Abstract
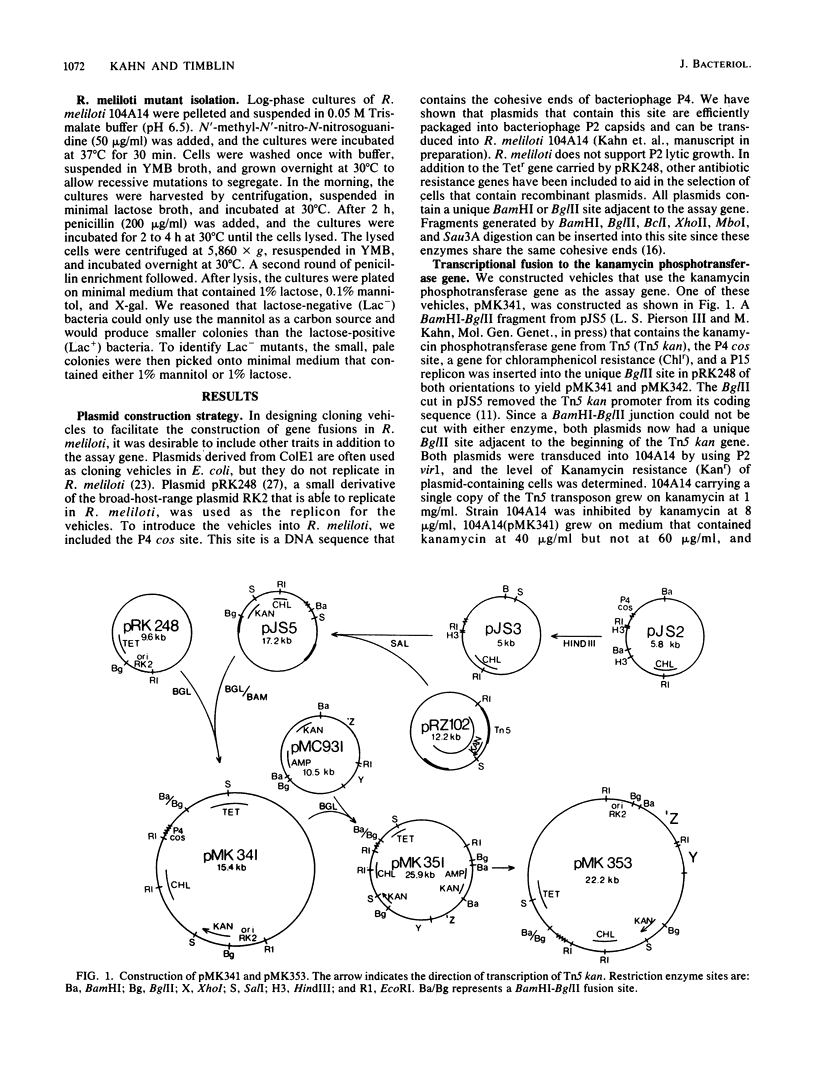
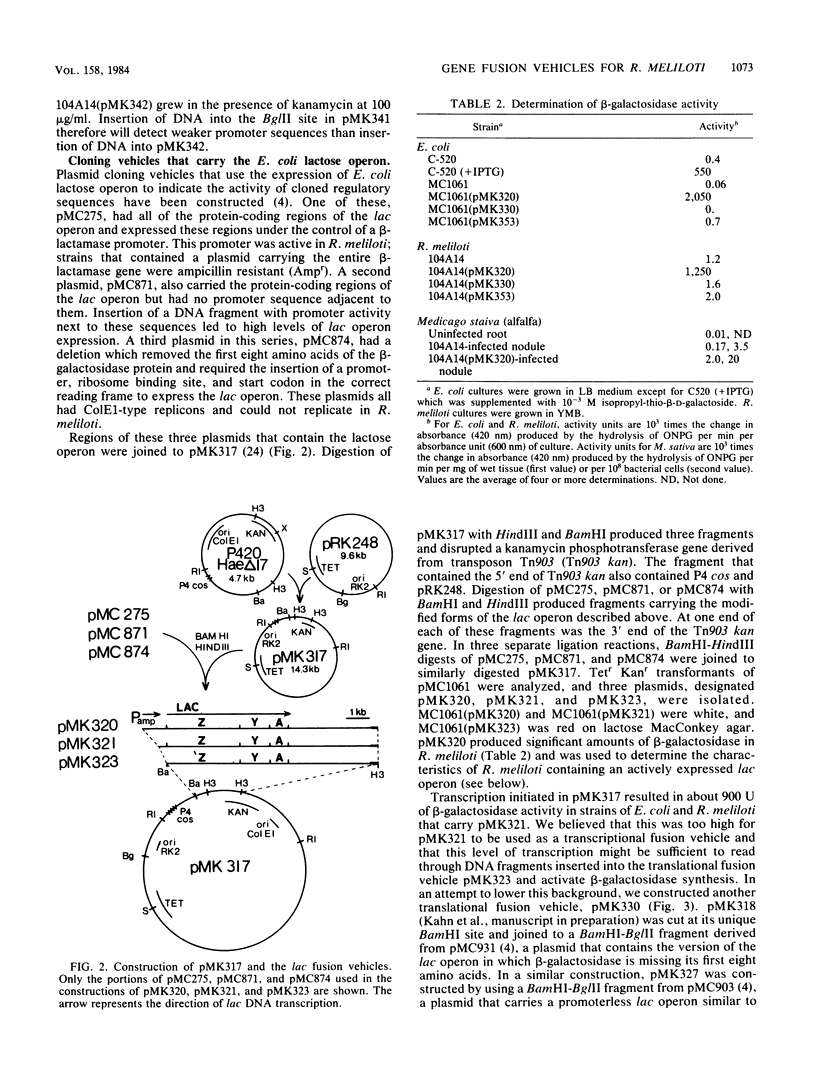
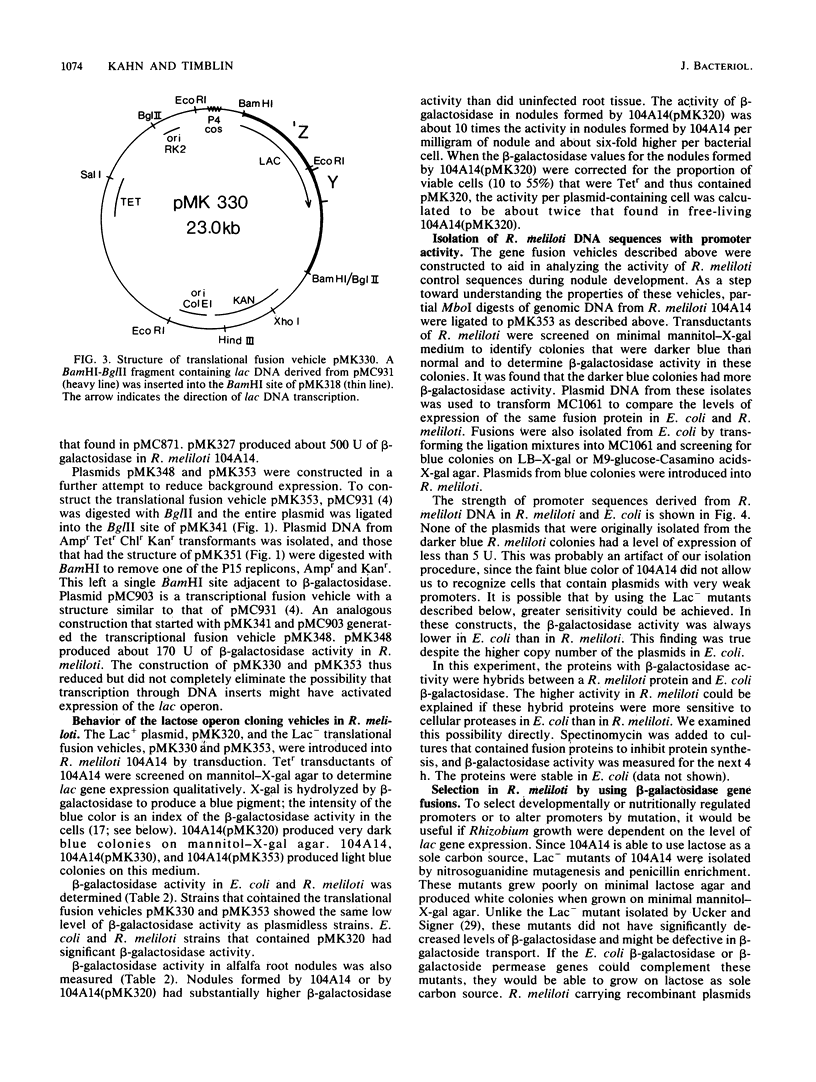
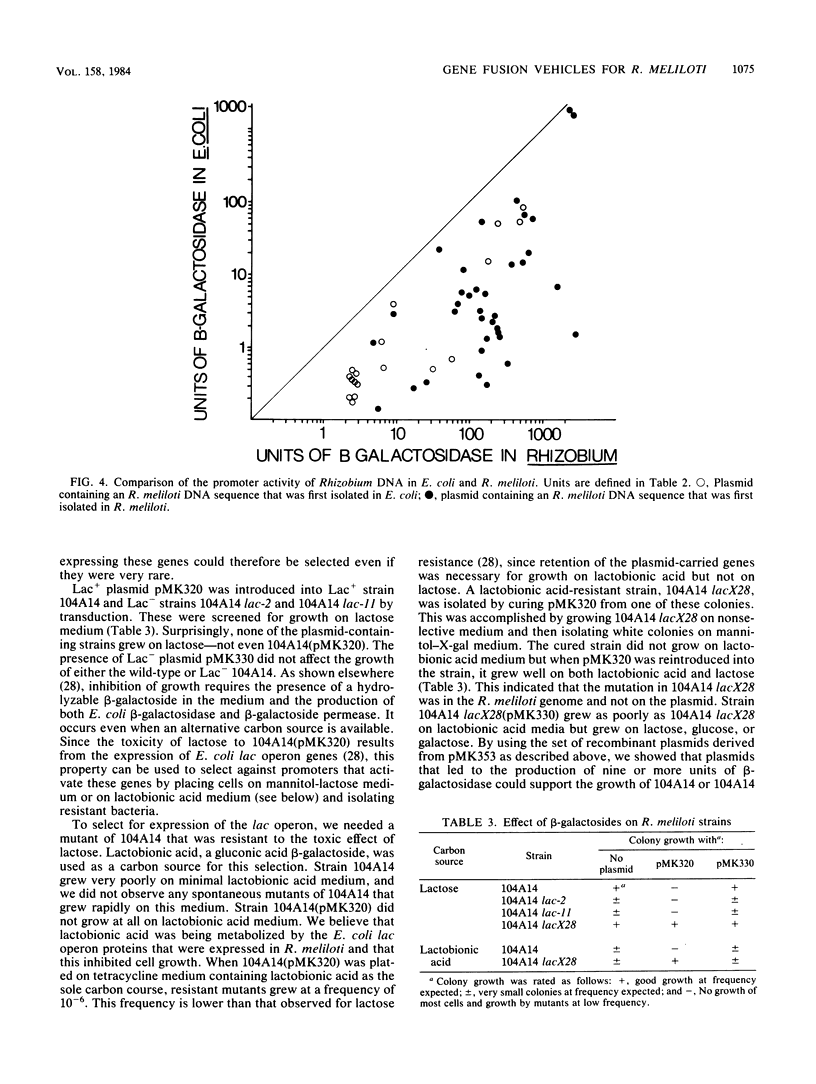
A set of plasmid cloning vehicles was developed to facilitate the construction of gene or operon fusions in Rhizobium meliloti. The vehicles also contain a broad-host-range replicon and could be introduced into bacteria either by transformation or by transduction, using bacteriophage P2. Insertion of foreign DNA into a unique restriction endonuclease cleavage site promotes the synthesis of either the Escherichia coli lactose operon or the kanamycin phosphotransferase gene from transposon Tn5. Expression of the lactose operon could be detected by observing the color of Rhizobium colonies on medium that contained a chromogenic indicator. We also determined the growth conditions that make it possible to select either for or against the expression of the E. coli lactose operon in R. meliloti. Recombinant plasmids were constructed by inserting MboI restriction fragments of R. meliloti DNA into one of the vehicles, pMK353 . Expression of beta-galactosidase by a number of these recombinants was measured in both R. meliloti and E. coli.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertani L. E., Bertani G. Genetics of P2 and related phages. Adv Genet. 1971;16:199–237. doi: 10.1016/s0065-2660(08)60359-4. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Dixon R., Eady R. R., Espin G., Hill S., Iaccarino M., Kahn D., Merrick M. Analysis of regulation of Klebsiella pneumoniae nitrogen fixation (nif) gene cluster with gene fusions. Nature. 1980 Jul 10;286(5769):128–132. doi: 10.1038/286128a0. [DOI] [PubMed] [Google Scholar]
- Geisselsoder J., Youdarian P., Dehò G., Chidambaram M., Goldstein R., Ljungquist E. Mutants of satellite virus P4 that cannot derepress their bacteriophage P2 helper. J Mol Biol. 1981 May 5;148(1):1–19. doi: 10.1016/0022-2836(81)90232-1. [DOI] [PubMed] [Google Scholar]
- Hill S., Kennedy C., Kavanagh E., Goldberg R. B., Hanau R. Nitrogen fixation gene (nifL) involved in oxygen regulation of nitrogenase synthesis in K. pneumoniae. Nature. 1981 Apr 2;290(5805):424–426. doi: 10.1038/290424a0. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Jorgensen R. A., Rothstein S. J., Reznikoff W. S. A restriction enzyme cleavage map of Tn5 and location of a region encoding neomycin resistance. Mol Gen Genet. 1979;177(1):65–72. doi: 10.1007/BF00267254. [DOI] [PubMed] [Google Scholar]
- Kahn M., Kolter R., Thomas C., Figurski D., Meyer R., Remaut E., Helinski D. R. Plasmid cloning vehicles derived from plasmids ColE1, F, R6K, and RK2. Methods Enzymol. 1979;68:268–280. doi: 10.1016/0076-6879(79)68019-9. [DOI] [PubMed] [Google Scholar]
- Kenyon C. J., Walker G. C. DNA-damaging agents stimulate gene expression at specific loci in Escherichia coli. Proc Natl Acad Sci U S A. 1980 May;77(5):2819–2823. doi: 10.1073/pnas.77.5.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss G. B., Kálmán Z. Transformation of Rhizobium meliloti 41 with plasmid DNA. J Bacteriol. 1982 May;150(2):465–470. doi: 10.1128/jb.150.2.465-470.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil D., Zhu J., Brill W. J. Regulation of nitrogen fixation in Klebsiella pneumoniae: isolation and characterization of strains with nif-lac fusions. J Bacteriol. 1981 Jan;145(1):348–357. doi: 10.1128/jb.145.1.348-357.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares J., Casadesús J., Bedmar E. J. Method for Testing Degree of Infectivity of Rhizobium meliloti Strains. Appl Environ Microbiol. 1980 May;39(5):967–970. doi: 10.1128/aem.39.5.967-970.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ow D. W., Ausubel F. M. Regulation of nitrogen metabolism genes by nifA gene product in Klebsiella pneumoniae. Nature. 1983 Jan 27;301(5898):307–313. doi: 10.1038/301307a0. [DOI] [PubMed] [Google Scholar]
- Selvaraj G., Iyer V. N. Genetic transformation of Rhizobium meliloti by plasmid DNA. Gene. 1981 Nov;15(2-3):279–283. doi: 10.1016/0378-1119(81)90137-2. [DOI] [PubMed] [Google Scholar]
- Somerville J. E., Kahn M. L. Cloning of the glutamine synthetase I gene from Rhizobium meliloti. J Bacteriol. 1983 Oct;156(1):168–176. doi: 10.1128/jb.156.1.168-176.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunshine M. G., Thorn M., Gibbs W., Calendar R., Kelly B. P2 phage amber mutants: characterization by use of a polarity suppressor. Virology. 1971 Dec;46(3):691–702. doi: 10.1016/0042-6822(71)90071-7. [DOI] [PubMed] [Google Scholar]
- Sutton W. D., Jepsen N. M., Shaw B. D. Changes in the Number, Viability, and Amino-acid-incorporating Activity of Rhizobium Bacteroids during Lupin Nodule Development. Plant Physiol. 1977 Apr;59(4):741–744. doi: 10.1104/pp.59.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C. M., Meyer R., Helinski D. R. Regions of broad-host-range plasmid RK2 which are essential for replication and maintenance. J Bacteriol. 1980 Jan;141(1):213–222. doi: 10.1128/jb.141.1.213-222.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timblin C. R., Kahn M. L. Lactose inhibits the growth of Rhizobium meliloti cells that contain an actively expressed Escherichia coli lactose operon. J Bacteriol. 1984 Jun;158(3):1204–1207. doi: 10.1128/jb.158.3.1204-1207.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucker D. S., Signer E. R. Catabolite-repression-like phenomenon in Rhizobium meliloti. J Bacteriol. 1978 Dec;136(3):1197–1200. doi: 10.1128/jb.136.3.1197-1200.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


