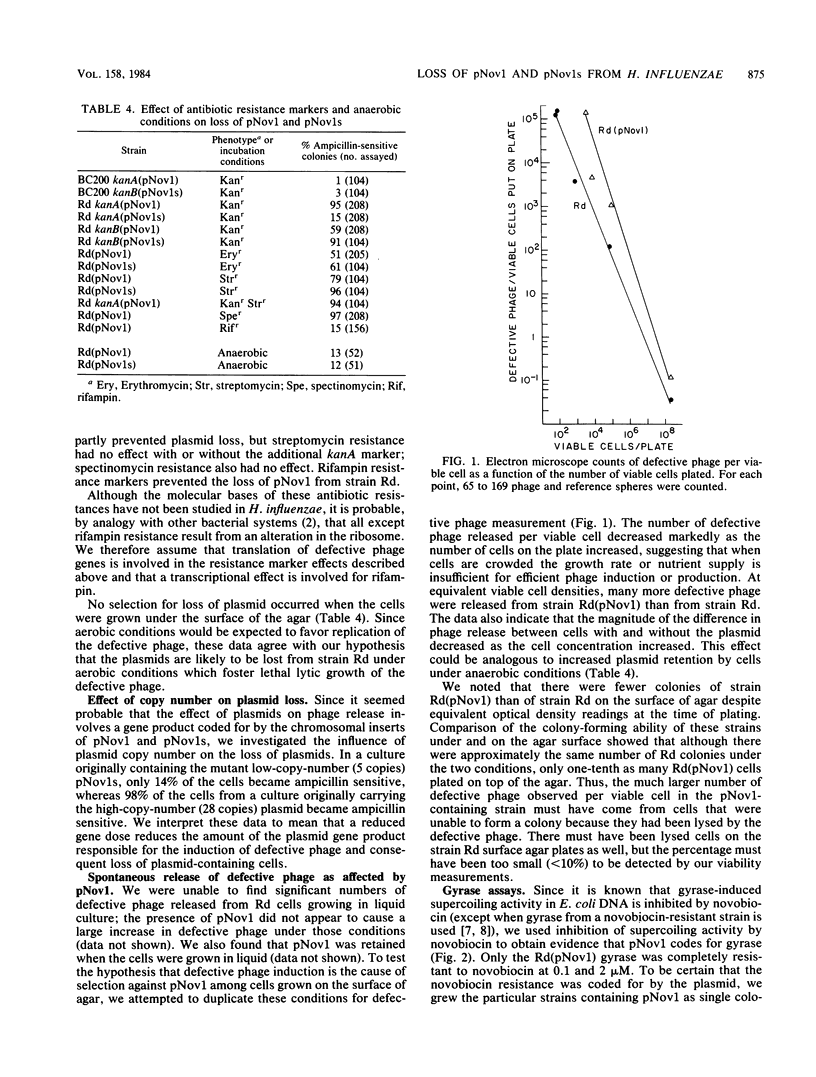
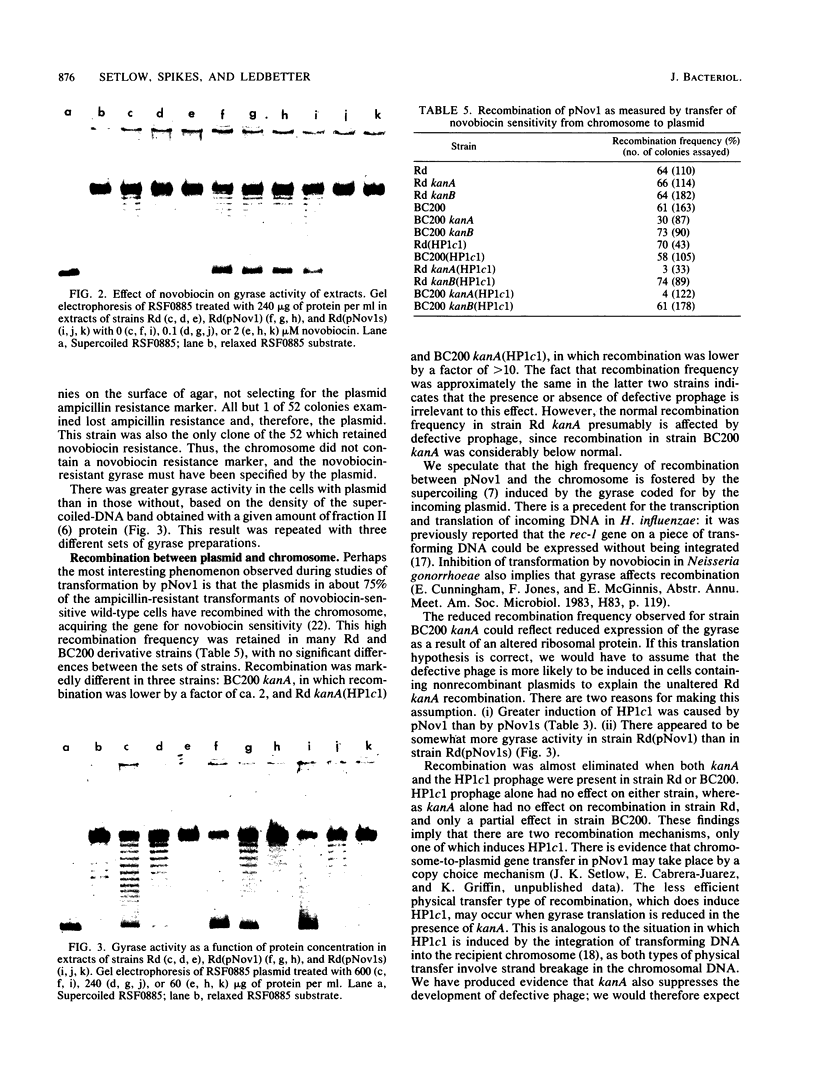
Plasmids pNov1 and pNov1s , coding for resistance and sensitivity to novobiocin, respectively, were readily lost from wild-type Haemophilus influenzae but retained in a strain lacking an inducible defective prophage. The plasmid loss could be partly or wholly eliminated by a low-copy-number mutation in the plasmid or by the presence of certain antibiotic resistance markers in the host chromosome. Release of both phage HP1c1 , measured by plaque assay, and defective phage, measured by electron microscopy, was increased when the plasmids were present. The frequency of recombination between pNov1 and the chromosome, causing the plasmid to be converted to pNov1s , could under some circumstances be decreased from the normal 60 to 70% to below 10% by the presence of a kanamycin resistance marker in the chromosome. This suggested that a gene product coded for by the plasmid, the expression of which was affected by the kanamycin resistance marker, was responsible for the high recombination frequency. Evidence was obtained from in vitro experiments that the gene product was a gyrase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnhart B. J., Cox S. H. Radiation-sensitive and radiation-resistant mutants of Haemophilus influenzae. J Bacteriol. 1968 Jul;96(1):280–282. doi: 10.1128/jb.96.1.280-282.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R., Davies J. Mechanisms of antibiotic resistance in bacteria. Annu Rev Biochem. 1973;42:471–506. doi: 10.1146/annurev.bi.42.070173.002351. [DOI] [PubMed] [Google Scholar]
- Boling M. E., Allison D. P., Setlow J. K. Bacteriophage of Haemophilus influenzae. 3. Morphology, DNA homology, and immunity properties of HPlcl, S2, and the defective bacteriophage from strain Rd. J Virol. 1973 Apr;11(4):585–591. doi: 10.1128/jvi.11.4.585-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura R. K. Biochemical analysis of the naturally repaired sections of bacteriophage T5 deoxyribonucleic acid. II. Conditions for nucleotide incorporation under nonpermissive conditions. Biochemistry. 1971 Nov 23;10(24):4381–4386. doi: 10.1021/bi00800a005. [DOI] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Nash H. A. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Ohmori H., Tomizawa J. DNA gyrase and DNA supercoiling. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):35–40. doi: 10.1101/sqb.1979.043.01.007. [DOI] [PubMed] [Google Scholar]
- Gellert M., O'Dea M. H., Itoh T., Tomizawa J. Novobiocin and coumermycin inhibit DNA supercoiling catalyzed by DNA gyrase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4474–4478. doi: 10.1073/pnas.73.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARM W., RUPERT C. S. INFECTION OF TRANSFORMABLE CELLS OF HAEMOPHILUS INFLUENZAE BY BACTERIOPHAGE AND BACTERIOPHAGE DNA. Z Vererbungsl. 1963 Dec 30;94:336–348. doi: 10.1007/BF00897593. [DOI] [PubMed] [Google Scholar]
- Higgins N. P., Peebles C. L., Sugino A., Cozzarelli N. R. Purification of subunits of Escherichia coli DNA gyrase and reconstitution of enzymatic activity. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1773–1777. doi: 10.1073/pnas.75.4.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball R. F., Boling M. E., Perdue S. W. Evidence that UV-inducible error-prone repair is absent in Haemophilus influenzae Rd, with a discussion of the relation to error-prone repair of alkylating-agent damage. Mutat Res. 1977 Aug;44(2):183–196. doi: 10.1016/0027-5107(77)90076-8. [DOI] [PubMed] [Google Scholar]
- LeClerc J. E., Setlow J. K. Effects of combining ultraviolet repair and recombination mutations in Haemophilus influenzae. Nat New Biol. 1973 Feb 7;241(110):172–174. doi: 10.1038/newbio241172a0. [DOI] [PubMed] [Google Scholar]
- Notani N. K., Setlow J. K., McCarthy D., Clayton N. L. Transformation of Haemophilus influenzae by plasmid RSF0885. J Bacteriol. 1981 Dec;148(3):812–816. doi: 10.1128/jb.148.3.812-816.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Beattie K. L., Boling M. E. Expression of a recombination gene on transforming DNA in a recombination-defective Haemophilus influenzae recipient cell. J Mol Biol. 1972 Jul 21;68(2):379–381. doi: 10.1016/0022-2836(72)90219-7. [DOI] [PubMed] [Google Scholar]
- Setlow J. K., Boling M. E., Allison D. P., Beattie K. L. Relationship between prophage induction and transformation in Haemophilus influenzae. J Bacteriol. 1973 Jul;115(1):153–161. doi: 10.1128/jb.115.1.153-161.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Boling M. E., Beattie K. L., Kimball R. F. A complex of recombination and repair genes in Haemophilus influenzae. J Mol Biol. 1972 Jul 21;68(2):361–378. doi: 10.1016/0022-2836(72)90218-5. [DOI] [PubMed] [Google Scholar]
- Setlow J. K., Brown D. C., Boling M. E., Mattingly A., Gordon M. P. Repair of deoxyribonucleic acid in Haemophilus influenzae. I. X-ray sensitivity of ultraviolet-sensitive mutants and their behavior as hosts to ultraviolet-irradiated bacteriophage and transforming deoxyribonucleic acid. J Bacteriol. 1968 Feb;95(2):546–558. doi: 10.1128/jb.95.2.546-558.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., McCarthy D., Clayton N. L. Novobiocin resistance marker in Haemophilus influenzae that is not expressed on a plasmid. J Bacteriol. 1982 Sep;151(3):1358–1362. doi: 10.1128/jb.151.3.1358-1362.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Notani N. K., McCarthy D., Clayton N. L. Transformation of Haemophilus influenzae by plasmid RSF0885 containing a cloned segment of chromosomal deoxyribonucleic acid. J Bacteriol. 1981 Dec;148(3):804–811. doi: 10.1128/jb.148.3.804-811.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachura I., Mckinley F. W., Leidy G., Alexander H. E. Incomplete bacteriophage-like particles in ultraviolet-irradiated haemophilus. J Bacteriol. 1969 May;98(2):818–820. doi: 10.1128/jb.98.2.818-820.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womble D. D., Taylor D. P., Rownd R. H. Method for obtaining more-accurate covalently closed circular plasmid-to-chromosome ratios from bacterial lysates by dye-buoyant density centrifugation. J Bacteriol. 1977 Apr;130(1):148–153. doi: 10.1128/jb.130.1.148-153.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]