Abstract
Natural killer (NK) cells express killer cell inhibitory receptors (KIRs) for major histocompatibility complex class I molecules. Engagement of these surface receptors inhibits NK cell cytotoxic programs. KIR can also be expressed on T cell subsets, and their engagement similarly results in inhibition of effector functions initiated by the CD3/T cell receptor complex. KIR genes belong to two distinct families: the immunoglobulin superfamily (IgSF KIRs) and dimeric C2 lectins (lectin-like KIRs). Whereas both IgSF (p58: CD158, p70, and p140) and lectin-like KIRs (CD94/NKG2A heterodimers) have been found in human, only lectin-like KIRs (all members of the Ly-49 family) have been described in the mouse. We have generated transgenic mice expressing an IgSF KIR, CD158b (p58.2), which recognizes HLA-Cw3. Our data show that CD158b is necessary and sufficient to confer specificity to NK cells, as well as to modulate T cell activation programs in vitro. In addition, we did not detect any adaptation of CD158b cell surface expression to that of HLA class I ligands in the CD158b × HLA-Cw3 double transgenic mice, in contrast to observations with Ly-49 in the mouse. Therefore, distinct strategies of selection/calibration appear to be used by IgSF and lectin-like KIRs. Finally, the transgenic expression of CD158b KIR prevents the in vivo rejection of H-2 mismatch bone marrow grafts, which express the cognate major histocompatibility class I HLA-Cw3 allele, demonstrating for the first time the in vivo implication of human IgSF KIRs in the negative regulation of NK cell function.
Natural killer (NK) cells can induce the lysis of target cells that either do not express, or express in a modified form, major histocompatibility complex (MHC) class I molecules (1, 2). A family of MHC class I-specific receptors, killer cell inhibitory receptors (KIRs), capable of inhibiting NK cell activation, has been described both in human and in mouse (1, 3, 4). The presence of both lectin-like KIRs and immunoglobulin superfamily (IgSF) KIRs in humans, as compared with the exclusive expression of lectin-like KIRs in mice, as well as the large degeneracy of MHC class I molecule recognition by human lectin-like KIRs, suggests that lectin-like KIRs appeared in mammals prior to IgSF KIRs. Despite these differences of genetic origin, both IgSF and lectin-like KIRs serve as T and NK cell surface receptors for MHC class I molecules, express intracytoplasmic immunoreceptor tyrosine based-inhibition motifs (5, 6), and transduce upon engagement with their ligands, inhibitory signals that impair T cell receptor-induced T cell activation (7, 8), NK cell antibody-dependent cell cytotoxicity, as well as NK cell natural cytotoxicity (9). In the mouse, it has been demonstrated that NK cells isolated from an irradiated H-2k/b host mediate the rejection of parental H-2b/b or H-2k/k bone marrow (10, 11). Mouse KIRs, members of the Ly-49 family, have been shown to be involved in this hybrid resistance phenomenon (12, 13). Indeed, distinct subsets of NK cells can be identified in a H-2k/b background, based on the single or coexpression of KIRs, which interact with autologous H-2b or H-2k molecules. The absence of KIRs that recognize H-2b on cells of a NK subset, which only expresses H-2k-specific KIRs, results in the lack of inhibitory signals induced by the recognition of H-2b, leading to the lysis of H-2b cells and to the rejection of H-2b bone marrow graft. In contrast to this established function of Ly-49 molecules in the mouse, the role of human IgSF KIR in the control of lymphocyte activation in vivo remains to be elucidated. We generated transgenic mice that express the human IgSF KIR, CD158b, a receptor for HLA-Cw3. Our results show that comparable to mouse Ly-49 molecules, the expression of CD158b can prevent H-2 mismatch bone marrow graft rejection. However, the mechanisms of selection/calibration of human Ig-like KIRs, which are required to optimize the recognition of MHC class I molecules as well as to ensure the tolerance to normal autologous cells, are revealed as being different than that used by lectin-like KIRs.
MATERIALS AND METHODS
Generation of CD158b Transgenic Mice.
The CD158b cDNA (cl 6.11) (14) was subcloned in the MHC class I promoter/immunoglobulin enhancer expression cassette pHSE3′-HindIII (15) and injected into fertilized C57BL/6 (B6) (H-2b/b) × CBA/J (H-2k/k) F2 eggs. Transgenic founder mice and their transgenic progenies were identified by PCR with primers specific for CD158b cDNA and by immunofluorescence analysis of peripheral blood lymphocytes (PBL) using biotinylated GL183 mAb (anti-CD158b) followed by phycoerythrin-conjugated streptavidin. Transgenic lines were established and maintained by crossing of founders with B6 mice. C57BL/6-HLA-Cw3 (H-2b/b) transgenic mice have been described (16). All the mice used in this study were between 6 and 24 weeks old and were maintained at the Animal Facility of the Centre d’Immunologie de Marseille–Luminy.
Immunofluorescence Analysis.
Spleen cells and PBL were stained as previously described and analyzed on a FACScan apparatus (Becton Dickinson) (17). The mAbs used in these experiments have been described elsewhere: fluorescein isothiocyanate (FITC)-conjugated anti-CD3ɛ (PharMingen), F4/326 (anti-HLA-C) (18), biotinylated GL183 (anti-CD158b) (19), as well as biotinylated anti-human CD2 and FITC-conjugated anti-human CD3, both used as negative controls (Immunotech, Marseille, France). 11.4.1 (anti-H-2Kk) and 20.8.4 (anti-H-2Kb) mAbs were used for the determination of the H-2 haplotype. Indirect immunofluorescence staining was carried out with FITC- or phycoerythrin-conjugated secondary antibodies of the appropriate species and isotype specificity, purchased from Southern Biotechnology Associates; tricolor (TC)-conjugated streptavidin was purchased from Caltag (South San Francisco, CA) and phycoerythrin-conjugated streptavidin from Immunotech.
Cytolytic Assay.
To increase the number of splenic NK cells, mice were injected i.p. with 200 μg of poly(I:C) (Pharmacia) 24 hr prior to sacrifice. Spleens were then harvested and single cell suspensions were prepared in RPMI 1640 medium containing 10% fetal calf serum. Erythrocytes were depleted by osmotic lysis and macrophages were removed by 1 hr adherence step on 6-well plates at a concentration of 5 × 106 cells/ml. These freshly isolated nonadherent splenocytes were used as effector cells in a 4-hr 51Cr release assay. The NK sensitive YAC-1 cell line, the murine mastocytoma cell line P815 (parental as well as transfected with the HLA-Cw3 allele) (19), and the human cell line LCL 721.221 [parental (221) as well as transfected with the HLA-Cw3 (221-Cw3) or HLA-Cw4 (221-Cw4) alleles] (20), were used as target cells. In these assays, 5 × 103 51Cr-labeled target cells were added to effector cells at various effector:target ratios in V-bottom 96-well plates (final volume 200 μl). After 4 hr at 37°C, 100 μl of supernatant was collected from each well and counted in a γ-counter for the determination of 51Cr release and percentage specific lysis (19).
Bone Marrow Grafts.
Recipient HLA-Cw3 (H-2b/b) transgenic, HLA-Cw3 (H-2k/b) transgenic, and CD158b × HLA-Cw3 (H-2k/b) transgenic mice were irradiated (950 rads from a 137Cs source) and inoculated intravenously with 5 × 106 T-depleted bone marrow cells from B6 HLA-Cw3 (H-2b/b) transgenic mice. Five days later, recipient mice were injected i.p. with 3 μCi 5-[125I]iodo-2′-deoxyuridine (125IdUdr, Amersham). Animals were killed 24 hr later and incorporated radioactivity in the spleen was measured in a γ counter (21).
RESULTS
Reconstitution of in Vitro KIR Inhibitory Function in NK and T Lymphocytes Expressing the CD158b Transgene.
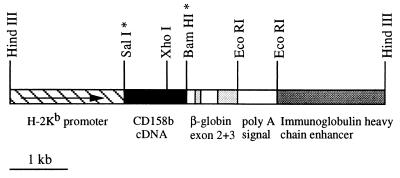
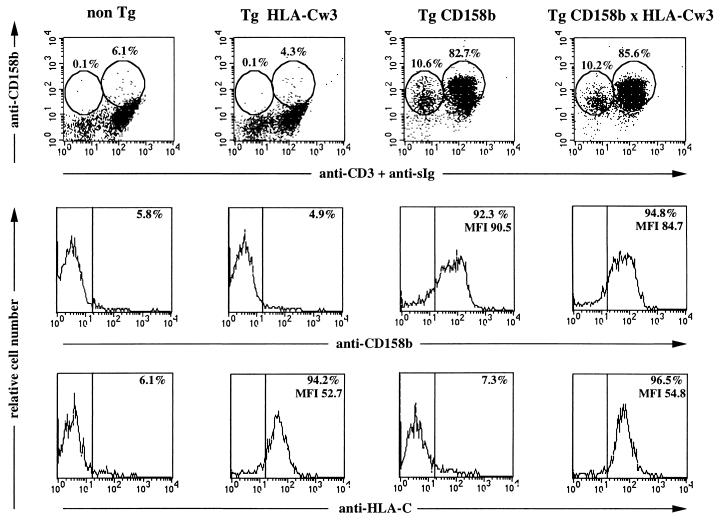
Four founder mice carrying the CD158b transgene (Tg CD158b) were generated using a MHC class I promoter/immunoglobulin enhancer expression cassette (Fig. 1). Analyses were performed on three independent transgenic lines (L26, L47, and L61) established following stable transmission of the CD158b transgene. In particular, the CD158b transgene was expressed on 85 ± 8% (mean ± SEM, n = 8) of PBL isolated from the Tg CD158b L61 mice, as determined by flow cytometry. The vast majority of T cells (95 ± 4% of CD3ɛ+ cells, n = 6) and NK cells (78 ± 4% of CD3ɛ−, sIg− cells, n = 3) expressed the CD158b transgene as shown for a representative Tg CD158b L61 mouse in Fig. 2. Similar results were obtained with splenocytes isolated from Tg CD158b L61 transgenic mice as compared with PBL (data not shown). Of note, we also detected human KIR on the surface of mouse B cells. This result indicates that the cell surface expression of KIR does not require any T/NK-specific molecular environment, as previously demonstrated in COS fibroblasts as well as in the RBL-2H3 mast cell line (14, 22).
Figure 1.
Schematic representation of the CD158b transgenic vector. The restriction sites marked with an asterisk were destroyed during plasmid construction.
Figure 2.
Cell surface expression of the CD158b transgene. PBL isolated from mice representative of the indicated mouse lines were examined by flow cytometry for the cell surface expression of CD158b, CD3ɛ, surface immunoglobulin (sIg), and HLA-Cw3; nontransgenic, non-Tg; HLA-Cw3 transgenic, Tg HLA-Cw3; CD158b transgenic, Tg CD158b (L61); HLA-Cw3 and CD158b transgenic, Tg CD158b × HLA-Cw3. Cells were stained with FITC-goat anti-mouse IgG; after saturation of free binding sites with mouse Ig, FITC-anti-CD3ɛ and biotinylated GL183 (anti-CD158b) mAbs were added. Biotinylated GL183 was revealed using TC streptavidin. For HLA-Cw3 expression cells were incubated with F4/326 mAb (anti-HLA-C) followed by a FITC-goat anti-mouse IgG. Percentage of positive stained cells in each circle is indicated. (Middle and Bottom) Percentage and mean of fluorescence intensity of CD158+ and HLA-Cw3+ cells are indicated.
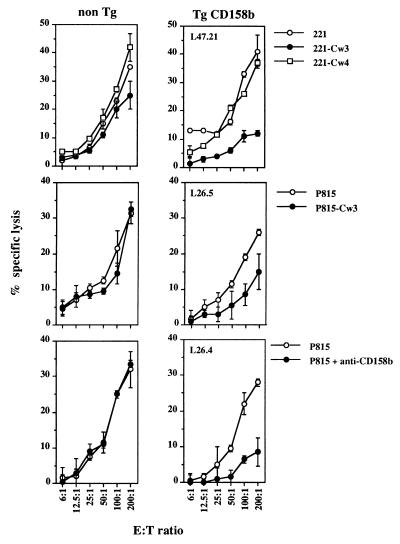
Splenocytes isolated from nontransgenic and CD158b transgenic mice were then analyzed for their ability to induce lysis of human HLA class I negative (221) and murine (P815) tumor cell lines transfected or not with HLA-Cw3. Splenocytes isolated from the CD158b Tg mice were unable to induce an efficient lysis of both 221-Cw3 and P815-Cw3 cells. By contrast, HLA-Cw3+ target cells were not protected from lysis exerted by splenocytes isolated from the nontransgenic mice. Of note, splenocytes that express or not the CD158b transgene were able to induce lysis of 221, 221-Cw4, and P815 cell lines (Fig. 3 Top and Middle). Thus, the expression of HLA-Cw3 at the surface of target cell line selectively inhibits the natural cytotoxicity of splenocytes that express the CD158b transgene. Cross-linking of CD158b using anti-CD158b mAb mimicked the effect of HLA-Cw3 (Fig. 3 Bottom), and consistent with observations performed in human NK clones, KIR engagement is always more efficient with anti-KIR mAb than with the cognate MHC class I molecule (23).
Figure 3.
In vitro cytotoxicity of splenic NK cells isolated from CD158b transgenic mice. Freshly isolated nonadherent splenocytes from CD158b transgenic (Tg CD158b) mice (L47 and L26 mouse lines) and nontransgenic littermate (non Tg) were tested for their ability to kill the indicated target cell lines in a standard 4-hr cytotoxicity assay. The following mice were used in this representative experiment: L47.21 (H-2k/b), L26.4 (H-2b/b), and L26.5 (H-2k/b).
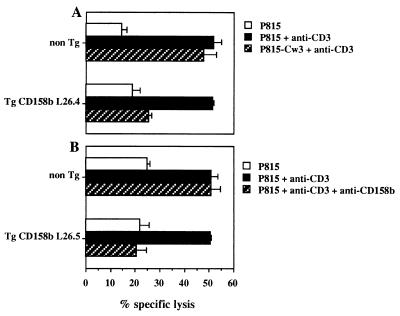
The function of the transgenic CD158b molecule expressed at the surface of mouse T cells was then analyzed. The CD3/T cell receptor complex was engaged using anti-CD3ɛ mAb in a redirected killing assay toward P815 cells. The engagement of CD158b by HLA-Cw3 (Fig. 4A) or by anti-CD158b mAb (Fig. 4B) inhibited the anti-CD3-mediated redirected killing of P815 by T cells from CD158b transgenic animals. The cell surface expression of HLA-Cw3 did not protect P815 cells from lysis by T cells isolated from nontransgenic littermates. Therefore, the transgenic expression of CD158b reconstitutes its inhibitory function on both T and NK cell activation programs in in vitro cytotoxicity assays.
Figure 4.
In vitro cytotoxicity of splenic T cells isolated from CD158b transgenic mice. Freshly isolated nonadherent splenocytes from CD158b transgenic mice (Tg CD158b, L26 mouse line) and nontransgenic littermates (non Tg) were tested in a redirected killing assay against P815 target cells at an effector:target ratio of 100:1. Anti-CD3 mAb-induced cytotoxicity was inhibited in Tg CD158b T cells upon CD158b engagement by HLA-Cw3 expressed on target cells (A) or by anti-CD158b mAb (B). Neither HLA-Cw3 expression (A) nor anti-CD158b mAb (B) could induce inhibition of anti-CD3 redirected target cell lysis by non-Tg T cells. The following mice were used in this representative experiment: L26.4 (H-2b/b) and L26.5 (H-2k/b).
CD158b Expression Is Not Influenced by the Expression of Its HLA-Cw3 Ligand in Vivo.
In an attempt to document the influence of the cognate MHC class I molecules on the cell surface expression of their KIR ligand, CD158b transgenic mice were crossed to mice transgenic for the CD158b ligand, HLA-Cw3. As shown in Fig. 2, no difference could be detected as to percentage of CD158b+ NK (CD3ɛ−, sIg− cells) and CD158b+ T/B cells (CD3ɛ+, sIg+) between the CD158b single transgenic and the CD158b × HLA-Cw3 double transgenic mice. In addition, no modulation of CD158b cell surface expression could be observed either, as assessed by the mean fluorescence intensity of CD158b: CD158b mean fluorescence intensity was 84 ± 10 and 78 ± 8 in PBL isolated from CD158b transgenic and CD158b × HLA-Cw3 double transgenic mice, respectively (P > 0.6). Similarly, the cell surface expression of HLA-Cw3 was unchanged between the single HLA-Cw3 transgenic mice when compared with the double CD158b × HLA-Cw3 transgenic mice (Fig. 2). Thus, in our experimental model, we cannot detect any adaptation of KIR cell surface expression to that of their HLA class I ligands.
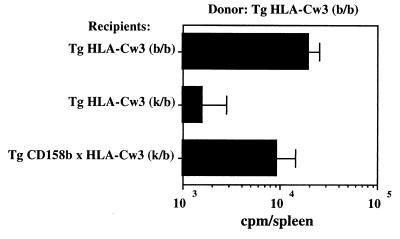
Prevention of HLA-Cw3+, H-2 Mismatched Bone Marrow Graft Rejection in CD158b Transgenic Mice.
It has been previously demonstrated that NK cells from an irradiated H-2k/b hybrid host mediate the rejection of mismatched H-2k/k or H-2b/b parental bone marrow grafts (10–12). The role of the CD158b KIR transgene was then tested in vivo for its ability to modulate the rejection of bone marrow graft in a similar hybrid resistance assay. Bone marrow grafts were prepared from HLA-Cw3 transgenic mice of H-2b/b haplotype. Syngenic H-2b/b HLA-Cw3 transgenic mice, H-2k/b HLA-Cw3 transgenic mice, and H-2k/b CD158b × HLA-Cw3 transgenic mice were used as hosts following lethal irradiation. The syngenic H-2b/b HLA-Cw3 graft was successful, whereas the H-2k/b HLA-Cw3 transgenic mice rejected H-2b/b bone marrow grafts (Fig. 5). This result confirms the H-2k/b hybrid resistance to H-2b/b parental grafts as a consequence of the lack of expression of inhibitory receptors for H-2b (potentially Ly-49C) on NK cell subsets from the H-2k/b HLA-Cw3 transgenic mice. By contrast, H-2b/b bone marrow grafts were not rejected in H-2k/b CD158b × HLA-Cw3 transgenic mice despite the mismatch at the H-2 locus. Therefore, the engagement of the transgenic CD158b KIR in hybrid host cells overcomes the lack of expression of endogenous KIRs, which recognize H-2b molecules. Moreover, these results demonstrate that the inhibitory signals generated upon engagement of CD158b with its HLA-Cw3 ligand override the signals initiated by the endogenous mouse activatory receptors expressed on NK cells, similar to CD158b dominant inhibition of endogenous activatory receptors (4). Since we and others have shown that human KIRs’ inhibitory function depends upon the recruitment of protein tyrosine phosphatases (i.e., SHP-1) by their intracytoplasmic immunoreceptor tyrosine-based inhibition motifs (5, 6, 24, 25), our results are in agreement with data indicating that both human and mouse NK cell activatory receptors use a common protein tyrosine kinase-dependent signaling pathway.
Figure 5.
CD158b transgenic mice are tolerant to graft of allogeneic bone marrow cells that express HLA-Cw3. Incorporation of 125IdUdr in donor marrow-derived cells in the spleen of irradiated recipients 6 days after bone marrow graft was used as an assay to determine the extent of donor cell proliferation. Results are expressed as mean cpm ± SEM of incorporated 125IdUdr obtained from three independent grafts.
DISCUSSION
The identification of KIRs revealed a novel strategy for T and NK cell control that is based on the promiscuous recognition of MHC class I molecules on antigen-presenting cells and target cells (26). Human KIRs belong to two unrelated families of molecules, IgSF (CD158, p70, p140) or dimeric C-type lectins (CD94-NKG2A/B), whereas only dimeric C-type lectins KIRs (Ly-49) have been described in the mouse (3). In vitro experiments using anti-KIR mAbs as well as KIR gene transfection have shown that engagement of human IgSF KIRs with their MHC class I ligands inhibit both T and NK cell activation programs. In vivo experiments in unmanipulated as well as transgenic mice have shown that the absence of mouse lectin KIRs is responsible for the F1 rejection of MHC class I mismatch parental bone marrow graft (13, 27–29). By contrast, no data are available relative to the role of human IgSF KIRs in vivo. Our data demonstrate that CD158b is sufficient to confer specificity to NK cells in vitro (Figs. 3 and 4) and in vivo (Fig. 5). The generation of human IgSF KIR transgenic mice reported here also provides several answers to central issues on the function and the selection of human KIRs.
First, these results represent the first experimental in vivo evidence that human IgSF KIRs control the host tolerance to MHC mismatch bone marrow grafts. In the hybrid resistance experimental system that we used (11), only NK cells from the hybrid F1 are responsible for the rejection of parental bone marrow grafts (Fig. 5). The inhibition of anti-CD3-induced T cell cytotoxicity by KIR engagement (Fig. 4) enlarges the spectrum of KIR inhibitory function, and reveals that both T and NK cells from the CD158b transgenic mice are unresponsive to any activatory stimuli when HLA-Cw3 interacts with CD158b. Therefore, our results provide an explanation for the necessity of selecting for a KIR expression confined to NK and T cell subsets. Indeed, the expression of KIR reacting with self-MHC on all T cells would prevent their response to antigen. Moreover, the distribution of KIRs on all NK cells rather than on NK cell subsets, as it naturally occurs, would render these cells insensitive to changes in the expression of only one MHC class I allele, which is a frequent alteration of MHC class I expression observed in vivo upon viral infection or malignant transformation (30). In this regard, our data are comparable to those generated in the Ly-49A transgenic mice (29).
Second, it is of note that in the double CD158b × HLA-Cw3 transgenic mice we cannot detect any adaptation of KIR cell surface expression to its MHC class I ligand (Fig. 2). This result is consistent with the lack of correlation between the level of expression of p70/NKB1 as well as the frequency of p70/NKB1+ cells, and the expression of cognate MHC class I molecules (i.e., HLA-Bw4) (31). In the mouse, a model of “receptor calibration” has been proposed based on the observation that the level of Ly-49 expression is down-regulated in the H-2 background corresponding to its ligand (e.g., H2-Dd for Ly-49A) (32). This adaptation of mouse KIR to their H-2 ligands selects for a low level of KIR cell surface expression and allows NK cells to detect subtle alteration of self-MHC class I expression. We can rule out the possibility that the use of an exogenous promoter for the generation of the CD158b transgenic mice might have influenced our observation, since a down-regulation of a Ly-49A transgene driven by the same promoter was detected in H-2d mice (33). Therefore, the absence of adaptation of CD158b KIR cell surface expression to HLA-Cw3 in the double CD158b × HLA-Cw3 transgenic mice would rather suggest that distinct strategies of selection/calibration are used by human IgSF KIRs and mouse lectin-like KIRs. In this regard, our results also indicate that the interaction between IgSF KIRs and their cognate MHC class I ligands exerts no role in the proliferation and differentiation of NK and T lymphocytes that express KIRs, in contrast to the inhibition of their cytotoxic programs. It is therefore possible that KIRs are unable to inhibit cytokine-induced lymphocyte proliferation once it is initiated, but rather selectively impair the signaling cascades that drive the cell cycle from G0 to G1, such as antigen-induced T cell activation. We have recently described that the coligation between KIRs and various activatory receptors is mandatory to KIR inhibitory function (22). Consistent with this observation, two factors are likely to determine the efficiency of KIR inhibitory function: (i) the intensity of the activatory signals and (ii) the ratio between the number of KIRs and the number of activatory receptors coexpressed on the same cell. The transgenic expression of KIR is up-regulated in peripheral T cells as compared with immature thymocytes (data not shown) and mimics the up-regulation of human IgSF KIRs during their progression from thymocytes to naive and memory T cells (4). The low expression of KIRs at early phases of T and NK cell development could thus account for their inability to inhibit T and NK cell differentiation. It remains also to be elucidated whether KIRs are coupled to an inhibitory signaling pathway (i.e., protein tyrosine phosphatases) only at a later stage of their differentiation programs and/or whether the signaling pathways that are coupled to the cytokine receptors involved in thymocytes/T cell and NK cell differentiation/proliferation are refractory to KIR inhibition.
Finally, it has been recently described that in patients receiving a haplo-identical bone marrow graft, a large fraction of the reconstituted T cell population expresses IgSF KIRs at their surface (34). It is tempting to speculate that expression of KIRs may prevent the development of an immune response mounted against the cells of the host. Taken together with the acceptance of HLA-Cw3+ H-2 mismatched bone marrow grafts by CD158b transgenic mice reported here, these results emphasize the potential implications of documenting and acting on KIR expression in the development of novel strategies of cellular therapy.
Acknowledgments
We thank Hanspeter Pircher as well as Günter Hämmerling and Jean-Pierre Abastado for the generous gift of the pHSE3′ vector and the HLA-Cw3 transgenic mice, respectively; Marie Malissen, Bernard Malissen, and Anne Gillet for early advice in the generation of transgenic mice; Michel Fougereau and Pierre Golstein for their continuous encouragement; G. Warcollier and M. Pontier for managing the mouse house; and Corinne Beziers-La Fosse for expert graphic art. This work is supported by institutional grants from Institut National de la Santé et de la Recherche Médicale, Centre National de la Recherche Scientifique, Ministère de l’Enseignement Supérieur et de la Recherche, and specific grants from Association pour la Recherche contre le Cancer (E.V.) and from Ligue Nationale contre le Cancer (E.V.). A.C. is supported by a fellowship of the Training and Mobility of Researcher Programme. A.M., L.M., and R.B. are supported by Associazione Italiana per la Ricerca sul Cancro, Consiglio Nazionale delle Ricerche, Istituto Superiore di Sanità, and Progetto Finalizzato Applicazioni Cliniche della Ricerche Oncologica (Italy). E.V. is a member of the Institut Universitaire de France.
ABBREVIATIONS
- FITC
fluorescein isothiocyanate
- IgSF
immunoglobulin superfamily
- KIR
killer cell inhibitory receptor
- MHC
major histocompatibility complex
- NK
natural killer
- TC
tricolor
- PBL
peripheral blood lymphocytes
References
- 1.Yokoyama W M. Curr Opin Immunol. 1995;7:110–120. doi: 10.1016/0952-7915(95)80036-0. [DOI] [PubMed] [Google Scholar]
- 2.Raulet D H, Held W. Cell. 1995;82:697–700. doi: 10.1016/0092-8674(95)90466-2. [DOI] [PubMed] [Google Scholar]
- 3.Lanier L L, Corliss B, Phillips J H. Immunol Rev. 1997;155:145–154. doi: 10.1111/j.1600-065x.1997.tb00947.x. [DOI] [PubMed] [Google Scholar]
- 4.Moretta A, Bottino C, Vitale M, Pende D, Biassoni R, Mingari M C, Moretta L. Annu Rev Immunol. 1996;14:619–648. doi: 10.1146/annurev.immunol.14.1.619. [DOI] [PubMed] [Google Scholar]
- 5.Olcese L, Lang P, Vély F, Cambiaggi A, Marguet D, Bléry M, Hippen K L, Biassoni R, Moretta A, Moretta L, Cambier J C, Vivier E. J Immunol. 1996;156:4531–4534. [PubMed] [Google Scholar]
- 6.Burshtyn D N, Scharenberg A M, Wagtmann N, Rajogopalan S, Berrada K, Yi T, Kinet J-P, Long E O. Immunity. 1996;4:77–85. doi: 10.1016/s1074-7613(00)80300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mingari M C, Vitale C, Cambiaggi A, Schiavetti F, Melioli G, Ferrini S, Poggi A. Int Immunol. 1995;7:697–703. doi: 10.1093/intimm/7.4.697. [DOI] [PubMed] [Google Scholar]
- 8.Phillips J H, Gumperz J E, Parham P, Lanier L L. Science. 1995;268:403–405. doi: 10.1126/science.7716542. [DOI] [PubMed] [Google Scholar]
- 9.Correa I, Corral L, Raulet D H. Eur J Immunol. 1994;24:1323–1331. doi: 10.1002/eji.1830240613. [DOI] [PubMed] [Google Scholar]
- 10.Cudkowicz G, Bennett M. J Exp Med. 1971;134:1513–1528. doi: 10.1084/jem.134.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kärre K, Ljunggren H G, Piontek G, Kiessling R. Nature (London) 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 12.Karlhofer F M, Ribaudo R K, Yokoyama W M. Nature (London) 1992;358:66–70. doi: 10.1038/358066a0. [DOI] [PubMed] [Google Scholar]
- 13.Yu Y Y L, George T, Dorfman J R, Roland J, Kumar V, Bennett M. Immunity. 1996;4:67–76. doi: 10.1016/s1074-7613(00)80299-x. [DOI] [PubMed] [Google Scholar]
- 14.Wagtmann N, Biassoni R, Cantoni C, Verdiani S, Malnati M, Vitale M, Bottino C, Moretta L, Moretta A, Long E O. Immunity. 1995;2:439–449. doi: 10.1016/1074-7613(95)90025-x. [DOI] [PubMed] [Google Scholar]
- 15.Pircher H, Mak T W, Lang R, Ballhausen W, Rüedi E, Hengartner H, Zinkernagel R M, Bürki K. EMBO J. 1989;8:719–727. doi: 10.1002/j.1460-2075.1989.tb03431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dill O, Kievits F, Koch S, Ivanyi P, Hämmerling G J. Proc Natl Acad Sci USA. 1988;85:5664–5668. doi: 10.1073/pnas.85.15.5664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Renard V, Ardouin L, Malissen M, Milon G, Lebastard M, Gillet A, Malissen B, Vivier E. Proc Natl Acad Sci USA. 1995;92:7545–7549. doi: 10.1073/pnas.92.16.7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marsh S G E, Krausa P, Bodmer J G. Tissue Antigens. 1990;36:180–186. doi: 10.1111/j.1399-0039.1990.tb01827.x. [DOI] [PubMed] [Google Scholar]
- 19.Ciccone E, Pende D, Viale O, Di Donato C, Orengo A M, Biassoni R, Verdiani S, Amoroso A, Moretta A, Moretta L. J Exp Med. 1992;176:963–971. doi: 10.1084/jem.176.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biassoni R, Falco M, Cambiaggi A, Costa P, Verdiani S, Pende D, Conte R, Di Donato C, Parham P, Moretta L. J Exp Med. 1995;182:605–609. doi: 10.1084/jem.182.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cudkowicz G, Bennett M. J Exp Med. 1971;134:1513–1528. doi: 10.1084/jem.134.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bléry M, Delon J, Trautmann A, Cambiaggi A, Olcese L, Biassoni R, Moretta L, Chavrier P, Moretta A, Daëron M, Vivier E. J Biol Chem. 1997;272:8989–8996. doi: 10.1074/jbc.272.14.8989. [DOI] [PubMed] [Google Scholar]
- 23.Moretta A, Vitale M, Bottino C, Orengo A M, Morelli M, Augugliaro R, Barbaresi M, Ciccone E, Moretta L. J Exp Med. 1993;178:597–604. doi: 10.1084/jem.178.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fry A M, Lanier L L, Weiss A. J Exp Med. 1996;184:295–300. doi: 10.1084/jem.184.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell K S, Dessing M, Lopez-Botet M, Cella M, Colonna M. J Exp Med. 1996;184:93–100. doi: 10.1084/jem.184.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Renard V, Cambiaggi A, Vély F, Bléry M, Olcese L, Olivero S, Bouchet M, Vivier E. Immunol Rev. 1997;155:205–221. doi: 10.1111/j.1600-065x.1997.tb00953.x. [DOI] [PubMed] [Google Scholar]
- 27.Öhlén C, Kling G, Höglund P, Hansson M, Scangos G, Bieberich C, Jay G, Kärre K. Science. 1989;246:666–668. doi: 10.1126/science.2814488. [DOI] [PubMed] [Google Scholar]
- 28.Yokoyama W M. J Exp Med. 1995;182:273–277. doi: 10.1084/jem.182.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Held W, Cado D, Raulet D H. J Exp Med. 1996;184:2037–2041. doi: 10.1084/jem.184.5.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garrido F, Ruiz-Cabello F, Cabrera T, Pérez-Villar J J, Lòpez-Botet M, Duggan-Keen M, Stern P L. Immunol Today. 1997;18:89–95. doi: 10.1016/s0167-5699(96)10075-x. [DOI] [PubMed] [Google Scholar]
- 31.Gumperz J E, Valiante N M, Parham P, Lanier L L, Tyan D. J Exp Med. 1996;183:1817–1827. doi: 10.1084/jem.183.4.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olsson M Y, Kärre K, Sentman C L. Proc Natl Acad Sci USA. 1995;92:1649–1653. doi: 10.1073/pnas.92.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raulet D H, Held W, Correa I, Dorfman J R, Wu M F, Corral L. Immunol Rev. 1997;155:41–52. doi: 10.1111/j.1600-065x.1997.tb00938.x. [DOI] [PubMed] [Google Scholar]
- 34.Albi N, Ruggeri L, Aversa F, Merigiola C, Tosti A, Tognellini R, Grossi C E, Martelli M F, Velardi A. Blood. 1996;87:3993–4000. [PubMed] [Google Scholar]