Abstract
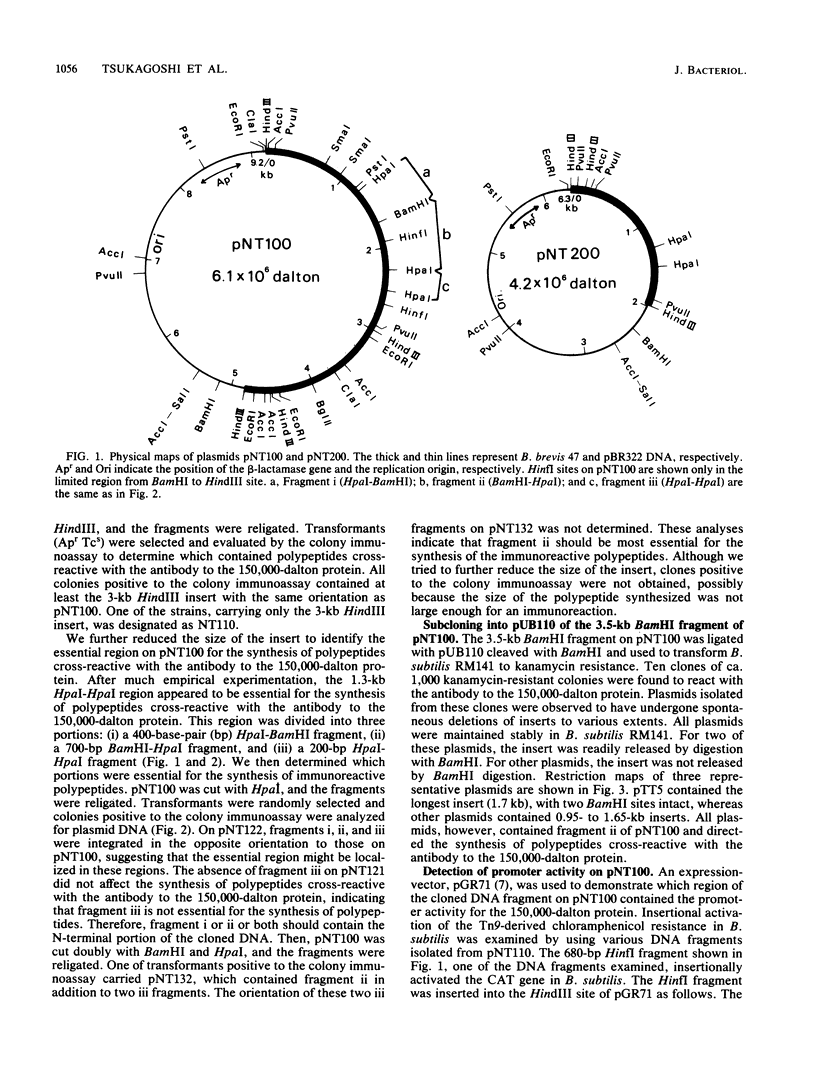
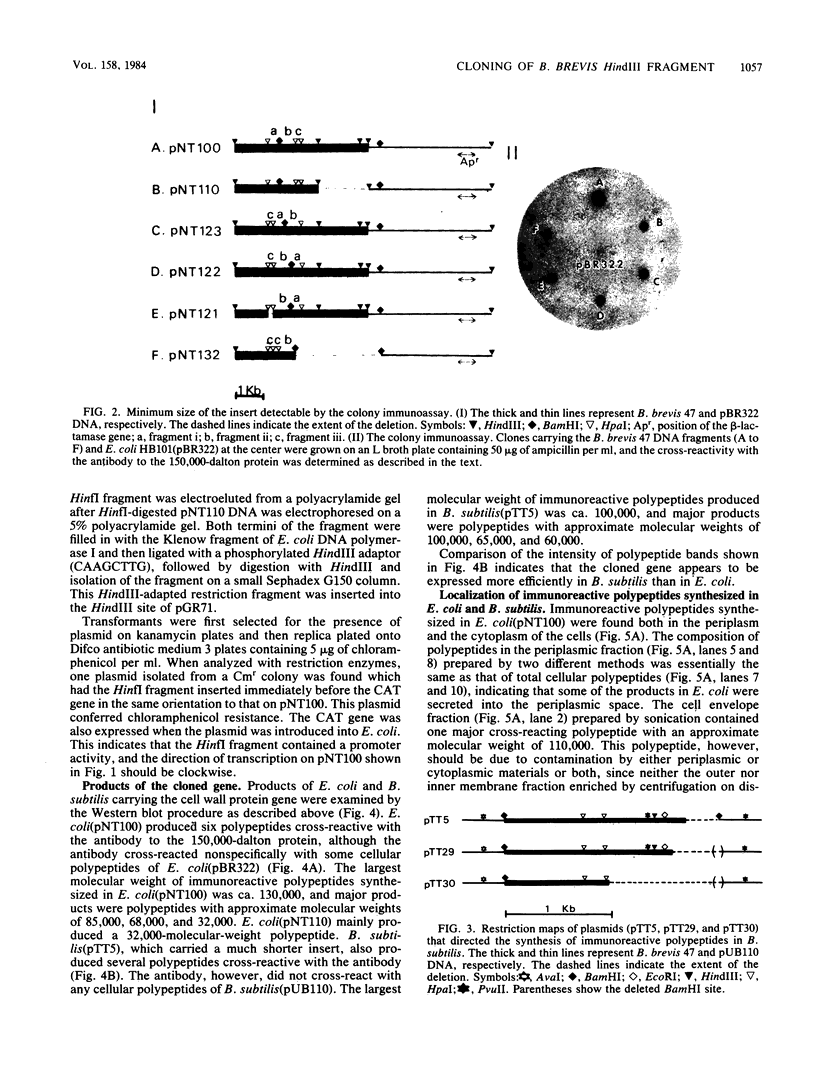
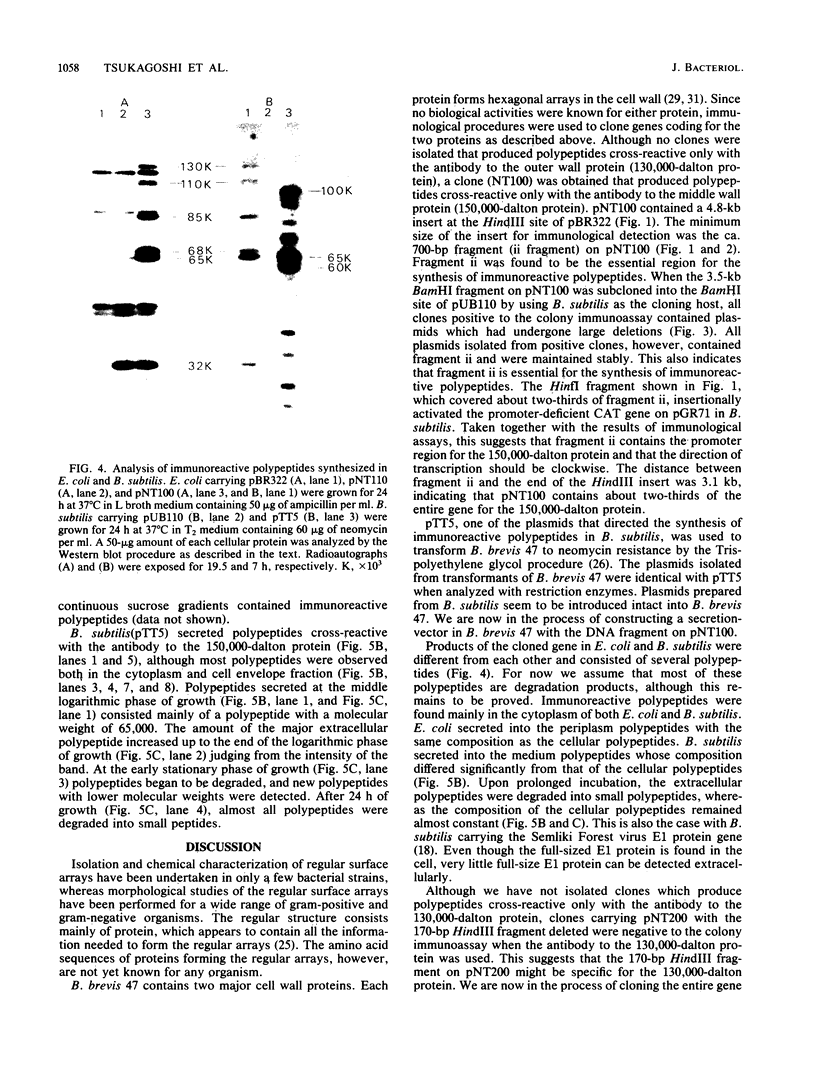
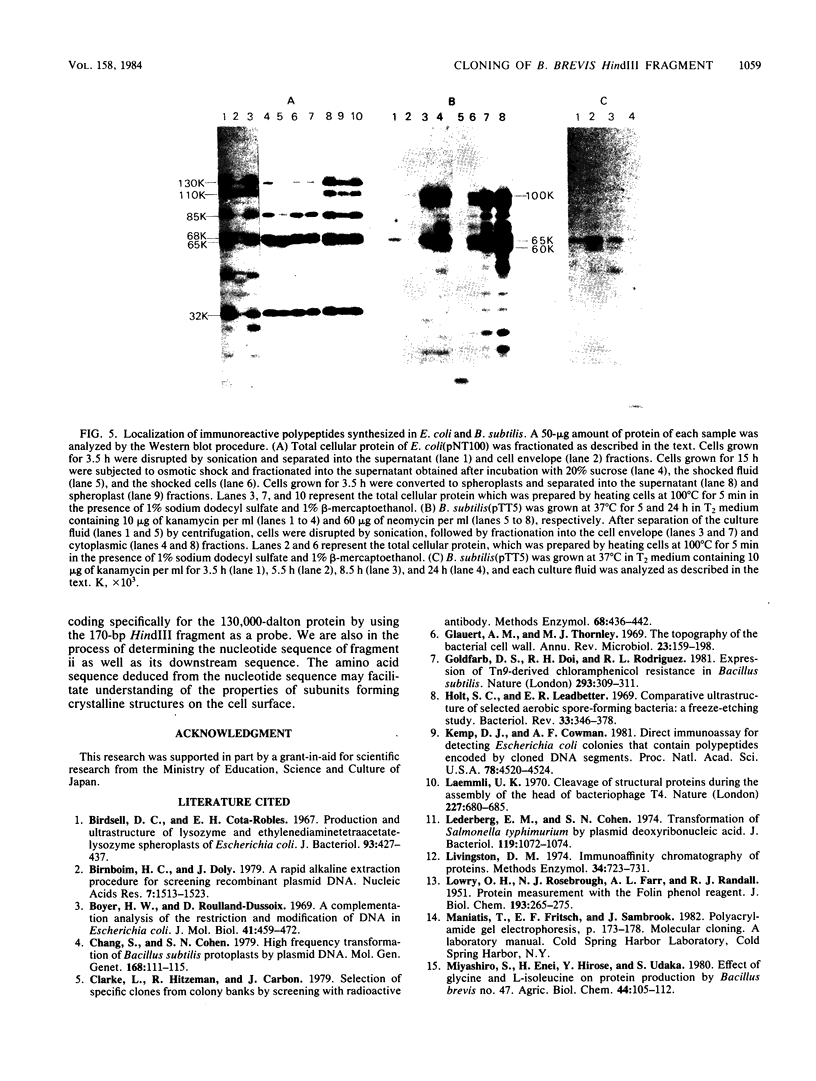
Bacillus brevis 47 contains two major cell wall proteins. Each protein forms a hexagonal array in the cell wall. A 4.8-kilobase HindIII fragment of B. brevis 47 DNA cloned into Escherichia coli with pBR322 as a vector directed the synthesis of polypeptides cross-reactive with antibody to the middle wall protein. A 700-base-pair BamHI-HpaI fragment was shown to be the essential region for the synthesis of immunoreactive polypeptides. Furthermore, this fragment appeared to contain the promoter activity. The 3.5-kilobase BamHI fragment covering the essential region as well as its downstream sequence was subcloned into the corresponding restriction site of pUB110 by using Bacillus subtilis as the cloning host. Both E. coli and B. subtilis carrying the cloned DNA synthesized several immunoreactive polypeptides which were mainly found in the cytoplasm. B. subtilis secreted polypeptides cross-reactive with antibody to the middle wall protein. These extracellular polypeptides were degraded upon prolonged culture.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birdsell D. C., Cota-Robles E. H. Production and ultrastructure of lysozyme and ethylenediaminetetraacetate-lysozyme spheroplasts of Escherichia coli. J Bacteriol. 1967 Jan;93(1):427–437. doi: 10.1128/jb.93.1.427-437.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Chang S., Cohen S. N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979 Jan 5;168(1):111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- Clarke L., Hitzeman R., Carbon J. Selection of specific clones from colony banks by screening with radioactive antibody. Methods Enzymol. 1979;68:436–442. doi: 10.1016/0076-6879(79)68033-3. [DOI] [PubMed] [Google Scholar]
- Glauert A. M., Thornley M. J. The topography of the bacterial cell wall. Annu Rev Microbiol. 1969;23:159–198. doi: 10.1146/annurev.mi.23.100169.001111. [DOI] [PubMed] [Google Scholar]
- Goldfarb D. S., Doi R. H., Rodriguez R. L. Expression of Tn9-derived chloramphenicol resistance in Bacillus subtilis. Nature. 1981 Sep 24;293(5830):309–311. doi: 10.1038/293309a0. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Cowman A. F. Direct immunoassay for detecting Escherichia coli colonies that contain polypeptides encoded by cloned DNA segments. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4520–4524. doi: 10.1073/pnas.78.7.4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston D. M. Immunoaffinity chromatography of proteins. Methods Enzymol. 1974;34:723–731. doi: 10.1016/s0076-6879(74)34094-3. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Ohmizu H., Sasaki T., Tsukagoshi N., Udaka S., Kaneda N., Yagi K. Major proteins released by a protein-producing bacterium, Bacillus brevis 47, are derived from cell wall protein. J Biochem. 1983 Oct;94(4):1077–1084. doi: 10.1093/oxfordjournals.jbchem.a134450. [DOI] [PubMed] [Google Scholar]
- Pettersson R. F., Lundström K., Chattopadhyaya J. B., Josephson S., Philipson L., Käriäinen L., Palva I. Chemical synthesis and molecular cloning of a STOP oligonucleotide encoding an UGA translation terminator in all three reading frames. Gene. 1983 Sep;24(1):15–27. doi: 10.1016/0378-1119(83)90127-0. [DOI] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Sadaie Y., Narui K. Repair deficiency, mutator activity, and thermal prophage inducibility in dna-8132 strains of Bacillus subtilis. J Bacteriol. 1976 Jun;126(3):1037–1041. doi: 10.1128/jb.126.3.1037-1041.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Yura T. Regulatory mutations conferring constitutive synthesis of major outer membrane proteins (OmpC and OmpF) in Escherichia coli. J Bacteriol. 1981 Jan;145(1):88–96. doi: 10.1128/jb.145.1.88-96.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B., Messner P. Crystalline surface layers on bacteria. Annu Rev Microbiol. 1983;37:311–339. doi: 10.1146/annurev.mi.37.100183.001523. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B. Regular arrays of macromolecules on bacterial cell walls: structure, chemistry, assembly, and function. Int Rev Cytol. 1978;53:1–62. doi: 10.1016/s0074-7696(08)62240-8. [DOI] [PubMed] [Google Scholar]
- Takahashi W., Yamagata H., Yamaguchi K., Tsukagoshi N., Udaka S. Genetic transformation of Bacillus brevis 47, a protein-secreting bacterium, by plasmid DNA. J Bacteriol. 1983 Dec;156(3):1130–1134. doi: 10.1128/jb.156.3.1130-1134.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Weisblum B. Construction of a colicin E1-R factor composite plasmid in vitro: means for amplification of deoxyribonucleic acid. J Bacteriol. 1975 Jan;121(1):354–362. doi: 10.1128/jb.121.1.354-362.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi A., Tsukagoshi N., Udaka S. Reassembly in vitro of hexagonal surface arrays in a protein-producing bacterium, Bacillus brevis 47. J Bacteriol. 1982 Sep;151(3):1485–1497. doi: 10.1128/jb.151.3.1485-1497.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukagoshi N., Ihara H., Yamagata H., Udaka S. Cloning and expression of a thermophilic alpha-amylase gene from Bacillus stearothermophilus in Escherichia coli. Mol Gen Genet. 1984;193(1):58–63. doi: 10.1007/BF00327414. [DOI] [PubMed] [Google Scholar]
- Tsukagoshi N., Yamada H., Tsuboi A., Udaka S., Katsura I. Hexagonal surface array in a protein-secreting bacterium, Bacillus brevis 47. Biochim Biophys Acta. 1982 Dec 8;693(1):134–142. doi: 10.1016/0005-2736(82)90479-5. [DOI] [PubMed] [Google Scholar]
- Yamada H., Tsukagoshi N., Udaka S. Morphological alterations of cell wall concomitant with protein release in a protein-producing bacterium, Bacillus brevis 47. J Bacteriol. 1981 Oct;148(1):322–332. doi: 10.1128/jb.148.1.322-332.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]