Abstract
Protein kinase C-ε (εPKC) induces neurite outgrowth in neuroblastoma cells but molecular mechanism of the εPKC-induced neurite outgrowth is not fully understood. Therefore, we investigated the ability of phosphatidylinositol 4,5-bisphosphate (PIP2) binding of εPKC and its correlation with the neurite extension. We found that full length εPKC bound to PIP2 in a 12-ο-tetradecanoylphorbol-13-acetate dependent manner, while the regulatory domain of εPKC (εRD) bound to PIP2 without any stimulation. To identify the PIP2 binding region, we made mutants lacking several regions from εRD, and examined their PIP2 binding activity. The mutants lacking variable region 1 (V1) bound to PIP2 stronger than intact εRD, while the mutants lacking pseudo-substrate or common region 1 (C1) lost the binding. The PIP2 binding ability of the V3-deleted mutant was weakened. Those PIP2 bindings of εPKC, εRD and the mutants well correlated to their neurite induction ability. In addition, a chimera of pleckstrin homology domain of phospholipase Cδ and the V3 region of εPKC revealed that PIP2 binding domain and the V3 region are sufficient for the neurite induction, and a first 16 amino acids in the V3 region was important for neurite extension. In conclusion, εPKC directly binds to PIP2 mainly through pseudo-substrate and common region 1, contributing to the neurite induction activity.
Keywords: actin; neurite outgrowth; neuroblastoma; phosphatidylinositol 4,5-bisphosphate; protein kinase C
Protein kinase C (PKC) plays pivotal roles in proliferation, differentiation, and apoptosis etc. The PKC family consists of at least 10 subtypes that are classified into three groups based on the structure of their regulatory domain (RD) (Nishizuka 1992; Shirai and Saito 2002; Newton 2006). Conventional PKCs (α, β1, β2, and γ) have two common regions, C1 domain and C2 domain, in the RD. The former is responsible for diacylglycerol (DAG) and phorbol ester binding, the latter binds to calcium. Thus, calcium and DAG are required for the activation of conventional PKCs. On the other hand, novel PKCs (ε, δ, η, and θ) are activated by DAG, but not by Ca2+ because novel PKCs lack the C2 domain. Atypical PKCs (ζ and λ/ι) are insensitive to both Ca2+ and DAG because of lack of the C2 domain and one of the C1 domains. Each subtype shows different enzymatic properties and distinct tissue and cellular distribution, suggesting specific functions of each PKC subtype (Ohno 1997), but the individual functions have not been fully understood.
Among them, εPKC is abundant in the central nervous system and is thought to play important roles in nervous system (Tanaka and Nishizuka 1994; Akita 2002). Specifically, εPKC is localized at nerve terminus and seems to mediate synaptic function (Saito et al. 1993; Prekeris et al. 1996). Indeed, εPKC induces neurites outgrowth in during neural differentiation with various stimulations (Hundle et al. 1995; Zeidman et al. 1999). For the neurite-induction ability, the actin-binding site (ABS) between the first half of C1 domain (C1A) and second half of C1 domain (C1B) is important (Prekeris et al. 1996, 1998;Zeidman et al. 2002). Interestingly, it has been shown that the neurite-induction ability of εPKC is independent of its catalytic activity; regulatory domain of εPKC (εRD) also induces neurite outgrowth (Zeidman et al. 1999). However, in the case of the εRD-mediated neurite induction, deletion of ABS is not effective; RD of an ABS-deleted mutant still had strong neurite induction ability, while full length of the ABS-deleted mutant had about 50% less activity (Zeidman et al. 2002). These results suggest that there is an additional factor, in addition to actin binding, important for the neurite induction by εPKC, especially by εRD.
Phosphatidylinositol 4,5-bisphosphate (PIP2) is membrane phospholipids. It is well established that PIP2 is involved in the regulation of the action through regulating actin-binding proteins (Toker 1998). To date, many actin-binding proteins which can bind to PIP2 have been reported (Toker 1998; Gorbatyuk et al. 2006). In addition, phospholipids including PIP2 can activate some PKC including εPKC (Toker et al. 1994) and direct interactions of phospholipids to some PKCs have been reported (Huang and Huang 1991; Sanchez-Bautistra et al. 2006). However, there is no direct evidence that εPKC binds to PIP2. We, therefore, investigated the possibility of the direct binding of εPKC to PIP2 and its correlation with the neurite outgrowth.
Materials and methods
Materials
SH-SY5Y cells were purchased and fetal bovine serum was obtained from Riken cell bank (Tokyo, Japan) and Sigma (St Louis, MO, USA), respectively. FuGENE 6 transfection reagent (Roche Molecular Biochemicals, Indianapolis, IN, USA) and PIP2 were obtained from Roche Molecular Biochemicals. Rabbit anti-green fluorescent protein (GFP) antibody was produced by us. GFP–fused pleckstrin homology domain (PHD) of phospholipase Cδ (PLCδ), named GFP–PHD here, was kindly donated by Dr Iino and Hirose et al. (1999).
Cell culture
SH-SY5Y cells were cultured in Dulbecco’s modified Eagles’ medium (Nacali tesque, Kyoto, Japan) and Dulbecco’s modified Eagles’ medium/Ham’s F-12 medium (1 : 1) (GIBCO, Grand Island, NY, USA) at 37°C in humidified atmosphere containing 5% CO2. The media contained 25 mmol/L glucose, and were buffered with 44 mmol/L NaHCO3 and supplemented with 10% fetal bovine serum (Sigma), 1% GlutaMAXTM-I (Invitrogen Corp., Carlsbad, CA, USA), penicillin (100 units/mL) and streptomycin (100 μg/mL) (Invitrogen Corp.).
Constructs of plasmids encoding GFP-fused εPKC and mutants
The plasmid encoding εPKC having GFP at its C terminus [full length εPKC (εFL)–GFP] was described previously (Shirai et al. 1998). A cDNA fragments encoding εRD with Bgl II site was produced by a PCR with cDNA for rat εPKC as the template. The sense primer was 5-TTAGATCTACCATGGTAGTGTTCAATGGCC-3, and the anti-sense was 5-GAAGATCTTCCTCGGTTGTCAAATGAC-3. The PCR product was subcloned into pTB701-GFP (described as BS340 in Shirai et al. 1998) for the expression in mammalian cells and into pGEX-4T-1 (Amersham Pharmacia Biotech, Buckinghamshire, UK) for bacterial expression of a glutathione S-transferase (GST)-fusion protein, respectively. The plasmids encoding mutants lacking variable region 1 (ΔV1), pseudo-substrate (ΔPS) region, C1, C1A, C1B domains, and V3 region were made using the amino terminus-deleted and domain-deleted mutants of εPKC described by Kashiwagi et al. (2002) as the template and primers described above. Similarly, PCR was performed using following primers to make mutants lacking some of V3 regions, and subcloned into Bgl II site of BS340. Anti-sense primers were 5-TTAGATCTTTCCTGGTCACAAGGGGA-3 for RRKK mutant II, 5-GAAGATCTTGACTTGGATCGGTCGTCTTC-3 for RRKK mutant, and 5-TTAGATCTTGGCCACTGTTGAT-3 for ΔRRKK, respectively. Furthermore, a site directed mutagenesis was performed according to manufacture’s recommended protocol with ExSite PCR-based site directed mutagenesis kit (Stratagene, La Jolla, CA, USA) to make the putative actin-binding site-Δ (ΔpABS) mutant. Rat εPKC cDNA was used as a template and the primers used were 5′-ATCAACAACATCCGGAAGGCC-3′ and 5′-ATCTTCCTGGTCACAAGGGGA-3′. All PCR products were verified by sequencing.
Constructs of plasmids encoding a chimera of PH domain of PLCδ and the V3 region of εPKC
A cDNA fragment encoding the V3 region of rat εPKC with Xho I sites were produced by PCR using the sense primer, 5-TTTCTAGAGGGGTGGACGCCAGAGGAATT-3, and the anti-sense primer, 5-CCGGGCCCTTATCCTCGGTTGTCAAATGA-3. A cDNA encoding PH domain was obtained by digestion of GFP–PHD by with Bam HI and Xho I. The PCR product of the εPKC V3 region and the cDNA encoding PHD were subcloned into pEGFPC1 (Clontech, Palo Alto, CA, USA). The PCR product was verified by its sequencing.
Transfection and confocal microscopy
Cells (2.0 × 106 cells/dish) on glass-bottom dish (MatTek Corp., Ashland, MA, USA) were transfected using 6 μL of FuGENETM6 transfection reagent (Roche Molecular Biochemicals) and 2 μg of DNA according to the manufacturer’s protocol. Transfected cells were cultured at 37°C for about 24 h before use. The fluorescence of GFP was observed under confocal laser scanning fluorescent microscopy (Carl Zeiss, Jena, Germany). The GFP-fluorescence was monitored at 488 nm argon laser excitation with 515 nm long pass barrier filter.
Evaluation of neurite induction
Twenty-four hours after the transfection, at least 100 cells expressing the GFP-fused εPKC or mutants were observed under confocal laser microscopy and the cells with neurites longer than the length of two cell bodies were counted. Three independent experiments were performed and data (mean + SEM) are presented as percentage of the cells having long neurites among the 100 cells expressing GFP-fused εPKC or mutants
Recombinant protein expression and purification
BL21 (DE3) pLys cells were transformed with the plasmids for GST–εRD and control vector according to the manufacture’s instruction (New England Biolabs, Beverly, MA, USA), and the expression of recombinant proteins was induced by 0.1 mmol/L isopropyl-1-thio-β-d-galactoside at 25°C for 4 h. The cells were then harvested and lysed in column buffer [20 mmol/L Tris–HCl (pH 7.0), 1 mmol/L EDTA, 1 mmol/L dithiothreitol, 5 mmol/L MgCl2, 250 mmol/L sucrose, 1% triton-X 100, 20 μg/mL leupeptin, and 1 mmol/L phenylmethylsulfonyl fluoride] with handy sonic (Tomy Seiko Co., Ltd, Tokyo, Japan). After ultra-centrifugation at 14 000 g for 30 min, the proteins were purified using Glutathione-Sepharose 4B (Amersham Pharmacia Biotech) according to the manufacture’s instruction.
Protein-lipid overlay assay
Various amount of PIP2 (2–500 pmol) were spotted on Hybond-C extra membrane (Amersham Biosciences). After dried up, the membrane was blocked with 3% fatty acid-free bovine serum albumin in Tris buffered saline with Tween 20 (TBST; 50 mmol/L Tris–HCl, 150 mmol/L NaCl, and 0.1% Tween 20, pH 7.5) for 1 h at 25°C, and then incubated with 20 nmol/L GST–RDεPKC or GST for 3 h at 25°C in the blocking solution. The membrane was washed five times for 1 h (each time) in TBST buffer and then incubated for 1 h with 1/1000 dilution of anti-GST polyclonal antibody (Sigma-Aldrich) in the blocking solution. After washing with TBST, the membrane was immersed in the blocking solution with anti-rabbit horseradish peroxidase conjugate (1/5000 dilution) (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for 1 h. After washing, the protein bound to the lipid was detected by enhanced chemiluminescence (Amersham Biosciences).
In the case of protein-lipid overlay (PLO) assay using GFP fusion proteins, we used lysate of COS-7 cells, which were transfected with various plasmids encoding εPKC and the mutants. The amount of the fusion proteins to be applied to the PLO assay was adjusted by western blot using GFP antibody.
Results
Effect of over-expression of εPKC on neurite extension
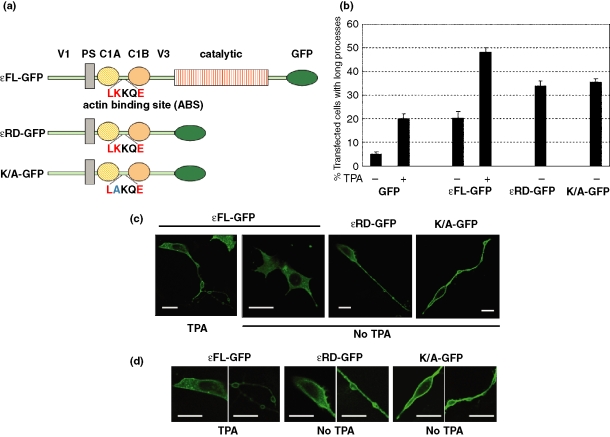
First, we tried to confirm the neurite induction by over-expression of εPKC in SH-SY5Y cells. Approximately 20% of the cells expressing εFL–GFP had long neurites, while only 5% of control cells expressing GFP showed long processes (Fig. 1b). εFL–GFP was expressed mainly in the cytoplasm under the normal conditions but TPA treatment induced translocation of εFL–GFP to the plasma membrane (Fig. 1c), resulting in the neurite extension; about 50% of the εFL–GFP cells possessed long neurites in the presence of TPA (Fig. 1b). Typical long neurite induced by εPKC had one or two neurites with varicose structures (Fig. 1c).
Fig. 1.
Induction of neurite outgrowth by over-expression of protein kinase C-ε (εPKC) and mutants. (a) Schematic illustration of full length εPKC (εFL), regulatory domain of εPKC (εRD) and regulatory domain of K/A mutant. Lysine in the actin-binding site of the K/A mutant was substituted by alanine. Consensus amino acids in the actin-binding motif were written in red. (b) Statistic analysis on the neurite induction ability of εPKC and the mutants. Twenty-four hours after the transfection, the cells with neurites longer than the length of two cell bodies were counted as described in Materials and methods. Three independent experiments were performed and the data (mean + SEM) are presented as percentage of the cells having long neurites among the cells expressing green fluorescent protein (GFP)-fused εPKC or mutants. (c) Typical neurites induced by GFP-tagged εFL, regulatory domain of εPKC (εRD), and KA mutant in the presence or absence of 12-ο-tetradecanoylphorbol-13-acetate (TPA) and their localizations. Plasmids encoding GFP-tagged εFL, εRD, and KA mutant were transfected into SHSY-5Y cells by lipofection and images were taken after 24 h after the transfection. Bars are 20 μm. (d) Magnified images of SH-SY5Y cells expressing GFP-tagged εFL, εRD, or KA mutant. The images shown in (c) are magnified to clearly show the plasma membrane localization of GFP–εFL in the presence of TPA, and of εRD or KA mutant without any stimulation. Bars are 20 μm.
By contrast, εRD–GFP showed membrane localization and strong neurite-induction activity even in the absence of TPA (Fig. 1b and c); 35% of the εRD cells had the typical long neurite. These results are basically consistent with previous findings by Larsson’s group.
To investigate the importance of actin-binding site between C1A and C1B in the neurite induction by εRD, we made a K/A mutant, in which lysine in the consensus sequence of the actin-binding site was substituted by alanine. RD of the K/A mutant showed both strong neurite induction activity and membrane localization as shown in Fig. 1b and c; about 35% of the cells expressing RD of the K/A mutant possessed long neurites. These results suggested that, except for the actin-binding site, unknown important factor is involved in the εRD-induced neurite induction
PIP2 binding of εPKC
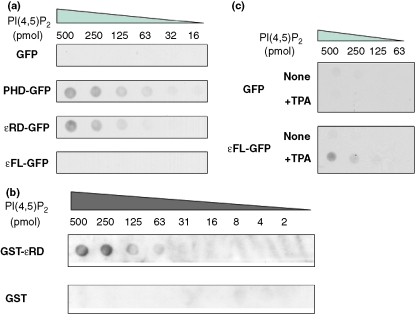
We, then, focused on PIP2 as a possible candidate for the important regulator of the εRD-induced neurite induction as described above. To examine whether εPKC can bind to PIP2, PLO assay was employed using GFP-fusion proteins from COS-7 cell lysate.εRD–GFP clearly binds to PIP2 as well as PH domain of phospholipase Cδ, which is well known to specifically bind to PIP2 (Fig. 2a). To confirm that εRD–GFP directly binds to PIP2, purified recombinant protein of GST–tagged εRD (GST–εRD) was used in the PLO assay. Similarly to the GFP-fusion protein form COS-7 cell lysate, the purified protein bound to PIP2 in a dose-dependent manner (Fig. 2b), indicating εRD can directly bind to PIP2 without any additional factors. By contrast, εFL did not show PIP2 binding under the same conditions. We, then, performed PLO assay in the presence of TPA because TPA induced the membrane localization of εPKC and neurite outgrowth as shown in Fig. 1. As expected, the TPA treatment enabled εFL to bind to PIP2 (Fig. 2c).
Fig. 2.
Phosphatidylinositol 4,5-bisphosphate (PIP2) binding of protein kinase C-ε (εPKC). (a) Binding of regulatory domain of εPKC (εRD) to PIP2. Various amount of PIP2 were spotted on the membrane and protein-lipid overlay (PLO) assay was performed using lysates from COS-7 cells expressing εRD–green fluorescent protein (GFP) or full length εPKC (εFL)–GFP. GFP and GFP–pleckstrin homology domain (PHD) of PLCδ were used as negative and positive control, respectively. (b) Direct binding of εRD to PIP2. Purified GST–εRD (10 nmol/L) was used for PLO assay to show direct binding. Same concentration of purified glutathione S-transferase (GST) was used as negative control. (c) 12-ο-tetradecanoylphorbol-13-acetate (TPA)-dependent PIP2 binding of full length εPKC (εFL). PLO assay was performed using lysates from COS-7 cells expressing εFL–GFP or GFP in the presence and absence of 200 nmol/L TPA.
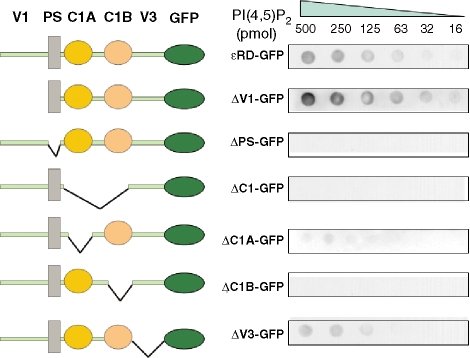
To identify the responsible region for the PIP2 binding, we made a series of mutants and investigated their PIP2 binding (Fig. 3). A mutant lacking V1 region from RD (ΔV1–GFP) showed stronger PIP2 binding than εRD–GFP, while deletion of V3 region weakened the PIP2 binding. Deletion of either PS or C1 domain completely abolished the PIP2 binding, indicating there is PIP2 binding region(s) in the PS and C1 domains. Furthermore, to investigate which domain, C1A or C1B, is critical for the PIP2 binding, we made C1A or C1B-deleted mutants. ΔC1A–GFP very faintly bound to PIP2, while ΔC1B–GFP lost the PIP2 binding. These results suggest that PS and C1 regions, relatively C1B than C1A, are important for the PIP2 binding.
Fig. 3.
Schematic illustration and phosphatidylinositol 4,5-bisphosphate (PIP2) binding ability of the domain-deleted mutant of protein kinase C-ε (εPKC). Protein-lipid overlay (PLO) assay was performed using lysates from COS-7 cells expressing green fluorescent protein (GFP)-fused regulatory domain of εPKC (εRD), and deletion mutants of variable region 1 (ΔV1), pseudo-substrate (ΔPS), common region 1-Δ (ΔC1), first half of C1 domain (ΔC1A), second half of C1 domain (ΔC1B), and ΔV3.
Importance of PIP2 binding for the neurite induction
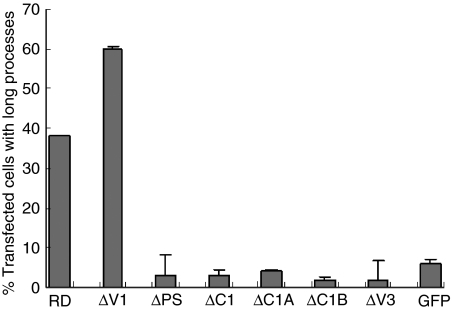
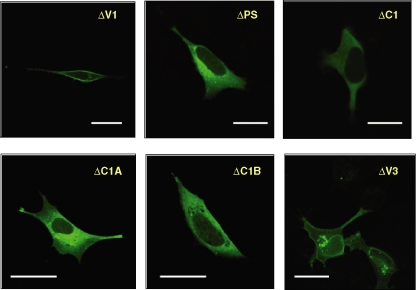
Next, the effects of over-expression of the deletion mutants on neurite induction were examined. ΔV1–GFP showed stronger neurite-induction activity than εRD–GFP; 60% of cells over-expressing of ΔV1–GFP possessed long neurite (Fig. 4). On the other hand, ΔPS, ΔC1, ΔC1A, ΔC1B–, and ΔV3–GFP lost ability to induce long neurite (Fig. 4). Localization of the mutants was also investigated. ΔV1–GFP showed very clear membrane localization, while ΔPS, ΔC1, ΔC1A, and ΔC1B–GFP were cytosolic. ΔV3–GFP was localized on the nuclear membrane with perinuclear accumulation, in addition to weak plasma membrane localization (Fig. 5).
Fig. 4.
Neurite induction ability of the domain-deleted mutant of protein kinase C-ε (εPKC). Twenty-four hours after the transfection of respective plasmids, the cells with neurites longer than the length of two cell bodies were counted. Three independent experiments were performed and at least 100 cells were counted at every experiment. Data (mean + SEM) are presented as percentage.
Fig. 5.
Localization of the domain-deleted mutant of protein kinase C-ε (εPKC). Plasmids encoding green fluorescent protein (GFP)-tagged mutants deleted variable region 1 (ΔV1), pseudo-substrate (ΔPS), common region 1-Δ (ΔC1), first half of C1 domain (ΔC1A), second half of C1 domain (ΔC1B), and ΔV3 were transfected into SHSY-5Y cells by lipofection and images were taken after 24 h after the transfection. Bars are 20 μm.
Table 1 summarizes the results of localization, PIP2 binding activity and neurite induction activity, indicating that the mutants without PIP2 binding activity clearly lost membrane location and neurite induction. On the other hand, the mutant having stronger PIP2 binding activity showed enhanced neurite induction ability. These results demonstrated that PIP2 binding is indispensable and important for the membrane localization and neurite induction of εPKC. In addition, as seen in the result of ΔV3–GFP, moderate PIP2 binding was not enough for the neurite induction, suggesting that strong PIP2 binding and/or V3 region are necessary.
Table 1.
Correlation between the phosphatidylinositol 4,5-bisphosphate (PIP2) binding, the plasma membrane localization, and the neurite extension of protein kinase C-ε (εPKC)
| PIP2 binding | Plasma membrane localization | Neurite extension | |
|---|---|---|---|
| εPKC without TPA | No | No | 20% |
| εPKC without TPA | ++ | Yes | 50% |
| Wild RD | ++ | Yes | 35% |
| RDK/A | ++ | Yes | 35% |
| RD ΔV1 | +++ | Yes | 60% |
| RD ΔPS | No | No | 6% |
| RD ΔC1 | No | No | 5% |
| RD ΔC1A | Very faint | No | 6% |
| RD ΔC1B | No | No | 2% |
| RD ΔV3 | + | Weak | 5% |
+++ and + represents stronger and moderate PIP2 binding, respectively. TPA, 12-ο-tetradecanoylphorbol-13-acetate; V1, variable regions 1 and 3; PS, pseudo-substrate; C1, common region 1; C1A, first half of C1 domain; C1B, second half of C1 domain; RD, regulatory domain.
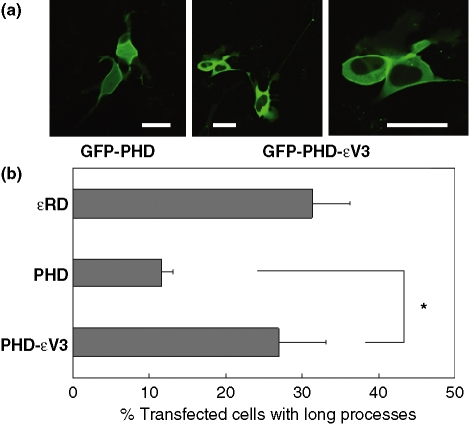
To confirm the importance of the PIP2 binding and V3 region for the neurite induction, we made a chimera of PH domain of PLCδ and V3 region of εPKC (PHD–εV3) and compared its neurite induction ability with that of PHD alone. Approximately 10% of the cells expressing PHD possessed long neurites, while 28% of the cells having PHD–εV3 showed comparable neurite induction ability to the εRD, although both proteins showed membrane localization (Fig. 6). These results indicate PIP2 binding and V3 region are sufficient for the neurite induction.
Fig. 6.
Effect of pleckstrin homology domain (PHD) of phospholipase C-δ (PLCδ) and the variable region 3 (V3) of protein kinase C-ε (εPKC) on neurite induction. (a) Localization of green fluorescent protein (GFP)–PHD and GFP–PHDεV3 in SY-SY5Y cells. Bars are 20 μm. (b) Statistical analysis on the neurite induction ability of GFP–PHD and GFP–PHDεV3. Twenty-four hours after the transfection, the cells with neurites longer than the length of two cell bodies were counted. Three independent experiments were performed and at least 100 cells were counted at every experiment. Data (mean + SEM) are presented as percentage. *p < 0.05.
Identification of subregion in the V3 region important for the neurite induction
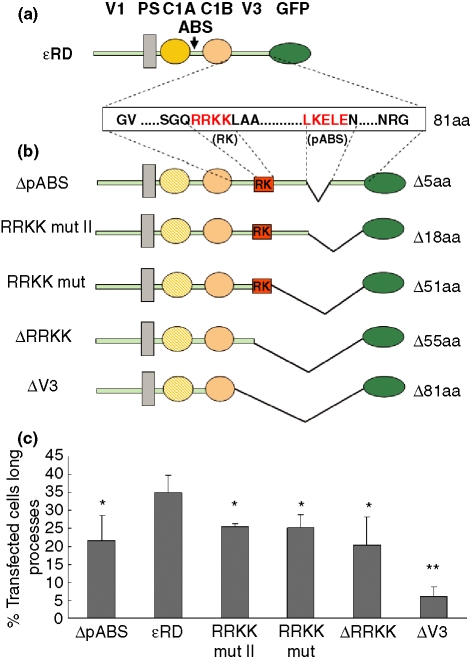
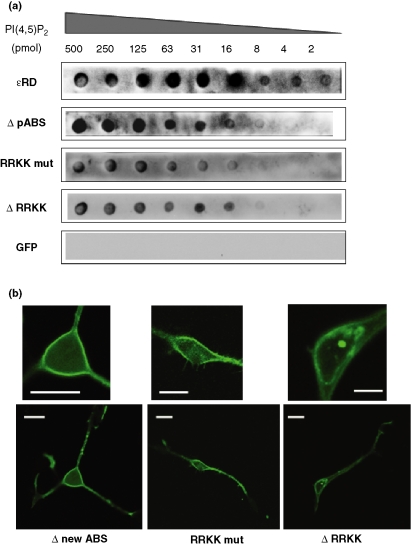
To identify subregion(s) of the V3 region important for the neurite induction, we focused on two regions; that are a cluster of basic amino acids corresponding to amino acids from 319 arginine to 322 lysine (RRKK) and a putative ABS corresponding to amino acids from 356 leucine to 360 glutamic acid (pABS), which has consensus actin-binding motif LKXXEX (Fig. 7a). We made a series of the mutants as shown in Fig. 7b and compared their neurite induction ability. Both RRKK and RRKK II mutants showed about 30% less neurite induction ability compared with the εRD; about 25% of the cells expressing the RRKK and RRKK II mutants had typical neurites. The PIP2 binding of RRKK was slightly weaker than εRD but were strongly enough (Fig. 8a). The PIP2 binding of RRKK II mutant was similar to that of RRKK mutant This suggested that pABS is somehow important for the neurite induction and PIP2 binding, because both RRKK and RRKK II mutants lack pABS. Consistently with this, the ability of the pABS deletion mutant to induce the neurite and its PIP2 binding was lower than that of εRD and similar to those of RRKK and RRKK II (Figs 7 and 8). On the other hand, deletion of the basic amino acid cluster form RRKK (ΔRRKK) did not significantly affect on the neurite induction ability and the PIP2 binding (Fig. 7). However, when further 16 amino acids were deleted from ΔRRKK mutant lost the neurite induction ability. The localization of these mutants was also investigated. As shown in Fig. 8b, ΔpABS and RRKK mutant were localized on the plasma membrane. The localization of RRKK II mutant was similar to that of RRKK mutant (data not shown). Some cells expressing ΔRRKK showed the nuclear membrane localization in addition to the plasma membrane localization like ΔV3 mutant (Fig. 8b), suggesting the basic amino acids are important for the plasma membrane localization.
Fig. 7.
Neurite induction ability of the protein kinase C-ε (εPKC) mutants lacking some of variable region 3 (V3). (a) V3 region of rat εPKC consists of 81 amino acids including a cluster of basic amino acids RRKK and putative actin-binding motif, LKELE. (b) Schematic illustration of the mutants. RK and putative actin-binding site (pABS) represent the cluster of basic amino acids and the putative actin-binding site in the V3 region, respectively. The numbers at the right side indicates the numbers of deleted amino acids from the V3 region. (c) Statistical analysis on the neurite induction ability of regulatory domain of εPKC (εRD) and the mutants. Twenty-four hours after the transfection, at least 100 cells expressing respective mutants were observed and the ratios of the cells with neurite longer than the length of two cell bodies to all observed cells were represented as percentage. Data are presented as mean ± SEM in three independent experiments. *Indicates the difference between εRD and the respective mutants was significant (p< 0.05). **Indicates the difference between ΔV3 and ΔRRKK was significant (p < 0.05).
Fig. 8.
Phosphatidylinositol 4,5-bisphosphate (PIP2) binding ability and localization of the protein kinase C-ε (εPKC) mutants lacking some of variable region 3 (V3). (a) Protein-lipid overlay (PLO) assay was performed using lysates from COS-7 cells expressing green fluorescent protein (GFP)-fused Δputative actin-binding site (pABS), RRKK mutant, or ΔRRKK. Regulatory domain of εPKC (εRD) and GFP were used as positive and negative control, respectively. (b) Plasmids encoding GFP-tagged ΔpABS, RRKK mutant, or ΔRRKK were transfected into SHSY-5Y cells by lipofection and images were taken after 24 h after the transfection. Upper images are magnified ones. Upper panels are magnified images. Bars are 20 μm.
These results revealed that the first 16 amino acids of the V3 region are important for the neurite induction and confirmed the importance of the PIP2 binding on the neurite induction.
Discussion
This is a first report to show that εPKC directly binds to PIP2. There are two types of interaction between PIP2 and proteins. One is the case that some proteins interact with the phosphoinositide through distinct domains including PH domain, FYVE, and ENTH, etc (DiNitto et al. 2003). The other case is that partner proteins bind to the lipid through an electrostatic mechanism involving basic amino acids. Generally, it is considered that the former interaction is more specific than the latter’s, although there are varieties of the specificity even in the case of the interaction between the distinct lipid-binding domains and PIP2 (DiNitto et al. 2003). Looking at the responsible region of εPKC for the PIP2 binding, specific motives for phosphoinositides like PH or five domains are not found, suggesting that electrostatic mechanism of basic amino acids are involved in the PIP2 binding of εPKC. Indeed, the PS and C1 regions of εPKC contain many basic amino acids and mutations on some of them reduced the PIP2 binding (data not shown). Especially, a number of basic amino acids in the PS region of εPKC are prominent among PKCs. Alternatively, higher structure of the C1 domain may be important as its structure is critical for DAG binding (Kazanietz et al. 1995; Xu et al. 1997). NMR and crystallography of the PS and C1 with PIP2 would be informative to understand precise mechanism of the PIP2 interaction. In the meantime, the characteristics of the PIP2 binding sites suggest that εPKC may bind to another phospholipids including PIP3. Interestingly, the regulations of εPKC by PIP3 have been reported (Toker et al. 1994; Moriya et al. 1996).
We also showed that the PIP2 binding ability of εPKC and mutants well correlated to their neurite induction abilities. For example, εFL induced neurites and bound to PIP2 in the presence of TPA, while any stimulation was not necessary for neurite induction and the PIP2 binding of εRD. In addition, the PIP2 binding abilities of the domain-deleted mutants of εPKC were parallel to their neurite induction abilities (Table 1 and Fig. 8). These results indicate that the PIP2 binding is important for the neurite induction. However, the PIP2 binding was not sufficient for the neurite outgrowth because PH domain of PLCδ did not induce the neurite outgrowth in SHSY-5Y cells. For the neurite induction, the V3 region of the εPKC was additionally necessary. It is evident that the V3 region alone was not enough either, because the mutant having the V3 regions, for example, ΔPS and ΔC1 lost the ability to induce neurite outgrowth. These results clearly show that both the PIP2 binding and εPKC V3 region are necessary and sufficient for the neurite induction. Importantly, the results using PHD–εV3 also proved that, at least, PIP2 is responsible lipid for the neurite induction even if εPKC could bind to another phospholipids, based on that PH domain of PLCδ specifically binds to PIP2.
In the V3 region of rat εPKC, we found pABS and it seemed to be somehow important for the neurite induction ability because deletion of the pABS reduced the ability about 20%. We, therefore, investigated whether the pABS binds to actin by ELISA and Biacore 3000 (GE Healthcare, Buckinghamshire, UK) using purified GST-fusion protein of the V3 region (GST–εV3) and pABS deleted one (GST–εV3ΔpABS). Unfortunately, however, we could not conclude that the V3 region binds to actin through the pABS because both GST–εV3 and GST–εV3ΔpABS showed tendency to bind to actin and the interaction was not so significant (data not shown). In addition, the pABS is not conserved in human εPKC although it is seen in mouse εPKC; the corresponding sequence of human is insufficient as actin-binding motif. In spite of this, human εPKC also induces the neurite. These facts suggest that pABS does not functions as ABS. Rather, the first 16 amino acids in the V3 region are important for the neurite induction of εRD. The importance of these amino acids for neurite induction is supported by Ling et al. (2005); they showed first 20 amino acids of V3 region are critical for the neurite induction ability of human εPKC.
Furthermore, plasma membrane localization appears to be important for the neurite induction for εPKC because all mutants which induced neurite outgrowth were localized on the plasma membrane, consistent with previous results (Zeidman et al. 1999, 2002). On the other hand, ΔV3 mutants, which lost the neurite induction activity, showed nuclear and Golgi membrane localization in addition to weak plasma membrane localization, suggesting V3 region and PIP2 binding may contribute to the membrane localization. Indeed, importance of a cluster of basic amino acids, RRKK, in the V3 region was shown (Fig. 8). However, the plasma membrane localization and the PIP2 binding were not sufficient for the neurite induction because GFP–PHD did not induce neurite although it localizes on the plasma membrane. These findings suggest recruitment of εPKC to specific site on the plasma membrane may be important for the neurite induction.
How do the PIP2 binding and the V3 region, especially the first 16 amino acids, participate in the neurite outgrowth? What we can do here is only speculation. One plausible function of the PIP2 binding of εPKC is to regulate the amount of PIP2 at local sites of the membrane. This may influence the function of actin-binding proteins, resulting in actin rearrangement and neurite induction. Other possibility is that the PIP2 binding of εPKC contributes to the inhibition of the ROCK-Rho A path way because Larsson’s group reported that Rho A and ROCK are involved in the εRD-induced neurite outgrowth (Trollér et al., 2004). Indeed, PIP2 activates Rho A by regulating open state of RhoA/RhoGDI complex (Faure et al., 1999). The PIP2 binding of εPKC may affect on this state by reducing amount of free PIP2 at specific site. On hand, the V3 region may bind to some proteins, the regulating RhoA/ROCK pathway and contributing to the recruitment of εPKC to appropriate site. A GTPase-activating protein for the Rho family, p190RhoGAP, is one interesting candidate to bind to the V3 because it binds to εPKC (Ling et al. 2005) and is relating to the regulation of Rho A. Intriguingly, the accumulation of p190 RhoGAP in lipid rafts regulates Rho activity, affecting in cytoskeletal structure (Mammoto et al. 2006). As discussed, the mechanism of εPKC-induced neurite induction is still puzzling but the PIP2 binding would be one piece to solve the puzzle, in addition to several proteins including actin, actin-binding proteins, Rho A, and Rho-regulating proteins as reported by Larsson group.
In conclusion, εPKC binds to PIP2 through the PS and C1 regions, contributing its neurite induction ability cooperating with the V3 region. The PIP2 binding gives new insight to understand mechanisms of εPKC functions including the neurite induction.
Acknowledgments
We would like to thank Dr Iino and Dr Hirose for providing a plasmid encoding PH domain of PLCδ and helpful discussions, and Dr Ueyama and Dr Kashiwagi for helpful discussions of our work. This work was supported by the 21st Century Center of Excellence Program of the Ministry of Education, Culture, Sports, Science.
Glossary
Abbreviations used
- ABS
actin-binding site
- C1
common region 1
- C1A
first half of C1 domain
- C1B
second half of C1 domain
- DAG
diacylglycerol
- εFL
full length εPKC
- GAP
GTPase-activating protein
- GFP
green fluorescent protein
- GST
glutathione S-transferase
- PHD
pleckstrin homology domain
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PKC
protein kinase C
- PLC
phospholipase C
- PLO
protein-lipid overlay
- PS
pseudo substrate
- ΔpABS
putative actin-binding site-Δ
- RD
regulatory domain
- TBST
Tris buffered saline with Tween 20
- TPA
12-ο-tetradecanoylphorbol-13-acetate
- V1 and V3
variable regions 1 and 3
- εRD
regulatory domain of εPKC
References
- Akita Y. Protein kinase C-ε: its unique structure and function. J. Biochem. 2002;132:847–852. doi: 10.1093/oxfordjournals.jbchem.a003296. [DOI] [PubMed] [Google Scholar]
- DiNitto JP, Cronin TC, Lambright DG. Membrane recognition and targeting by lipid-binding domains. Sci. STKE. 2003;213:re16. doi: 10.1126/stke.2132003re16. [DOI] [PubMed] [Google Scholar]
- Fauré J, Vignais PV, Dagher MC. Phosphoinositide-dependent activation of Rho A involves partial opening of the RhoA/Rho-GDI complex. Eur. J. Biochem. 1999;262:879–889. doi: 10.1046/j.1432-1327.1999.00458.x. [DOI] [PubMed] [Google Scholar]
- Gorbatyuk V, Norworthy NJ, Robson SA, Bains NPS, Maciejewski MW, Ramedios CG, King GF. Mapping the phosphoinositide-binding site on chick cofilin explains how PIP2 regulates the cofilin actin interaction. Mol. Cell. 2006;24:511–522. doi: 10.1016/j.molcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Hirose K, Kadowaki S, Tanabe M, Takeshima H, Iino M. Spatiotemporal dynamics of inositol 1,4,5-triphosphate that underlies complex Ca2+ mobilization patterns. Science. 1999;284:1527–1530. doi: 10.1126/science.284.5419.1527. [DOI] [PubMed] [Google Scholar]
- Huang FL, Huang KP. Interaction of protein kinase C isozymes with phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 1991;266:8727–8733. [PubMed] [Google Scholar]
- Hundle B, McMahon T, Dagar J, Messing RO. Overexpression of epsilon-protein kinase C enhances nerve growth factor-induced phosphorylation of mitogen-activated protein kinases and neurite outgrowth. J. Biol. Chem. 1995;270:30134–30140. doi: 10.1074/jbc.270.50.30134. [DOI] [PubMed] [Google Scholar]
- Kashiwagi K, Shirai Y, Kuriyama M, Sakai N, Saito N. Importance of C1B domain for lipid messenger-induced targeting of protein kinase C. J. Biol. Chem. 2002;277:18037–18045. doi: 10.1074/jbc.M111761200. [DOI] [PubMed] [Google Scholar]
- Kazanietz M, Wang S, Milne GW, Lewin NE, Liu HL, Blumberg PM. Residues in the second cysteine-rich region of protein kinase C δ relevant to phorbol ester binding as revealed by site-directed mutagenesis. J. Biol. Chem. 1995;270:21852–21859. doi: 10.1074/jbc.270.37.21852. [DOI] [PubMed] [Google Scholar]
- Ling M, Trollér U, Zeidman R, Stensman H, Schultz A, Larsson C. Identification of conserved amino acids N-terminal of the PKCεC1b domain crucial for protein kinase Cε-mediated induction of neurite outgrowth. J. Biol. Chem. 2005;280:17910–17919. doi: 10.1074/jbc.M412036200. [DOI] [PubMed] [Google Scholar]
- Mammoto A, Huang S, Ingber DE. Filamin links cell shape and cytoskeletal structure to Rho regulation by controlling accumulation of p190RhoGAP in lipid rafts. J. Cell Sci. 2006;120:456–467. doi: 10.1242/jcs.03353. [DOI] [PubMed] [Google Scholar]
- Moriya S, Kazalauskas A, Akimoto K, Hirai S, Mizuno K, Takenawa T, Fukui Y, Watanabe Y, Ozaki S, Ohno S. Platelet-drived growth factor activates protein kinase C ε through redundant and independent signaling pathways involving phospholipase Cγ or phosphatydilinositol 3-kinase. Proc. Natl Acad. Sci. USA. 1996;93:151–155. doi: 10.1073/pnas.93.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton AC. The ins and outs of protein kinase C. In: Newton AC, editor. Methods in Molecular Biology. Vol. 233. Totowa, NJ, USA: Humana Press; 2006. pp. 3–7. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein Kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Ohno Y. The distinct biological potential of PKC isotypes. In: Parker PJ, Dekker LV, editors. Protein kinase C. Heidelberg: Springer-Verlag GmbH & Co; 1997. pp. 75–95. [Google Scholar]
- Prekeris R, Mayhew MW, Cooper JB, Terrian DM. Identification and localization of an actin-binding motif that is unique to the epsilon isoform of protein kinase C and participates in the regulation of synaptic function. J. Cell Biol. 1996;132:77–90. doi: 10.1083/jcb.132.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prekeris R, Hernandez RM, Mayhew MW, White MK, Terrian DM. Molecular analysis of the interactions between protein kinase C-ε and filamentous actin. J. Biol. Chem. 1998;273:26790–26798. doi: 10.1074/jbc.273.41.26790. [DOI] [PubMed] [Google Scholar]
- Saito N, Itouji A, Totani Y, Osawa I, Koide H, Fujisawa N, Ogita K, Tanaka C. Cellular and intracellular localization of ε-subspecies of protein kinase C in the rat brain: presynaptic localization of the e-subspecies. Brain Res. 1993;607:241–248. doi: 10.1016/0006-8993(93)91512-q. [DOI] [PubMed] [Google Scholar]
- Sanchez-Bautistra S, Marin-Vincente C, Gomez-Fernanzez JC, Corblan-Grcia S. The C2 domain of PKCa is a Ca2+-dependent PtdIns(4,5)P2 sensing domain: a new insight into an old pathway. J. Mol. Biol. 2006;362:901–914. doi: 10.1016/j.jmb.2006.07.093. [DOI] [PubMed] [Google Scholar]
- Shirai Y, Saito N. Activation mechanism of protein kinase C: maturation, catalytic activation, and targeting. J. Biochem. 2002;132:663–668. doi: 10.1093/oxfordjournals.jbchem.a003271. [DOI] [PubMed] [Google Scholar]
- Shirai Y, Kashiwagi K, Yagi K, Sakai N, Saito N. Distinct effect of fatty acids on translocation of r- and e-subspecies of protein kinase C. J. Cell Biol. 1998;143:511–521. doi: 10.1083/jcb.143.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka C, Nishizuka Y. The protein kinase C family for neuronal signaling. Annu. Rev. Neurosci. 1994;17:551–567. doi: 10.1146/annurev.ne.17.030194.003003. [DOI] [PubMed] [Google Scholar]
- Toker A. The synthesis and cellular roles of phosphatidylinositol 4,5-bisphosphate. Curr. Opin. Cell Biol. 1998;10:254–261. doi: 10.1016/s0955-0674(98)80148-8. [DOI] [PubMed] [Google Scholar]
- Toker A, Meyer M, Reddy KK, Flack JR, Aneja R, Aneja S, Parra A, Burns DJ, Balla LM, Cantley LC. Activation of protein kinase C family members by the novel polyphosphoinositides PtdINs-3,4P2 and PtdINs-3,4,5P3. J. Biol. Chem. 1994;269:32358–32367. [PubMed] [Google Scholar]
- Trollér U, Raghunath A, Larsson C. A possible role for p190 RhoGAP in PKCɛ-induced morphological effects. Cell. Signal. 2004;16:245–252. doi: 10.1016/s0898-6568(03)00135-9. [DOI] [PubMed] [Google Scholar]
- Xu RX, Pawelczk T, Xia T, Brown SC. NMR structure of a protein kinase C-γ phorbol-binding domain and study of protein-lipid micelle interactions. Biochemistry. 1997;36:10709–10717. doi: 10.1021/bi970833a. [DOI] [PubMed] [Google Scholar]
- Zeidman R, Löfgren B, Påhlman S, Larsson C. PKCε, via its regulatory domain and independently of its catalytic domain, induces neurite-like processes in neuroblastoma cells. J. Cell Biol. 1999;145:713–726. doi: 10.1083/jcb.145.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidman R, Trollér U, Raghunath A, Påhlman S, Larsson C. Protein kinase C ε actin-binding site is important for neurite outgrowth during neuronal differentiation. Mol. Biol. Cell. 2002;13:12–24. doi: 10.1091/mbc.01-04-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]