Abstract
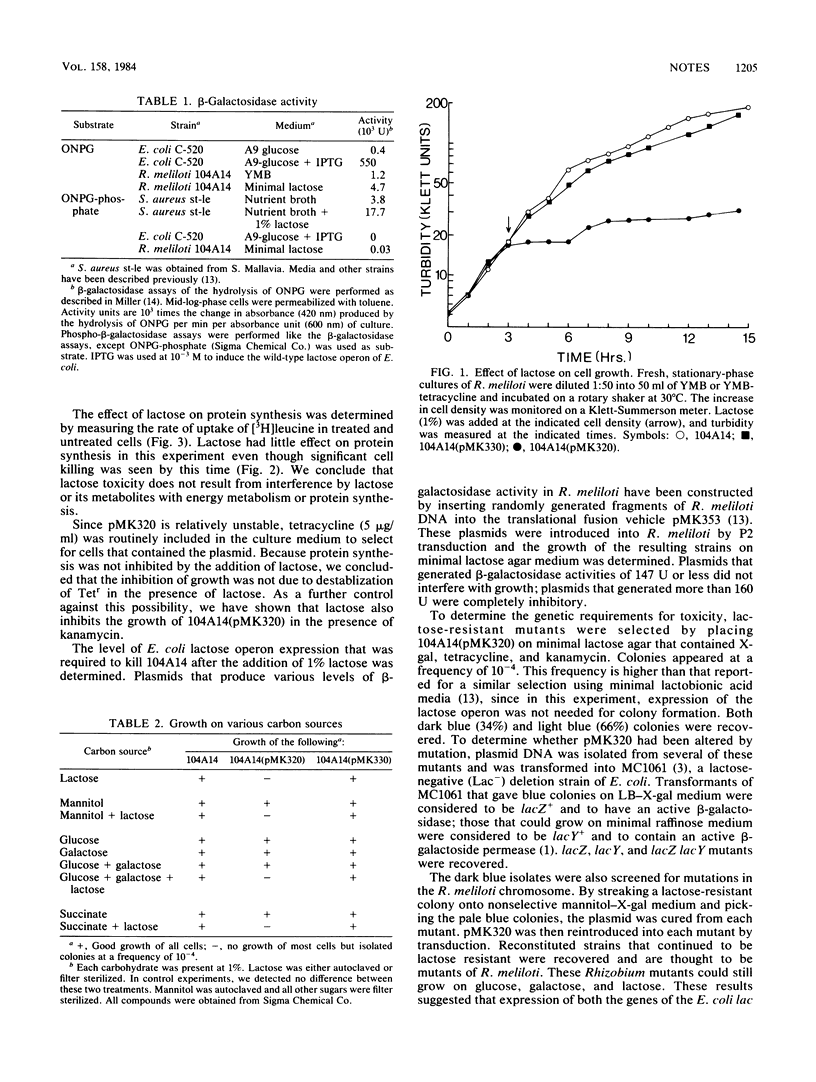
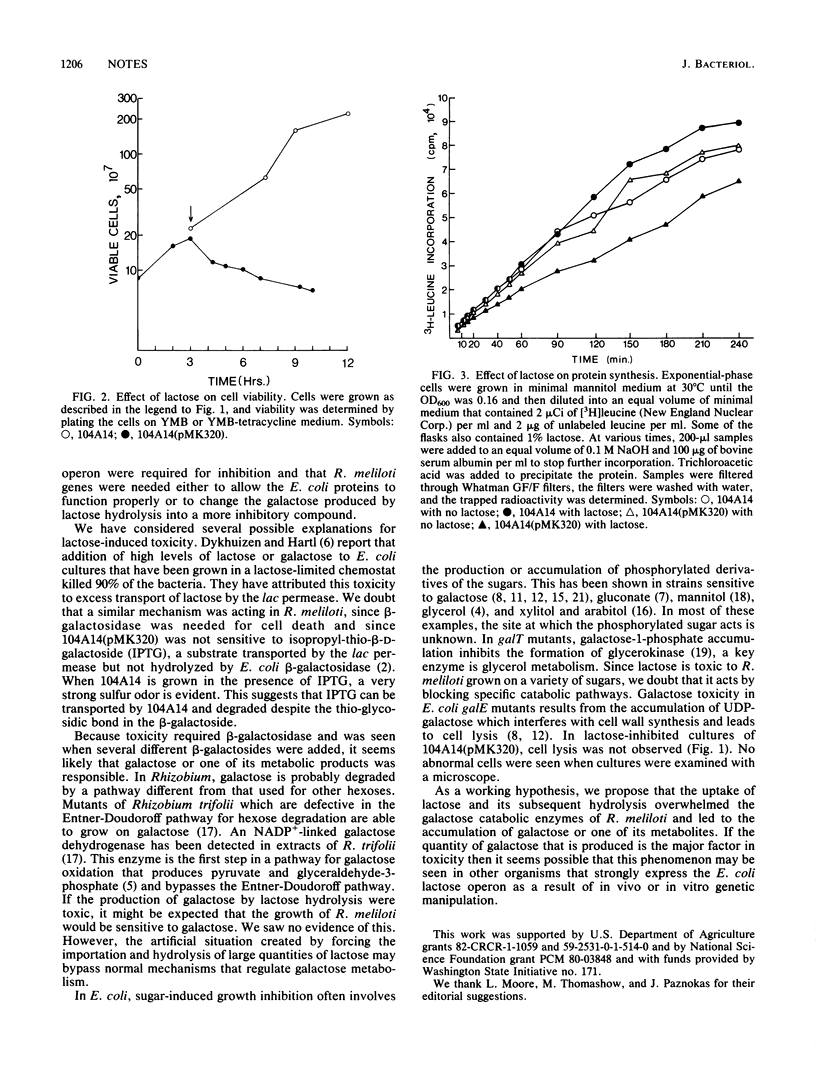
Expression of the Escherichia coli lactose operon in Rhizobium meliloti 104A14 made the cells sensitive to the addition of the beta-galactosides lactose, phenyl-beta-D-galactoside, and lactobionic acid. Growth stopped when the beta-galactoside was added and viability decreased modestly during the next few hours, but little cell lysis was observed and the cells appeared normal. Protein synthesis was not inhibited. Growth was inhibited only when beta-galactosidase expression was greater than 160 U. Lactose-resistant mutants had defects in the plasmid-carried E. coli beta-galactosidase or beta-galactoside permease and in the R. meliloti genome. We speculate that uncontrolled production of galactose by the action of the lactose operon proteins was responsible for growth inhibition.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arditti R. R., Scaife J. G., Beckwith J. R. The nature of mutants in the lac promoter region. J Mol Biol. 1968 Dec;38(3):421–426. doi: 10.1016/0022-2836(68)90396-3. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R., Koch J. P., Hayashi S., Lin E. C. Growth stasis by accumulated L-alpha-glycerophosphate in Escherichia coli. J Bacteriol. 1965 Nov;90(5):1325–1329. doi: 10.1128/jb.90.5.1325-1329.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE LEY J., DOUDOROFF M. The metabolism of D-galactose in Pseudomonas saccharophila. J Biol Chem. 1957 Aug;227(2):745–757. [PubMed] [Google Scholar]
- Dykhuizen D., Hartl D. Transport by the lactose permease of Escherichia coli as the basis of lactose killing. J Bacteriol. 1978 Sep;135(3):876–882. doi: 10.1128/jb.135.3.876-882.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUKASAWA T., NIKAIDO H. Galactose-sensitive mutants of Salmonella. II. Bacteriolysis induced by galactose. Biochim Biophys Acta. 1961 Apr 15;48:470–483. doi: 10.1016/0006-3002(61)90045-2. [DOI] [PubMed] [Google Scholar]
- Fradkin J. E., Fraenkel D. G. 2-keto-3-deoxygluconate 6-phosphate aldolase mutants of Escherichia coli. J Bacteriol. 1971 Dec;108(3):1277–1283. doi: 10.1128/jb.108.3.1277-1283.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. G. Lactose metabolism involving phospho-beta-galactosidase in Klebsiella. J Bacteriol. 1979 Jun;138(3):691–698. doi: 10.1128/jb.138.3.691-698.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengstenberg W., Egan J. B., Morse M. L. Carbohydrate transport in Staphylococcus aureus. VI. The nature of the derivatives accumulated. J Biol Chem. 1968 Apr 25;243(8):1881–1885. [PubMed] [Google Scholar]
- KALCKAR H. M. Hereditary defects in galactose metabolism in man and microorganisms. Fed Proc. 1960 Dec;19:984–990. [PubMed] [Google Scholar]
- Kahn M. L., Timblin C. R. Gene fusion vehicles for the analysis of gene expression in Rhizobium meliloti. J Bacteriol. 1984 Jun;158(3):1070–1077. doi: 10.1128/jb.158.3.1070-1077.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalckar H. M., Kurahashi K., Jordan E. HEREDITARY DEFECTS IN GALACTOSE METABOLISM IN ESCHERICHIA COLI MUTANTS, I. DETERMINATION OF ENZYME ACTIVITIES. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1776–1786. doi: 10.1073/pnas.45.12.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIKAIDO H. Galactose-sensitive mutants of Salmonella. I. Metabolism of galactose. Biochim Biophys Acta. 1961 Apr 15;48:460–469. doi: 10.1016/0006-3002(61)90044-0. [DOI] [PubMed] [Google Scholar]
- Reiner A. M. Xylitol and D-arabitol toxicities due to derepressed fructose, galactitol, and sorbitol phosphotransferases of Escherichia coli. J Bacteriol. 1977 Oct;132(1):166–173. doi: 10.1128/jb.132.1.166-173.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUNDARARAJAN T. A. INTERFERENCE WITH GLYCEROKINASE INDUCTION IN MUTANTS OF E. COLI ACCUMULATING GAL-1-P. Proc Natl Acad Sci U S A. 1963 Sep;50:463–469. doi: 10.1073/pnas.50.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon E., Lin E. C. Mutations affecting the dissimilation of mannitol by Escherichia coli K-12. J Bacteriol. 1972 Aug;111(2):566–574. doi: 10.1128/jb.111.2.566-574.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucker D. S., Signer E. R. Catabolite-repression-like phenomenon in Rhizobium meliloti. J Bacteriol. 1978 Dec;136(3):1197–1200. doi: 10.1128/jb.136.3.1197-1200.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarmolinsky M. B., Wiesmeyer H., Kalckar H. M., Jordan E. HEREDITARY DEFECTS IN GALACTOSE METABOLISM IN ESCHERICHIA COLI MUTANTS, II. GALACTOSE-INDUCED SENSITIVITY. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1786–1791. doi: 10.1073/pnas.45.12.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]


