Abstract
North American monarch butterflies (Danaus plexippus) undergo a spectacular fall migration. In contrast to summer butterflies, migrants are juvenile hormone (JH) deficient, which leads to reproductive diapause and increased longevity. Migrants also utilize time-compensated sun compass orientation to help them navigate to their overwintering grounds. Here, we describe a brain expressed sequence tag (EST) resource to identify genes involved in migratory behaviors. A brain EST library was constructed from summer and migrating butterflies. Of 9,484 unique sequences, 6068 had positive hits with the non-redundant protein database; the EST database likely represents ∼52% of the gene-encoding potential of the monarch genome. The brain transcriptome was cataloged using Gene Ontology and compared to Drosophila. Monarch genes were well represented, including those implicated in behavior. Three genes involved in increased JH activity (allatotropin, juvenile hormone acid methyltransfersase, and takeout) were upregulated in summer butterflies, compared to migrants. The locomotion-relevant turtle gene was marginally upregulated in migrants, while the foraging and single-minded genes were not differentially regulated. Many of the genes important for the monarch circadian clock mechanism (involved in sun compass orientation) were in the EST resource, including the newly identified cryptochrome 2. The EST database also revealed a novel Na+/K+ ATPase allele predicted to be more resistant to the toxic effects of milkweed than that reported previously. Potential genetic markers were identified from 3,486 EST contigs and included 1599 double-hit single nucleotide polymorphisms (SNPs) and 98 microsatellite polymorphisms. These data provide a template of the brain transcriptome for the monarch butterfly. Our “snap-shot” analysis of the differential regulation of candidate genes between summer and migratory butterflies suggests that unbiased, comprehensive transcriptional profiling will inform the molecular basis of migration. The identified SNPs and microsatellite polymorphisms can be used as genetic markers to address questions of population and subspecies structure.
Introduction
The monarch butterfly (Danaus plexippus) is arguably the world's most captivating and well-known butterfly species [1]. Monarchs are renowned for their orange and black-edged wings, milkweed-derived chemical defenses, and involvement in mimicry with viceroy butterflies. But the monarch's most notable claim to fame is the spectacular fall migration of its North American populations.
The migratory state is characterized by reproductive diapause, a condition in which the butterflies exhibit refractory mating behavior and arrested reproductive development, as migrants need to conserve energy for the long journey [2]. Migrants also have increased abdominal fat stores, a marked increase in longevity, and an overwhelming urge to fly south. Diapause persists in Eastern North American migrants at the overwintering sites in Mexico until the early spring when the butterflies reproduce and take wing northward to lay fertilized eggs on newly emerged milkweed plants (genus Asclepias) in the southern United States. Another two to three generations of reproductively competent, short-lived “summer” butterflies follow the progressive, northward emergence of milkweed to reestablish, by late summer, the most northerly reaches (in southern Canada) of the eastern population of monarch butterflies. In the fall, decreasing daylength helps trigger the migratory generation and, once again, the long journey south begins [2]–[4].
As in Drosophila melanogaster, juvenile hormone (JH) is a key regulator of adult reproductive activity and longevity in monarch butterflies [5]. In migratory monarchs, JH levels are significantly reduced, reproductive development is curtailed, and longevity is increased-from a life span of a few weeks in summer butterflies to several months in migrants. Moreover, experimental manipulation of JH in adult butterflies causes predictable changes in reproductive activity and longevity. Thus, reproductive diapause and increased longevity, phenotypic markers of the migratory state, are induced by JH deficiency. JH synthesis is likely regulated by insulin-like peptides originating from neurosecretory cells in the pars intercerebralis [6].
The circadian clock plays a vital role in monarch migration by providing the timing component of time-compensated sun compass orientation [7]–[10], which contributes to navigation to the overwintering grounds. The remarkable navigational abilities of monarch butterflies are part of a genetic program, as the migrants are always on their maiden voyage, and those that make the trip south are at least two generations removed from the previous generation of migrants [3]. Here, we describe an expressed sequence tag (EST) resource, as a tool for ultimately identifying genes involved in migratory behaviors, as well as in other aspects of the biology of monarch butterflies.
Results and Discussion
Monarch brain EST database
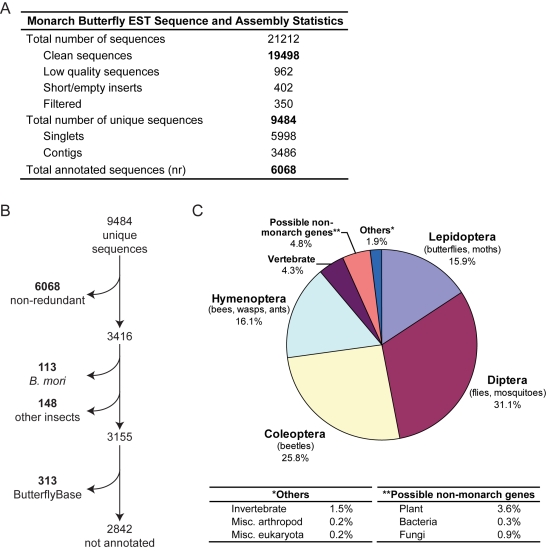
Nearly 300 monarch brains were collected from a mix of summer reproductive animals and fall migrating animals (Table 1) to create a cDNA library (average insert size 1.7 kb). Library clones were sequenced at the 5′ ends to create the brain EST database. The average read length was 741 base pairs. Out of 21,212 sequence reads, 19,498 were classified as “clean” sequences (GenBank accession numbers EY255129–EY274705) (Dataset S1). These were assembled into 3,486 contigs and 5,998 singlets, resulting in a total of 9,484 unique sequences (Fig 1A). The monarch butterfly EST Information Management Application (ESTIMA) can be found at: http://titan.biotec.uiuc.edu/cgi-bin/ESTWebsite/estima_startseqSetbutterfly
Table 1. Monarch Butterflies Collected for the cDNA Library.
| State* | Capture date | #Males | #Females | Total |
| MA | August 11, 2004 | 19 | 17 | 36 |
| MA | August 12, 2004 | 20 | 19 | 39 |
| MA | August 14, 2004 | 20 | 21 | 41 |
| MN | September 5, 2004 | 10 | 29 | 39 |
| MN | September 6, 2004 | 20 | 21 | 41 |
| MN | September 7, 2004 | 30 | 10 | 40 |
| TX | October 19, 2004 | 20 | 20 | 40 |
| TX | October 20, 2004 | 8 | 14 | 22 |
| Total | 147 | 151 | 298 |
Within the United States of America. MA, Massachusetts; MN, Minnesota; TX, Texas.
Figure 1. Overview of the monarch brain EST database.
A. Sequencing the monarch brain cDNA clones and assembly into contigs. B. Annotating the monarch EST database (described in text). C. The 6068 ESTs annotated against the non-redundant protein database are represented in the pie-chart according to the best matching sequences.
Database matches
The 9,484 unique sequences were compared to the non-redundant (nr) protein database (NCBI) using the BLASTX algorithm. Of these, 6068 matched an nr entry at E≤1×10−5 (Fig. 1B). Nearly 16% of these sequences had a best hit among the Lepidoptera, but surprisingly 31% had a best hit within the Diptera (Fig. 1C). This discrepancy is likely due to the fact that many dipteran genomes have been sequenced, and the only lepidopteran genome available is that of the commercial silkworm Bombyx mori. The annotation also revealed a small number of sequences that are similar to plants. These are mostly likely due to pollen contaminants in the brain dissections. In addition, a small number of bacterial and fungal genes were identified; these probably represent parasitic infections of some of our summer butterflies. Three sequences with similarity to Nosema species were discovered in the annotation. Nosema is known to be an infectious microsporidian in Lepidoptera [11].
Of the 3416 ESTs that did not have a match with the nr database, 113 had at least one match with the B. mori UniGene database (E≤1×10−5), and 148 had at least one hit with one or more of the following protein databases (NCBI): Tribolium castaneum, Flybase, Apis mellifera, and Anopheles gambiae. Of the remaining 3155 ESTs, 313 had a hit with the ButterflyBase v2.9 (Consortium for Comparative Genomics of Lepidoptera; http://heliconius.cap.ed.ac.uk/butterfly/db/index.php) (Fig. 1B). The ButterflyBase database used for the search includes EST sequences from 20 lepidopteran species, excluding B. mori. To determine if the remaining 2842 sequences with no matches have the potential to encode proteins, we used the OrfPredictor web server (https://fungalgenome.concordia.ca/tools/OrfPredictor.html; [12]). A total of 2563 ESTs (90%) were predicted to contain an ORF of 30 amino acids or longer, and 2473 (96%) of these were encoded on the plus strand, which is expected as the library was directionally cloned. Many of these non-annotated ESTs may represent genes unique to butterflies, the butterfly family Nymphalidae, and/or monarchs.
Number of genes
Our EST database likely represents a large portion of the gene-encoding potential of the monarch genome. After the sequences similar to plant, bacterial, and fungal genes were removed from the unique sequences tally, 9024 monarch sequences still remain. The unique sequence tally may be a modest overestimate (∼20%) of the actual number of monarch genes in the database, however, because assembly into contigs is not perfect [13]. Using a conservative estimate of 7219 unique genes, our database could represent ∼39% of the monarch protein-coding sequences, compared to the B. mori genome (18,510 genes predicted; Table 2). Yet, the monarch butterfly has the smallest genome of the lepidopterans examined (based on 59 lepidopterans from Animal Genome Size Database http://www.genomesize.com [14]), and is more similar in size to that of the mosquito A. gambiae, which is predicted to contain 13,683 genes (Table 2). Therefore, compared to the mosquito genome, our EST database could represent ∼52% of the genes in the monarch genome. Furthermore, as our EST database is based on a brain library, it is likely that our EST database represents more than 52% of the genes expressed in brain.
Table 2. Genome Sizes and Predicted Protein Coding Gene Numbers from Insect Genomes.
| Organism | Genome Size Based on C-value | Gene Number |
| Bombyx mori | 509-518 Mb | 18,510 |
| Drosophila melanogaster | 117-176 Mb | 13,854 |
| Anopheles gambiae | 264 Mb | 13,683 |
| Apis mellifera | 166-342 Mb | 10,157 |
| Tribolium castaneum | 196-205 Mb | 9,132 |
| Danaus plexippus | 284 Mb | — |
C-values are from Animal Genome Size Database [14]. The estimated gene numbers are from [50] for B. mori, [51] for D. melanogaster, [52] for A. gambiae, [53] for A. mellifera, and http://www.bioinformatics.ksu.edu/BeetleBase/index.shtml for T. castaneum.
Note: The Honeybee Genome Sequencing Consortium believes that their gene number is an underestimate.
Functional annotation
Putative functional roles of the 6068 genes were analyzed by Gene Ontology (GO) annotation and compared to the Drosophila annotation (Table 3). The number of genes in the GO groups under Molecular Function and Biological Process was well represented in monarch EST resource. There were 148 genes grouped under behavior, which included 51 genes involved in learning and memory, 74 genes involved in locomotor activity, and 8 genes involved in visual behavior.
Table 3. Gene Ontology for Annotated Monarch Genes.
| Gene Ontology Terms | Danaus Plexippus | Drosophila Genome (FlyBase) |
| Molecular function | 4158 | 8849 |
| antioxidant activity (GO:0016209) | 22 | 34 |
| auxiliary transport protein activity (GO:0015457) | 2 | 6 |
| binding (GO:0005488) | 2565 | 2474 |
| catalytic activity (GO:0003824) | 2120 | 3299 |
| chaperone regulator activity (GO:0030188) | 1 | 1 |
| enzyme regulator activity (GO:0030234) | 237 | 269 |
| molecular transducer activity (GO:0060089) | 371 | 515 |
| motor activity (GO:0003774) | 45 | 72 |
| structural molecule activity (GO:0005198) | 402 | 405 |
| transcription regulator activity (GO:0030528) | 419 | 609 |
| transcription factor activity (GO:0003700) | 147 | 300 |
| translation regulator activity (GO:0045182) | 72 | 83 |
| transporter activity (GO:0005215) | 545 | 650 |
| Biological process | 4097 | 7393 |
| biological adhesion (GO:0022610) | 216 | 152 |
| biological regulation (GO:0065007) | 1119 | 1455 |
| cellular process (GO:0009987) | 3626 | 4953 |
| developmental process (GO:0032502) | 1192 | 2244 |
| establishment of localization (GO:0051234) | 1026 | 732 |
| growth (GO:0040007) | 77 | 130 |
| immune system process (GO:0002376) | 108 | 197 |
| localization (GO:0051179 | 1206 | 1074 |
| locomotion (GO:0040011) | 7 | 12 |
| maintenance of localization (GO:0051235) | 24 | 23 |
| metabolic process (GO:0008152) | 2816 | 3148 |
| multicellular organismal process (GO:0032501) | 1135 | 2366 |
| multi-organism process (GO:0051704) | 34 | 209 |
| pigmentation (GO:0043473) | 26 | 68 |
| reproduction (GO:0000003) | 373 | 698 |
| reproductive process (GO:0022414) | 86 | 203 |
| response to stimulus (GO:0050896) | 584 | 975 |
| behavior (GO:0007610) | 148 | 452 |
| adult behavior (GO:0030534) | 38 | 108 |
| chemosensory behavior (GO:0007635) | 37 | 169 |
| feeding behavior (GO:0007631) | 5 | 20 |
| grooming behavior (GO:0007625) | 4 | 10 |
| hatching behavior (GO:0035187) | 1 | 1 |
| larval behavior (GO:0030537) | 17 | 29 |
| learning and/or memory (GO:0007611) | 51 | 94 |
| locomotory behavior (GO:0007626) | 74 | 158 |
| mechanosensory behavior (GO:0007638) | 2 | 26 |
| regulation of behavior (GO:0050795) | 1 | 18 |
| reproductive behavior (GO:0019098) | 37 | 87 |
| rhythmic behavior (GO:0007622) | 24 | 34 |
| visual behavior (GO:0007632) | 8 | 11 |
| rhythmic process (GO:0048511) | 34 | 48 |
Number of assembled monarch (D. plexippus) sequences that were assigned into GO categories of Molecular function and Biological process based on BLASTX homology. The Drosophila number is provided as a reference.
Using the EST database as a tool to investigate migration
Previous studies have focused on the physiological (e.g., reproductive diapause, increased longevity, cold tolerance, fat body hypertrophy) and behavioral (e.g., directional flight) aspects of monarch migration [2]–[4]. We are interested in expanding this knowledge to the molecular level, and the EST database is a powerful tool, as it will allow us to utilize microarray technology to identify candidate genes involved in all aspects of migration, with emphasis on those involved in migratory behavior.
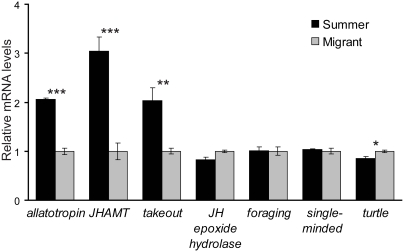
As a prelude to microarray studies, we used a candidate gene approach, along with real-time polymerase chain reaction (qPCR), to evaluate potential differences in gene expression between summer and migratory butterflies using whole head homogenates. We examined the expression of four genes identified in the EST database that are involved in JH activity. The four genes are allatotropin, a neuropeptide that can stimulate JH synthesis in the corpora allata [15]; juvenile hormone acid methyltransferase, the enzyme that mediates the final step in JH biosynthesis [16]; takeout, a potential JH binding protein that is an output gene of the circadian clock and is implicated in feeding homeostasis [17]; and juvenile hormone epoxide hydrolase, an enzyme involved in JH degradation [18].
Consistent with increased JH activity in summer butterflies, allatotropin, juvenile hormone acid methlyltransferase, and takeout were each up regulated significantly in summer animals, compared to migrants (allatotropin and juvenile hormone acid methyltransferase, p<0.001; takeout, p<0.01) (Fig. 2). The levels of expression of the juvenile hormone epoxide hydrolase gene were not significantly different between migrants and summer monarchs (p>0.05), however. It has been reported that flight may help keep JH levels low during migration by enhancing JH degradation through the activity of JH esterase [19], which was not represented in our database.
Figure 2. Expression profiles of selected genes between summer and migratory butterflies.
Relative expression of the mRNA levels of allatotropin, juvenile hormone acid methyltransferase (JHAMT), takeout, juvenile hormone (JH) epoxide hydrolase, foraging, single-minded, and turtle were examined by qPCR. The analysis was performed on RNA from summer monarchs (three 12-animals sets of head RNA collected during summer 2005) and on RNA from migratory monarchs (three 12-animal sets of head RNA collected during fall 2005, and three 12-animals sets of head RNA collected during fall 2006). Only the 2005 RNA from migratory butterflies was used for analysis of single-minded and turtle gene expression. The results were normalized with rp49 and then averaged. The average level of each gene in the migrants was normalized to 1.0 for graphing. *** p<0.001, ** p<0.01, * p<0.05
We also examined the expression of the EST-identified monarch homologs for three genes involved in locomotor behavior, foraging, single-minded, and turtle. The foraging gene encodes a cyclic nucleotide-dependent protein kinase that was of particular interest because it has been shown to induce foraging behavior in bees [20], and some of the navigational activities of foraging bees resemble those of migratory monarchs (e.g., use of time-compensated sun compass orientation). The single-minded gene encodes a PAS-containing transcription factor involved in midline CNS development [21], and it is important for normal adult walking behavior and locomotion in flies [22]; single-minded mutant adult flies have defects in the central complex, which is an important integration center of visual and skylight information from eyes, and may be the actual site of the sun compass [23], [24]. The turtle gene encodes a CNS-specific member of the Ig superfamily that is required for coordinated motor control in Drosophila [25].
Interestingly the expression of turtle was significantly increased by 15% in migrants versus summer monarchs (p<0.05), making it a candidate gene involved in migratory locomotor behavior (Fig. 2). The expression of the forager and single-minded genes, however, were not significantly different between migrant and summer butterflies (p>0.05).
The results are consistent with the differential regulation of JH activity between summer and migratory butterflies and further suggest that turtle may be a candidate “migration” gene. However, the marginal increase in turtle expression in migrants needs to be re-examined in brains, as whole head extracts may not accurately reflect expression in brain. In addition, the brain distribution of expression of any candidate migration gene will need to be compared between migrant and summer butterflies.
Circadian clock genes
The circadian clock in brain plays an important role in monarch migration by providing the timing component of time-compensated sun compass orientation [7]–[10], which contributes to successful navigation to the overwintering grounds. It is also possible that the circadian clock is involved in the induction of butterfly migration, as migration is initiated in the fall, in part, by decreasing daylength [26].
The EST database has allowed us to identify 8 monarch homologs out of the 12 genes involved in the core clock of Drosophila (Table 4). This included a Drosophila-like cryptochrome, designated insect cry1. Importantly, a novel, vertebrate-like cryptochrome, designated insect cry2, which is not present in Drosophila, was discovered in the monarch EST database [27]. This second cry encodes a light-insensitive protein that has potent repressive activity on the transcription factors CLOCK and CYCLE, which, as heterodimers, drive the intracellular transcriptional feedback loop that appears to be the critical gear of the molecular clock in all animals studied. The discovery of cry2 has thus provided novel insights into the molecular nature of the monarch butterfly circadian clock in particular [28] and the diversity of insect clocks in general, as cry2 exists in the genomes of all non-drosophilid insects so far examined [29].
Table 4. Clock Genes Represented in Monarch EST Database.
| Gene | EST Database | Proposed Function in Drosophila clock |
| period | — | Clock gene |
| timeless | — | Clock gene |
| Clock | — | Transcription factor |
| cycle | BF01012B2H07.f1 | Transcription factor |
| cryptochrome1 | BF14.3182.C1.Contig3165 | Circadian photoreceptor |
| cryptochrome2 | BF01037B1G10.f1BF01044A2E01.f1 | N/A |
| casein kinase II α | BF14.2950.C1.Contig2954 | Phosphorylates PERIOD |
| casein kinase II β | BF14.801.C1.Contig886 | Forms tetramer with alpha subunit (α2β2) |
| shaggy | BF14.370.C1.Contig413* | Phosphorylates TIMELESS |
| double-time | BF01044A2C10.f1* | Phosphorylates PERIOD |
| vrille | BF14.1188.C1.Contig1279 | Represses Clk transcription |
| Pdp1ε | BF01047A2E04.f1* | Activates Clk transcription |
| slimb | — | Ubiquitin-proteasome degradation of PERIOD |
3′ UTR only.
For clock genes not found in the EST database, we cloned the complete open reading frames and 3′untranslated regions, which were then used to search the database using BLASTN. The proposed functions in Drosophila clock are from [54].
A novel Na+/K+ ATPase allele for chemical defense
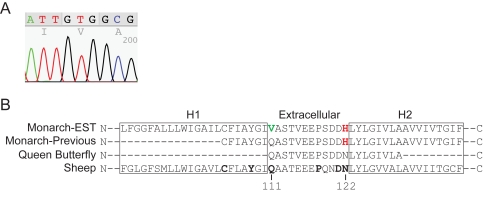
The utility of our EST resource for evaluating genes involved in the non-migratory aspects of monarch butterfly biology was apparent with the identification of ESTs encoding a new allele of a P type Na+/K+ ATPase (Fig. 3). The discovery of this novel allele bears directly on the chemical defense system of monarchs, as detailed below.
Figure 3. Na+/K+ ATPase in monarch butterflies.
A. Electropherogram of valine codon in the BF01056B1B12.fl EST. B. Partial sequence of the Na+/K+ ATPase from the monarch, queen butterfly (Danaus gilippus), and sheep. H1 and H2 are transmembrane domains. The residues in bold in the sheep sequence have been shown by mutagenesis to confer ouabain resistance when mutated, and positions 111 and 122 are indicated [35]. The Monarch-EST sequence is from two EST clones (BF01056B1B12.fl & BF01017B2C11.fl) from the monarch EST database. The Monarch-Previous sequence is the previously reported monarch sequence which has an amino acid change at one critical site (shown in red) [32], [34]. Both of the EST sequences have a second amino acid change at a second critical site (shown in green). The queen butterfly sequence has neither change [34].
An intriguing aspect of monarch biology is the ability of the larvae to consume milkweed, which contains large amounts of cardiac glycosides. In most invertebrates and vertebrates, these compounds bind to and inhibit a ubiquitous P type Na+/K+ ATPase. Cardiac glycosides can cause death, because this sodium/potassium pump is essential for proper cardiac function. Monarchs store cardiac glycosides in their bodies through adulthood, and it acts as a chemical defense against predators [30], [31]. However, it has been shown that the monarch ATPase is resistant to inhibition by the cardiac glycoside, ouabain [32]. Furthermore, sequencing an extracellular domain involved in ouabain sensitivity revealed an amino acid change at a critical site (H122). Site-directed mutatgenesis of the naturally ouabain-sensitive Drosophila ATPase at this position (N122H) created a less sensitive enzyme [33]. Sequencing the extracellular domain from milkweed-feeding species closely related to monarch (i.e., the queen butterfly, Danaus gilippus) revealed that this amino-acid change was unique to D. plexippus [34].
We found two ESTs in the monarch database with high sequence similarity to this P type Na+/K+ ATPase. When these ESTs were translated and aligned with the previously reported monarch sequence, an additional amino acid change was identified within this ouabain-sensitive domain (Fig. 3). This change is a result of not one but two nucleotide transversions; the CAG codon encoding glutamine is replaced by the GTG codon encoding valine (CA→GT). Interestingly, this particular position (amino acid 111) also has been shown to be important for ouabain sensitivity; amino acid substitutions produced by a random mutagenesis in the sheep α1 Na+/K+ ATPase at this site conferred ouabain resistance (Q111L, Q111R, Q111H) [35]. Lastly, when both position 111 and 122 were mutated in the same clone, ouabain resistance was higher than when a single mutation was present [36]. It is quite likely that the Na+/K+ ATPase variant present in the EST database is more resistant to ouabain than the allele previously reported.
Single nucleotide polymorphisms and microsatellites as genetic markers
The identification of single nucleotide polymorphisms (SNPs) and microsatellite polymorphisms will be useful for population studies of monarch butterflies at the species and subspecies levels. As nearly 300 individual wild butterflies collected from three states (Massachusetts, Minnesota, and Texas) were used to construct the EST library (Table 1), high polymorphism levels are expected to be present within the library. We took advantage of this expectation to identify SNPs and polymorphisms between microsatellite sequences.
To find reliable SNPs, we used a “double-hit” criterion in which each allelic variant must be represented by two or more ESTs (see Methods). Indeed, 1599 double-hit SNPs were identified from the 3,486 contigs (Dataset S2). To find microsatellites, we searched for tandem repeat sequences of 2, 3, 4, and 5 nucleotide repeats within our EST database. We identified 1333 potential microsatellites, and 98 of these exhibited polymorphism (Table 5, Dataset S2).
Table 5. Microsatellites Found in Monarch ESTs.
| Repeat Size | Repeat # | Total | Polymorphic* |
| 2 | >5 | 511 | 61 |
| 3 | >3 | 739 | 29 |
| 4 | >3 | 64 | 3 |
| 5 | >3 | 19 | 5 |
Number of microsatellites that exhibit polymorphism.
These SNPs and microsatellite polymorphisms can be used to more extensively address the long-standing question of the population structure of North American monarchs. Tagging studies have shown that monarchs from the Eastern United States of America (USA) overwinter in Mexico, while monarchs from the Western USA (west of the Rocky Mountains) overwinter in California [37]. Thus, it has been hypothesized that the Eastern and Western monarchs are two geographically isolated populations. Prior genetic studies using mtDNA [38], [39] have shown that Eastern and Western (and non-migrating South American) monarchs are rather homogenous with no clear population structure. [Also, Eanes and Koehn [40] found little variation in allozyme alleles within Eastern monarchs].
In addition to the issues of population structure, the SNPs and microsatellite polymorphisms found in our EST database will be useful for analyzing genetic differences between naturally occurring migrating (North American) and non-migrating (South American) subspecies [41], [42]. Furthermore, the SNP data could be used to identify genes that are evolving under natural selection (e.g., [43]).
Conclusions
To our knowledge, the monarch brain EST resource provides the first analysis of a brain transcriptome for any butterfly species. Our results show that the EST database will be valuable for examining the molecular control of many aspects of monarch butterfly biology. Likewise, the results suggest that extensive, unbiased analysis of differential gene expression between summer and migratory butterflies using high-density microarrays of all 9484 unique sequences will be informative for uncovering the genes involved in migratory behaviors. The SNPs and microsatellite polymorphisms offer important genetic markers for more rigorous analysis of North American monarch population structure and subspecies differences between migrating and non-migrating monarchs, than has been possible previously. Our monarch EST resource adds significantly to the expanding, comparative genomic data already available in Lepidoptera [44]. The resource also sets the stage for the cloning of the monarch butterfly genome.
Materials and Methods
Monarchs used for cDNA library
A total of 298 monarch butterfly brains were collected to construct the cDNA library (Table 1). Mid-summer, late-summer, and fall butterflies were obtained to ensure transcripts from both reproductive and diapuasing/migratory animals were represented in the library. Mid-summer butterflies were caught between August 11–14, 2004, near Greenfield, Massachusetts, USA (latitude 42°59′N, longitude 72°60′W) by Fred Gagnon, late-summer butterflies were caught between September 5–7, 2004, near Cannon Falls, Minnesota, USA (latitude 44°52′N, longitude 92°90′W) by Tim Murphy, and migrating butterflies were collected from roosts between October 19–10, 2004 near Eagle Pass, Texas, USA (latitude 28°71′N, longitude 100°49′W) by Carol Cullar. Mid-summer butterflies were housed in cages outside, and late-summer and fall butterflies were housed in glassine envelopes in incubators with controlled temperature (18°C), humidity (70%), and lighting (which mimicked the prevailing outdoor light-dark conditions) for less than one week prior to brain collections. The butterflies were fed 15% sucrose every other day.
Brains were collected in both the morning and the afternoon to increase chances of including circadian-controlled transcripts. Fresh brains were dissected in 0.5× RNAlater (Ambion). Brains did not include the photoreceptor layer of the eye.
To confirm that the Texas butterflies were in diapause, the female abdomens were dissected to determine reproductive status; none contained mature oocytes.
cDNA library construction, sequencing, and analysis
The W. M. Keck Center for Comparative and Functional Genomics (University of Illinois at Urbana-Champaign) carried out the following using the protocol of [45]:
Total RNA was extracted from each group of brains above using Trizol (Invitrogen), and equal amounts of RNA from mid-summer, late-summer, and fall (migratory) butterflies were pooled. PolyA+ RNA was purified from the total RNA mix using the Oligotex Direct mRNA kit (Qiagen). The mRNA was reverse transcribed using a polydT primer with a tag sequence appended. Double-stranded cDNAs larger than 800 bp were directionally cloned into a NotI and EcoRI digested pBS II SK(+) vector (Stratagene). After normalizing the primary library, 10,176 clones were sequenced to a redundancy of 41%. The average insert size of 12 clones was 1.7 kb (based on PCR of inserts). This library was then subtracted, and another 11,063 clones were sequenced.
The 5′ ends of the inserts were sequenced with a single pass. Sequences with a length of more than 200 base pairs after the quality trimming process were considered “high-quality”, while sequences that failed at this stage were called “low quality”. Next, the vector sequence was removed. If the remaining sequence length was less than 200 base pairs, then the sequence was called “short insert” and was removed from further analysis. Lastly, sequences were “filtered” for possible contaminants such as the E. coli genome, vector DNA, mitochondrial DNA, ribosomal RNA, and viral DNA using BLASTN. The remaining sequences were the “clean” sequence set. The raw sequences from the “clean” set (available in Dataset S1) were assembled into contigs using Phrap, and the vector sequences were trimmed from the contigs. All contigs were inspected manually using Consed, and a non-redundant database search detected false contigs.
Differential gene expression studies between summer and migratory butterflies
Summer butterflies were reared outdoors in western Massachusetts by Fred Gagnon. Adults were held in cages outside until mating was observed, which is indicative of mature reproductive status. On September 1, 2005 whole heads from 36 butterflies were collected and divided into three 12-animal sets for total RNA analysis.
Migrating butterflies were caught in Texas by Carol Cullar (October 17, 2005; October 16, 2006) and housed in an incubator for one week at 18°C prior to head collections. To confirm diapause status, 10 female abdomens were dissected and no mature oocytes were found. In addition, five male abdomens were dissected, and ejaculatory duct/tubular gland wet weights were less than 16 mg. Overwintering males have low reproductive organ weights [46], while males housed in summer conditions (25°C, 16 hrs light per day) have ejaculatory duct/tubular gland wet weights that average 32.4 mg [47]. Whole heads were collected from 36 of the 2005 migrants and 36 of the 2006 group; each of the two groups was divided into three 12-animal sets for RNA analysis.
Total RNA was prepared from each set of summer or migrating heads using Trizol (Invitrogen), and pigments were removed from the total RNA using charcoal purification.
Real-time PCR was performed using Taqman PCR primer/probe sets, and rp49 was used as control. For each candidate gene, the EST used for primer design was: allatotropin, BF01058B2A04.f1; juvenile hormone acid methyltransferase, BF01030B2G02.f1; takeout, BF01062B1H01.f1; juvenile hormone epoxide hydrolase, BF01057A1H08.f1; foraging, BF01042B1A03.f1; single-minded, BF01007X1C02.f1; and turtle, BF01052B1D11.f1. The primers and probe for rp49 were described previously [7]. The other primers and probes were as follows (F = forward primer, R = reverse primer, P = probe, all 5′-3′); allatotropinF: CCCGAGGGTTGGTAAACTTCA, allatotropinR : GGCTCGTGTTGCTCAATCCT, allatotropinP: FAM- AGCCCGTAGCTTTGGAAAACGCGA-BHQ1; juvenile hormone acid methyltransferaseF: GAACATCACGCCATGGATAACA, juvenile hormone acid methyltransferaseR: CGAAGTTCATCAGGCAGTTCAC, juvenile hormone acid methyltransferaseP: FAM-CAGCTTCACGCGGCTCGACATAGA-TAMRA; takeoutF: TCAGAACCAGTGCTACATTTTAAGGA, takeoutR: TGTTGTATCCATTTTAAACCCAGAAA, takeoutP: FAM-CTAACGGTTACAGGATTGAAGGGTCA-BHQ1; juvenile hormone epoxide hydrolaseF: ATGATTTAAGGGAGAGGTTGCTACA, juvenile hormone epoxide hydrolaseR: AACCGTAAGTGAAGCCTGAATTTTC, juvenile hormone epoxide hydrolaseP: FAM-TCGGCCATTTCAGCCTCCTC-BHQ1; foragingF: CCTTCAACCAGCTTATCTC, foragingR: TCATCGCCAACATCCT, foragingP: FAM- ACGCTCGATGAAATCCGCACCA-BHQ1; single-mindedF: GCCGTCACCGAGCTGAAG, single-mindedR: TGGCGTCCAGGAAGATGAG, single-mindedP: FAM-ATGTTCATGTTCCGCGCCTCGC-TAMRA; and turtleF: GGGTCAAACACAAGGCCATAAC, turtleR: ACGGACAGTATGATGGCCACTA, turtleP: FAM-TCGTTGGAGGGATATTGTTCTTC-TAMRA.
SNP and microsatellite identification
To identify SNPs in the EST database, trimmed EST sequences were assembled into contigs using Phrap developed by Phil Green (University of Washington) (http://www.phrap.org/). SNPs were predicted using the SEAN program (http://zebrafish.doc.ic.ac.uk/Sean/) [48]. To reduce the number of false SNPs due to sequencing or reverse transcription errors, the search for SNPs was restricted to contig regions with at least four-fold coverage, and a SNP was defined as a base variation that is present in at least two EST sequences. To remove sequences with potential sequencing errors, 15 base pairs on either side of the polymorphic position were compared to the consensus; if a second polymorphism was detected, this sequence read was eliminated from the analysis.
Microsatellite repeats were identified using a custom PERL script [49] on Phrap assembled contigs and singlet sequences. The location and size of each microsatellite is listed in the supplemental material. Default cutoffs (more than 5 repeats for 2bp, more than 3 repeats for 3bp, 4bp, and 5bp) were used for positive identification. Polymorphisms were detected by visual inspection of all microsatellites using a contig viewer program (sean.jar) provided in the SEAN program package. Summaries and details for both SNPs and microsatellites are provided in Supporting Dataset S2.
Supporting Information
Monarch Butterfly ESTs. A compressed FASTA file contains all the EST sequences in the monarch database.
(4.67 MB ZIP)
SNPs and Microsatellites in the Monarch EST Database. A compressed file contains detailed information of SNPs and mcirosatellites in the monarch EST database, which includes summaries for both SNPs and microsatellites. Access to the sequence information and position for each SNP and microsatellite is also provided.
(17.63 MB ZIP)
Acknowledgments
We thank the members of the Keck Center for Comparative and Functional Genomics at the University of Illinois at Urbana-Champaign for constructing the monarch brain EST resource and for their tireless help with this project. We also thank Danielle Metterville for technical assistance; Carol Cullar, Fred Gagnon, and Tim Murphy for supplying the monarch butterflies; Patricia Beldade and Anthony Long for providing the PERL script for the microsatellite analysis; and Adriana Briscoe for helpful discussions and suggesting the SNP and microsatellite analyses.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by NIH R01 NS047141 and funds from the University of Massachusetts Medical School.
References
- 1.Urquhart FA. Toronto: University of Toronto Press; 1960. The monarch butterfly. p. xxiv, 361. [Google Scholar]
- 2.Oberhauser KS, Solensky MJ, editors . Ithaca: Cornell University Press; 2004. Monarch butterfly biology & conservation. p. viii, 248. [Google Scholar]
- 3.Brower L. Monarch butterfly orientation: missing pieces of a magnificent puzzle. J Exp Biol. 1996;199:93–103. doi: 10.1242/jeb.199.1.93. [DOI] [PubMed] [Google Scholar]
- 4.Reppert SM. A colorful model of the circadian clock. Cell. 2006;124:233–236. doi: 10.1016/j.cell.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Herman WS, Tatar M. Juvenile hormone regulation of longevity in the migratory monarch butterfly. Proc Biol Sci. 2001;268:2509–2514. doi: 10.1098/rspb.2001.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- 7.Froy O, Gotter AL, Casselman AL, Reppert SM. Illuminating the circadian clock in monarch butterfly migration. Science. 2003;300:1303–1305. doi: 10.1126/science.1084874. [DOI] [PubMed] [Google Scholar]
- 8.Mouritsen H, Frost BJ. Virtual migration in tethered flying monarch butterflies reveals their orientation mechanisms. Proc Natl Acad Sci U S A. 2002;99:10162–10166. doi: 10.1073/pnas.152137299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez SM, Taylor OR, Jander R. A sun compass in monarch butterflies. Nature. 1997;387:29. [Google Scholar]
- 10.Sauman I, Briscoe AD, Zhu H, Shi D, Froy O, et al. Connecting the navigational clock to sun compass input in monarch butterfly brain. Neuron. 2005;46:457–467. doi: 10.1016/j.neuron.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Johny S, Kanginakudru S, Muralirangan MC, Nagaraju J. Morphological and molecular characterization of a new microsporidian (Protozoa: Microsporidia) isolated from Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae). Parasitology. 2006;132:803–814. doi: 10.1017/S0031182006009863. [DOI] [PubMed] [Google Scholar]
- 12.Min XJ, Butler G, Storms R, Tsang A. OrfPredictor: predicting protein-coding regions in EST-derived sequences. Nucleic Acids Res. 2005;33:W677–680. doi: 10.1093/nar/gki394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitfield CW, Band MR, Bonaldo MF, Kumar CG, Liu L, et al. Annotated expressed sequence tags and cDNA microarrays for studies of brain and behavior in the honey bee. Genome Res. 2002;12:555–566. doi: 10.1101/gr.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gregory TR, Nicol JA, Tamm H, Kullman B, Kullman K, et al. Eukaryotic genome size databases. Nucleic Acids Res. 2007;35:D332–338. doi: 10.1093/nar/gkl828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li S, Ouyang YC, Ostrowski E, Borst DW. Allatotropin regulation of juvenile hormone synthesis by the corpora allata from the lubber grasshopper, Romalea microptera. Peptides. 2005;26:63–72. doi: 10.1016/j.peptides.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 16.Shinoda T, Itoyama K. Juvenile hormone acid methyltransferase: a key regulatory enzyme for insect metamorphosis. Proc Natl Acad Sci U S A. 2003;100:11986–11991. doi: 10.1073/pnas.2134232100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarov-Blat L, So WV, Liu L, Rosbash M. The Drosophila takeout gene is a novel molecular link between circadian rhythms and feeding behavior. Cell. 2000;101:647–656. doi: 10.1016/s0092-8674(00)80876-4. [DOI] [PubMed] [Google Scholar]
- 18.Newman JW, Morisseau C, Hammock BD. Epoxide hydrolases: their roles and interactions with lipid metabolism. Prog Lipid Res. 2005;44:1–51. doi: 10.1016/j.plipres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Lessman CA, Herman WS. Flight enhances juvenile hormone inactivation in Danaus plexippus plexippus L. (Lepidoptera: Danaidae) Experientia. 1981;37:599–601. [Google Scholar]
- 20.Ben-Shahar Y, Robichon A, Sokolowski MB, Robinson GE. Influence of gene action across different time scales on behavior. Science. 2002;296:741–744. doi: 10.1126/science.1069911. [DOI] [PubMed] [Google Scholar]
- 21.Thomas JB, Crews ST, Goodman CS. Molecular genetics of the single-minded locus: a gene involved in the development of the Drosophila nervous system. Cell. 1988;52:133–141. doi: 10.1016/0092-8674(88)90537-5. [DOI] [PubMed] [Google Scholar]
- 22.Pielage J, Steffes G, Lau DC, Parente BA, Crews ST, et al. Novel behavioral and developmental defects associated with Drosophila single-minded. Dev Biol. 2002;249:283–299. doi: 10.1006/dbio.2002.0770. [DOI] [PubMed] [Google Scholar]
- 23.Heinze S, Homberg U. Maplike representation of celestial E-vector orientations in the brain of an insect. Science. 2007;315:995–997. doi: 10.1126/science.1135531. [DOI] [PubMed] [Google Scholar]
- 24.Liu G, Seiler H, Wen A, Zars T, Ito K, et al. Distinct memory traces for two visual features in the Drosophila brain. Nature. 2006;439:551–556. doi: 10.1038/nature04381. [DOI] [PubMed] [Google Scholar]
- 25.Bodily KD, Morrison CM, Renden RB, Broadie K. A novel member of the Ig superfamily, turtle, is a CNS-specific protein required for coordinated motor control. J Neurosci. 2001;21:3113–3125. doi: 10.1523/JNEUROSCI.21-09-03113.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goehring L, Oberhauser KS. Effects of photoperiod, temperature, and host plant age on induction of reproductive diapause and development time in Danaus plexippus. Ecological Entomology. 2002;27:674–685. [Google Scholar]
- 27.Zhu H, Yuan Q, Briscoe AD, Froy O, Casselman A, et al. The two CRYs of the butterfly. Curr Biol. 2005;15:R953–954. doi: 10.1016/j.cub.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 28.Zhu H, Sauman I, Yuan Q, Casselman A, Emery-Le M, et al. Cryptochromes define a novel circadian clock mechanism in monarch butterflies that may underlie sun compass navigation. PLoS Biol. 2008;6:e4. doi: 10.1371/journal.pbio.0060004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan Q, Metterville D, Briscoe AD, Reppert SM. Insect cryptochromes: gene duplication and loss define diverse ways to construct insect circadian clocks. Mol Biol Evol. 2007;24:948–955. doi: 10.1093/molbev/msm011. [DOI] [PubMed] [Google Scholar]
- 30.Malcolm SB, Brower LP. Evolutionary and ecological implications of cardenolide sequestration in the monarch butterfly. Experientia. 1989;45:284–295. [Google Scholar]
- 31.Parsons JA. A Digitalis-Like Toxin in the Monarch Butterfly, Danaus plexippus L. J Physiol. 1965;178:290–304. doi: 10.1113/jphysiol.1965.sp007628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holzinger F, Frick C, Wink M. Molecular basis for the insensitivity of the Monarch (Danaus plexippus) to cardiac glycosides. FEBS Lett. 1992;314:477–480. doi: 10.1016/0014-5793(92)81530-y. [DOI] [PubMed] [Google Scholar]
- 33.Holzinger F, Wink M. Mediation of cardiac glycoside insensitivity in the Monarch butterfly (Danaus plexippus): Role of an amino acid substitution in the ouabain binding site of Na+, K+ -ATPase. Journal of Chemical Ecology. 1996;22:1921–1937. doi: 10.1007/BF02028512. [DOI] [PubMed] [Google Scholar]
- 34.Mebs D, Zehner R, Schneider M. Molecular studies on the ouabain binding site of the Na+, K+-ATPase in milkweed butterflies. Chemoecology. 2000;10:201–203. [Google Scholar]
- 35.Croyle ML, Woo AL, Lingrel JB. Extensive random mutagenesis analysis of the Na+/K+-ATPase alpha subunit identifies known and previously unidentified amino acid residues that alter ouabain sensitivity–implications for ouabain binding. Eur J Biochem. 1997;248:488–495. doi: 10.1111/j.1432-1033.1997.00488.x. [DOI] [PubMed] [Google Scholar]
- 36.Price EM, Rice DA, Lingrel JB. Structure-function studies of Na,K-ATPase. Site-directed mutagenesis of the border residues from the H1-H2 extracellular domain of the alpha subunit. J Biol Chem. 1990;265:6638–6641. [PubMed] [Google Scholar]
- 37.Brower LP. Understanding and misunderstanding the migration of the monarch butterfly (Nymphalidae) in North America: 1857–1995. Journal of the Lepidopterists' Society. 1995;49:304–385. [Google Scholar]
- 38.Brower AVZ, Boyce TM. Mitochondrial DNA variation in monarch butterflies. Evolution. 1991;45:1281–1286. doi: 10.1111/j.1558-5646.1991.tb04393.x. [DOI] [PubMed] [Google Scholar]
- 39.Brower AVZ, Jeansonne MM. Geographical populations and “subspecies” of new world monarch butterflies (Nymphalidae) share a recent origin and are not phylogenetically distinct. Annals of the Entomological Society of America. 2004;97:519–523. [Google Scholar]
- 40.Eanes WF, Koehn RK. An analysis of genetic structure in the monarch butterfly danaus-plexippus. Evolution. 1978;32:784–797. doi: 10.1111/j.1558-5646.1978.tb04633.x. [DOI] [PubMed] [Google Scholar]
- 41.Ackery PR, Vane-Wright RI. London: British Museum (Natural History); Comstock Pub. Associates; 1984. Milkweed butterflies, their cladistics and biology : being an account of the natural history of the Danainae, a subfamily of the Lepidoptera, Nymphalidae. p. vii, 425. [Google Scholar]
- 42.Smith DS, Miller LD, Miller JY, Lewington R. Oxford; New York: Oxford Univeristy Press; 1994. The butterflies of the West Indies and south Florida. p. viii, 264. [Google Scholar]
- 43.Wheat CW, Watt WB, Pollock DD, Schulte PM. From DNA to Fitness Differences: Sequences and Structures of Adaptive Variants of Colias Phosphoglucose Isomerase (PGI). Mol Biol Evol. 2006;23:499–512. doi: 10.1093/molbev/msj062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papanicolaou A, Gebauer-Jung S, Blaxter ML, Owen McMillan W, Jiggins CD. ButterflyBase: a platform for lepidopteran genomics. Nucleic Acids Res Advanced online publication. 2007 doi: 10.1093/nar/gkm853. doi: 10.1093/nar/gkm853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonaldo MF, Lennon G, Soares MB. Normalization and subtraction: two approaches to facilitate gene discovery. Genome Res. 1996;6:791–806. doi: 10.1101/gr.6.9.791. [DOI] [PubMed] [Google Scholar]
- 46.Herman WS. Hormonally mediated events in adult monarch butterflies. Marine Science Supplement (Migration: Mechanisms and Adaptive Significance) 1985;27:799–815. [Google Scholar]
- 47.Herman WS. Endocrine regulation of post eclosion enlargement of the male and female reproductive glands in monarch butterflies. General & Comparative Endocrinology. 1975;26:534–540. doi: 10.1016/0016-6480(75)90176-8. [DOI] [PubMed] [Google Scholar]
- 48.Huntley D, Baldo A, Johri S, Sergot M. SEAN: SNP prediction and display program utilizing EST sequence clusters. Bioinformatics. 2006;22:495–496. doi: 10.1093/bioinformatics/btk006. [DOI] [PubMed] [Google Scholar]
- 49.Beldade P, Rudd S, Gruber JD, Long AD. A wing expressed sequence tag resource for Bicyclus anynana butterflies, an evo-devo model. BMC Genomics. 2006;7:130. doi: 10.1186/1471-2164-7-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xia Q, Zhou Z, Lu C, Cheng D, Dai F, et al. A draft sequence for the genome of the domesticated silkworm (Bombyx mori). Science. 2004;306:1937–1940. doi: 10.1126/science.1102210. [DOI] [PubMed] [Google Scholar]
- 51.Crosby MA, Goodman JL, Strelets VB, Zhang P, Gelbart WM. FlyBase: genomes by the dozen. Nucleic Acids Res. 2007;35:D486–491. doi: 10.1093/nar/gkl827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holt RA, Subramanian GM, Halpern A, Sutton GG, Charlab R, et al. The genome sequence of the malaria mosquito Anopheles gambiae. Science. 2002;298:129–149. doi: 10.1126/science.1076181. [DOI] [PubMed] [Google Scholar]
- 53.Consortium HGS. Insights into social insects from the genome of the honeybee Apis mellifera. Nature. 2006;443:931–949. doi: 10.1038/nature05260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Collins B, Blau J. Even a stopped clock tells the right time twice a day: circadian timekeeping in Drosophila. Pflugers Arch. 2007;454:857–867. doi: 10.1007/s00424-006-0188-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Monarch Butterfly ESTs. A compressed FASTA file contains all the EST sequences in the monarch database.
(4.67 MB ZIP)
SNPs and Microsatellites in the Monarch EST Database. A compressed file contains detailed information of SNPs and mcirosatellites in the monarch EST database, which includes summaries for both SNPs and microsatellites. Access to the sequence information and position for each SNP and microsatellite is also provided.
(17.63 MB ZIP)