Abstract
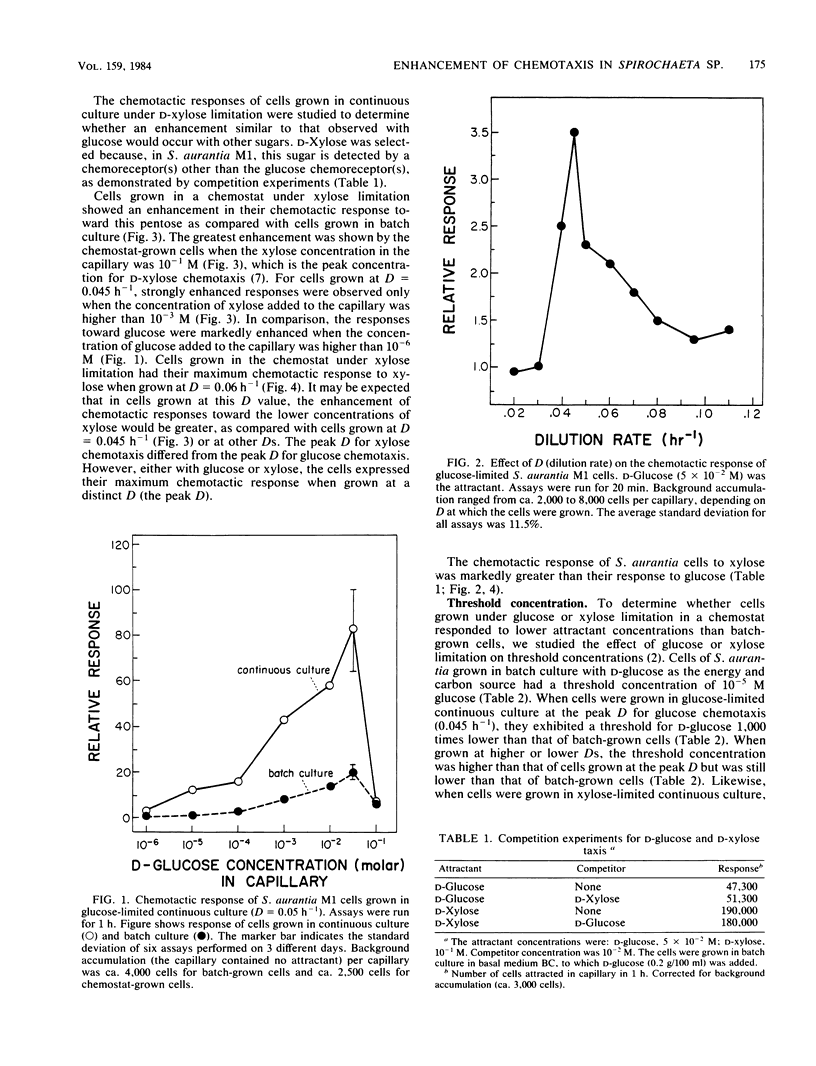
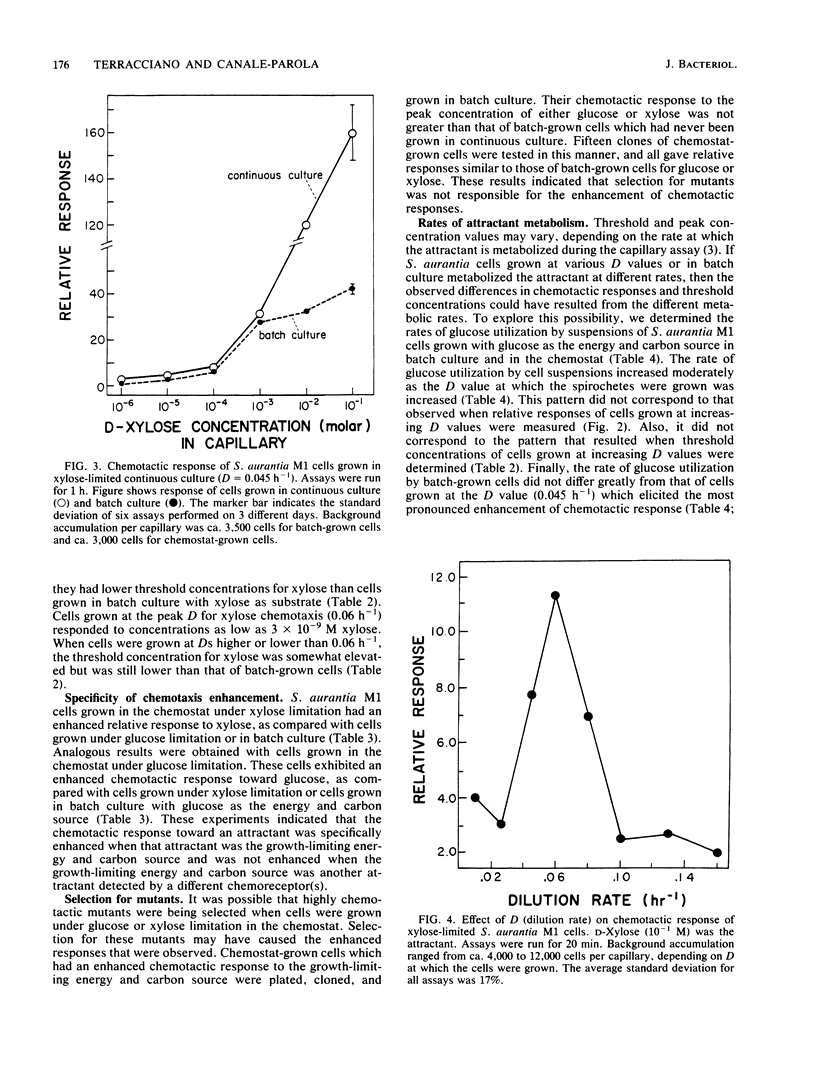
Spirochaeta aurantia M1 cells were grown in a chemostat under conditions of energy and carbon source limitation. The chemotactic responses of the chemostat-grown cells were compared with those of S. aurantia cells grown in batch culture in the presence of excess energy and carbon source. Chemotactic responses were measured by determining the number of cells that entered a capillary tube containing a solution of attractant. S. aurantia cells grown in the chemostat under energy and carbon source limitation exhibited enhanced chemotactic responses and detected lower concentrations of attractant, as compared with cells grown in batch culture. The chemotactic response toward an attractant was specifically enhanced when that attractant was the growth-limiting energy and carbon source. The medium used contained either D-glucose or D-xylose as the sole energy and carbon source. Cells had the greatest chemotactic response toward glucose when grown at a dilution rate (D) of 0.045 h-1 under glucose limitation and toward xylose when grown at D = 0.06 h-1 under xylose limitation. When cells were grown under glucose limitation (D = 0.045 h-1), they sensed concentrations of attractant (glucose) ca. 1,000 times lower than those sensed by batch-grown cells. A similar enhancement of sensing ability (toward xylose) was observed in cells grown under xylose limitation. The results indicated that S. aurantia cells are able to regulate their chemosensory system in response to nutrient limitation. Maximum enhancement of chemotaxis occurs in cells growing at very low concentrations of energy and carbon source. Most likely, this property provides the spirochetes with competitive advantages when the availability of nutrients becomes severely limited in their habitats.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973 Jan;74(1):77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- Adler J. Chemoreceptors in bacteria. Science. 1969 Dec 26;166(3913):1588–1597. doi: 10.1126/science.166.3913.1588. [DOI] [PubMed] [Google Scholar]
- Adler J., Hazelbauer G. L., Dahl M. M. Chemotaxis toward sugars in Escherichia coli. J Bacteriol. 1973 Sep;115(3):824–847. doi: 10.1128/jb.115.3.824-847.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breznak J. A., Canale-Parola E. Morphology and physiology of Spirochaeta aurantia strains isolated from aquatic habitats. Arch Microbiol. 1975 Sep 30;105(1):1–12. doi: 10.1007/BF00447104. [DOI] [PubMed] [Google Scholar]
- Goulbourne E. A., Jr, Greenberg E. P. Chemotaxis of Spirochaeta aurantia: involvement of membrane potential in chemosensory signal transduction. J Bacteriol. 1981 Dec;148(3):837–844. doi: 10.1128/jb.148.3.837-844.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulbourne E. A., Jr, Greenberg E. P. Relationship between proton motive force and motility in Spirochaeta aurantia. J Bacteriol. 1980 Sep;143(3):1450–1457. doi: 10.1128/jb.143.3.1450-1457.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg E. P., Canale-Parola E. Chemotaxis in Spirochaeta aurantia. J Bacteriol. 1977 Apr;130(1):485–494. doi: 10.1128/jb.130.1.485-494.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder W., Dijkhuizen L. Physiological responses to nutrient limitation. Annu Rev Microbiol. 1983;37:1–23. doi: 10.1146/annurev.mi.37.100183.000245. [DOI] [PubMed] [Google Scholar]
- Hazelbauer G. L., Harayama S. Sensory transduction in bacterial chemotaxis. Int Rev Cytol. 1983;81:33–70. doi: 10.1016/s0074-7696(08)62334-7. [DOI] [PubMed] [Google Scholar]
- Hazelbauer G. L. The binding of maltose to 'virgin' maltose-binding protein is biphasic. Eur J Biochem. 1975 Dec 15;60(2):445–449. doi: 10.1111/j.1432-1033.1975.tb21022.x. [DOI] [PubMed] [Google Scholar]
- Koman A., Harayama S., Hazelbauer G. L. Relation of chemotactic response to the amount of receptor: evidence for different efficiencies of signal transduction. J Bacteriol. 1979 Jun;138(3):739–747. doi: 10.1128/jb.138.3.739-747.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A., Veldkamp H. Physiological basis of the selective advantage of a Spirillum sp. in a carbon-limited environment. J Gen Microbiol. 1978 Apr;105(2):187–197. doi: 10.1099/00221287-105-2-187. [DOI] [PubMed] [Google Scholar]
- Mesibov R., Ordal G. W., Adler J. The range of attractant concentrations for bacterial chemotaxis and the threshold and size of response over this range. Weber law and related phenomena. J Gen Physiol. 1973 Aug;62(2):203–223. doi: 10.1085/jgp.62.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange P. G., Koshland D. E., Jr Receptor interactions in a signalling system: competition between ribose receptor and galactose receptor in the chemotaxis response. Proc Natl Acad Sci U S A. 1976 Mar;73(3):762–766. doi: 10.1073/pnas.73.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempest D. W., Neijssel O. M. Metabolic compromises involved in the growth of microorganisms in nutrient-limited (chemostat) environments. Basic Life Sci. 1981;18:335–356. doi: 10.1007/978-1-4684-3980-9_20. [DOI] [PubMed] [Google Scholar]



