Abstract
The combination treatment of peginterferon alpha-2a (PEG-IFN alpha-2a; Pegasys®) plus ribavirin (RBV) is recommended as a standard care for HCV infections. Side effects and aspects of efficacy and safety have to be balanced. This study evaluates clinical practice data on safety and efficacy of HCV treatment with PEG-IFN in combination with RBV over 24 and 48 weeks. This study was a phase III, multi-centre, open-label study with two treatment groups: PEG-IFN in combination with RBV for 24 or 48 weeks. The allocation to the treatment groups was at the discretion of the investigator; 309 patients entered active treatment: 90 patients received PEG-IFN plus RBV for 24 weeks and 219 patients PEG-IFN plus RBV for 48 weeks. A sustained virological response (SVR) was achieved in 48.9% of all patients. Genotype 1 patients with a 48-week combination treatment achieved an SVR of 39.9%. In the 48-week group a low baseline viral load was associated with a higher SVR rate (47.0% vs. 32.4%). For genotype 2 or 3 patients, the SVR was 67.9%. For these patients there was no relevant difference between patients with low and high viral loads; 97.7% of the patients experienced at least one adverse event. The incidence of serious adverse events was distinctly lower in the 24-week group (4.4% vs. 10.5%). This investigation confirms the well-known risk–benefit ratio found in controlled studies in a clinical practice setting. The safety profile is similar and shows the highest incidence of adverse events in the first 12 weeks of treatment.
Keywords: hepatitis C, pegylated interferon, polymerase chain reaction, sustained virological response
Introduction
Hepatitis C is a global health problem, with 170 million carriers worldwide. The distribution is heterogeneous, with an estimated 9 million chronic infections in western Europe and 66–75% of all HCV-infected patients having not been identified [1]. The hepatitis C virus (HCV) is a ribonucleic acid (RNA) virus of the family Flaviviridae which first was identified in 1989 [2,3]. The virus is transmitted primarily through large or repeated direct exposure to blood [4].
The HCV infection evolves in different ways for different patients. The major stepping points in individual histories of HCV include progression from acute infection to long-term infection, from long-term disease to cirrhosis, and from cirrhosis to decompensation or hepatocellular carcinoma [1,5].
The first drug with shown bioactivity against HCV infection was interferon (IFN) alpha, but with limited effectiveness: IFN monotherapy results in only 15–20% sustained response rates [6]. A substantial improvement of response of approximately twofold over IFN monotherapy was noted with the combination of IFN and ribavirin (RBV) [7,8]. The main reason for this still unsatisfactory response to IFN is its short half-life of approximately 8 h. Thus, in patients treated with IFN alpha three times weekly, an intermittent increase in viral load can be observed on treatment-free days [9].
To improve the effectiveness of HCV treatment, a covalent attachment of a 40-kDa branched-chain polyethylene glycol moiety to IFN alpha-2a was achieved, which produces peginterferon (PEG-IFN) alpha-2a, a compound that has sustained absorption, a slower rate of clearance and a longer half-life than unmodified IFN alpha [10,11]. These properties allow weekly dosing and enhanced clinical efficacy compared with unmodified IFN [10,12,13]. As with unmodified IFN, the combination treatment of PEG-IFN alpha-2a with RBV enhances efficacy over PEG-IFN alpha-2a monotherapy [10,14]. The availability of this drug regimen that eradicates the hepatitis C virus in more than half of the treated patients provides the medical community with a powerful new weapon, which is recommended as the standard cure for HCV infections [15–17].
All patients with long-term hepatitis C are potential candidates for antiviral therapy. The risks and benefits of anti-HCV treatment must be determined for each patient based on the individual's disease stage, symptoms, comorbid conditions, risk factors, and the likelihood of adherence and side effects. For managing side effects, aspects of efficacy and safety have to be balanced. One of the factors influencing safety appears to be the duration of treatment as the time of exposure to medication. Efficacy results have shown that treatment duration may be individualized by the HCV genotype of the patients: whereas patients with HCV genotype 1 require treatment for 48 weeks, those with HCV genotype 2 or 3 seem to be adequately treated in 24 weeks [18,19].
While the economic aspects of shorter treatment duration are well accepted, the safety profile of the combination treatment of PEG-IFN alpha-2a plus RBV over time remains unclear: What are the consequences not only in clinical study settings but also in clinical practice? To improve current safety and efficacy data and thus provide a better basis for treatment decisions, this study should evaluate clinical practice data on HCV treatment with PEG-IFN alpha-2a (Pegasys®, Roche Pharma AG, Grenzach-Wyhlen, Germany) in combination with RBV in patients with chronic hepatitis C in a setting as close to clinical practice as possible.
Methods
This data set is based on a German phase III, multi-centre, open-label study with two treatment groups:
PEG-IFN alpha-2a in combination with RBV for 24 weeks;
PEG-IFN alpha-2a in combination with RBV for 48 weeks.
The treatment periods in both groups were followed by an untreated 24-week follow-up period. The allocation to the treatment groups was at the discretion of the investigator. Patients in the 48-week treatment group with detectable HCV-RNA at week 24 were considered as non-responders and stopping of treatment was recommended.
The recommended dosage was 180 μg once weekly for PEG-IFN alpha-2a and 800 mg/day in split doses for RBV. However, in the meantime the weight-based dosing of RBV in genotype 1 and 4 patients has been established. Even though dose adjustment is highly recommended, some physicians still did stick to their former treatment regimen. Inclusion criteria were age of at least 18 years, quantifiable HCV-RNA, permanently increased alanine aminotransferase (ALT) serum levels and compensated liver disease. A sustained virological response (SVR) was defined as non-detectable HCV-RNA (e.g. <50 IU/mL by the Roche AMPLICOR® HCV Test, v2.0; Roche Molecular Systems, Inc., Branchburg, NJ, USA) 24 weeks after completion of the treatment period.
Statistical analysis
Treatment assignment to the two treatment groups was at the discretion of the physician; therefore, only descriptive statistical methods were used to analyse the results of the study. In accordance with the intent-to-treat principles, all patients who received at least one dose of study medication were included in the analysis of efficacy and safety data. Adverse events were assigned preferred terms and categorized into system organ class (SOC) according to MedDRA (Medical Dictionary for Regulatory Activities; http://www.meddramsso.com/MSSOWeb/index.htm). Stepwise multiple logistic regression analysis was used to explore the prognostic factors for an SVR across both treatment groups. In the stepwise model-building process, a variable was added to the model if the adjusted chi-squared statistic was significant at the 0.1 level and a variable was deleted from the model if the Wald chi-squared statistic was not significant at the 0.05 level. The following baseline disease and demographic factors were considered for entry into the model: age, gender (male vs female), body mass index, body surface area, log10 pre-treatment HCV-RNA titre, baseline ALT quotient, HCV genotype (1 vs non-1) and histological diagnosis (cirrhotic vs non-cirrhotic). Furthermore, the assigned treatment duration (24 vs 48 weeks of treatment) was considered. If the assumption of linearity in the logit for continuous variables was not obvious, HCV-RNA was dichotomized using a cutpoint of 400 000 IU/mL for all final models. If the assumption of the linearity of the logit for continuous variables was violated, appropriate categories (e.g. high and low virus load) were investigated to describe the association between the prognostic factor and SVR (see Fig. 1).
Fig. 1.
Patients’ flow chart
Results
Patients
In all, 320 patients in 35 German centres were screened and 309 patients entered active treatment with PEG-IFN alpha-2a in combination with RBV:
90 patients for 24 weeks;
219 patients for 48 weeks.
Of the patients, 58% were male with mean age of 41.5 years and mean weight of 75.7 kg. In 63% of the patients, genotype 1 was present and genotype 2 or 3 in 33% of the patients. In the 24-week treatment group, 93% of the patients presented with genotype 2 or 3, and in the 48-week treatment group 86% with genotype 1; 8.2% of the patients with histological data at baseline (21/309) were suffering from cirrhosis and details of the Ishak modified histology activity index [HAI; 20] showed a mean score of 5.6; 86% of the patients were treatment-naïve. The baseline characteristics of the two combination therapy groups were comparable (see Table 1).
Table 1.
Baseline characteristics
| PEG-IFN + RBV – 24 weeks (n = 90) | PEG-IFN + RBV – 48 weeks (n = 219) | Total (n = 309) | |
|---|---|---|---|
| Demography | |||
| Sex* | |||
| Male | 59 (65.6) | 119 (54.3) | 178 (57.6) |
| Female | 31 (34.4) | 100 (45.7) | 131 (42.4) |
| Age† (years) | 38.6 ± 10.3 | 42.7 ± 12.5 | 41.5 ± 12.1 |
| Weight† (kg) | 77.2 ± 15.4 | 75.0 ± 13.9 | 75.7 ± 14.4 |
| BMI† (kg/m2) | 25.6 ± 4.6 | 25.2 ± 4.0 | 25.3 ± 4.2 |
| Virological/biochemical parameters | |||
| Genotype* | |||
| 1 | 6 (6.7) | 188 (86.2) | 194 (63.0) |
| 2 | 14 (15.6) | 1 (0.5) | 15 (4.9) |
| 3 | 70 (77.8) | 17 (7.8) | 87 (28.2) |
| Other§ | 0 (0.0) | 12 (5.5) | 13 (3.9) |
| HCV-RNA‡ (103IU/mL) | 1188 (2–12 300) | 1023 (1–33 333) | 1071 (1–33 333) |
| ALT† (U/L) | 73.2 ± 55.6 (n = 89) | 56.3 ± 37.5 (n = 216) | 61.3 ± 44.1 (n = 305) |
| Histology | n = 75 | n = 182 | n = 257 |
| Cirrhosis* | 7 (9.3) | 14 (7.7) | 21 (8.2) |
| Total HAI score† | 5.5 ± 3.3 (n = 31) | 5.6 ± 3.9 (n = 89) | 5.6 ± 3.7 (n = 120) |
| Mode of HCV infection | |||
| Transfusion* | 5 (5.6) | 40 (18.3) | 45 (14.6) |
| Intravenous drug use* | 40 (44.4) | 51 (23.3) | 91 (29.4) |
| Percutan./sexual exp.* | 4 (4.4) | 11 (5.0) | 15 (4.9) |
| Unknown* | 35 (38.9) | 103 (47.0) | 138 (44.7) |
| Other* | 6 (6.7) | 14 (6.4) | 20 (6.5) |
n (%);
mean ± standard deviation;
mean (min–max).
In one further patient in the 48-week group genotype was missing.
Treatment
Duration of treatment
The mean duration of treatment with PEG-IFN alpha-2a was 23.1 weeks in the 24-week group and 41.7 weeks in the 48-week group; 14.4% (13/90) of the patients of the 24-week group and 41.1% (90/219) of the patients of the 48-week group prematurely withdrew from treatment (total: 103/309 patients, 33.3%). As far as the reasons were known, the most frequent were:
non-response (10.7%, 33/309);
adverse event (7.1%, 22/309);
lost to follow-up (5.5%, 17/309);
personal reasons (3.6%, 11/309).
The large difference between the two treatment groups is mainly triggered by the study design, i.e. the longer treatment period and the treatment recommendation to stop treatment in the 48-week arm, when the HCV-RNA test at week 24 was positive. During the follow-up period, 15% of the patients withdrew prematurely (13% in the 48-week group, 19% in the 24-week group).
Dose
The mean dose of PEG-IFN alpha-2a until termination of study medication was 178 μg/week in the 24-week group and 171 μg/week in the 48-week group. The median was 180 μg in both groups. For RBV, the mean dose was 796 mg/day in the 24-week group and 780 mg/day in the 48-week group. Treatment was modified or stopped at least once in 61.2% (134/219) of the patients of the 48-week group and in 34.4% (31/90) of the patients of the 24-week group.
Safety
Adverse events
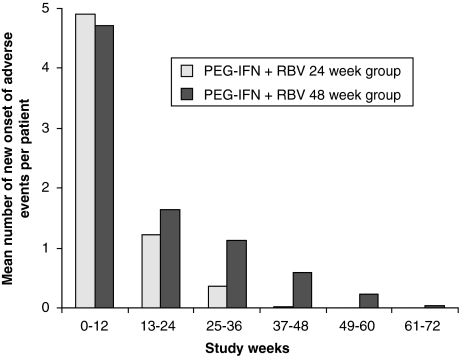
n all, 97.7% of the patients experienced at least one adverse event. A majority of these events were treatment-related (in 95.1% of the patients). The most frequently reported adverse events were those commonly associated with IFN therapy: headache, influenza-like illness, fatigue, leucopenia, alopecia, nausea and depression (see Table 2). Each of these disorders was reported in more than 22% of the patients. The incidence of these events was similar in both treatment groups. There was no specific organ system showing a higher incidence of adverse events in both treatment groups. In the 48-week group, anaemia, cough and bronchitis were more frequent (22.4%, 9.1% and 7.8%, respectively) than in the 24-week group (8.9%, 3.3% and 2.2%, respectively). The incidence rate of adverse events was highest during the first 12 weeks of therapy. Thereafter, the number of new onsets of adverse events decreased rapidly (see Fig. 2). Generally, the prevalence of adverse events declined rapidly after the end of treatment.
Table 2.
Common adverse events (≥10% of patients) during treatment and follow-up period
| Adverse event | PEG-IFN + RBV – 24 weeks (n = 90) | PEG-IFN + RBV – 48 weeks (n = 219) | Total (n = 309) |
|---|---|---|---|
| Headache | 38 (42.2) | 82 (37.4) | 120 (38.8) |
| Influenza like illness | 30 (33.3) | 72 (32.9) | 102 (33.0) |
| Fatigue | 26 (28.9) | 55 (25.1) | 81 (26.2) |
| Leucopenia | 18 (20.0) | 60 (27.4) | 78 (25.2) |
| Alopecia | 14 (15.6) | 57 (26.0) | 71 (23.0) |
| Depression | 19 (21.1) | 52 (23.7) | 71 (23.0) |
| Nausea | 17 (18.9) | 52 (23.7) | 69 (22.3) |
| Anemia | 8 (8.9) | 49 (22.4) | 57 (18.4) |
| Pyrexia | 17 (18.9) | 36 (16.4) | 53 (17.2) |
| Thrombocytopenia | 11 (12.2) | 42 (19.2) | 53 (17.2) |
| Pruritus | 12 (13.3) | 39 (17.8) | 51 (16.5) |
| Weight decreased | 12 (13.3) | 39 (17.8) | 51 (16.5) |
| Diarrhoea | 13 (14.4) | 35 (16.0) | 48 (15.5) |
| Influenza | 13 (14.4) | 30 (13.7) | 43 (13.9) |
| Arthralgia | 8 (8.9) | 33 (15.1) | 41 (13.3) |
| Myalgia | 11 (12.2) | 29 (13.2) | 40 (12.9) |
| Insomnia | 8 (8.9) | 31 (14.2) | 39 (12.6) |
| Asthenia | 10 (11.1) | 22 (10.0) | 32 (10.4) |
Values given in parentheses are percentages.
Fig. 2.
Incidence of adverse events during treatment and follow-up phase
Psychiatric adverse events
In 146 (47.2%) patients of the overall population (total 251), psychiatric adverse events were reported (47.8% and 47.0% in the 24- and 48-week arms, respectively). Most of these were depressive events (71 patients, 23.0%) including depressive disorders and mood alterations with depressive symptoms. As for the other adverse events, the maximal incidence of depressive adverse events was during the first 12 weeks of treatment.
A majority of the depressive events (72/87) resolved during the study; 25 of the 72 resolved events were treated pharmacologically, in most cases with serotonin-reuptake inhibitors or noradrenalin-reuptake inhibitors. Of the remaining 15 cases, eight were treated pharmacologically. Disturbance of sleep was the most frequent psychiatric adverse event next to depression (75 events, of which two were serious adverse events). The incidence of depression did not differ in patients with former intravenous drug use compared with patients who got infected by, e.g. blood transfusion (data not shown).
Laboratory parameters
With the exception of median ALT and aspartate aminotransferase (AST) serum levels, which were elevated as expected in patients suffering from hepatitis C, median serum levels of all other laboratory parameters were within the normal range at baseline. Their course during treatment was comparable in the treatment groups.
Median values of white blood count and platelets as well as median haemoglobin concentration decreased in all groups during treatment and returned to baseline after treatment. Median ALT and AST levels decreased during treatment and were within the normal range or slightly above at the end of treatment. The median decrease was slightly more pronounced in the 24-week group, reflecting the better therapeutic response of patients, with genotype 2 or 3 being the majority in this group.
Serious adverse events
Nine per cent of the patients experienced at least one serious adverse event (SAE). The proportion of patients with at least one SAE was higher in the PEG-IFN plus RBV 48-week group (10.5%) than in the PEG-IFN plus RBV 24-week group (4.4%). The most frequent SAEs were psychiatric disorders (2.3%), infections (1.9%), and cardiac and gastrointestinal disorders (1.6%).
Drug-related SAEs were reported in 5.5% of the patients. Most frequent were psychiatric disorders being reported in more than 2% of the patients in both treatment groups and being the only drug-related serious adverse event in the 24-week group. In the 48-week group, only gastrointestinal, immune system, cardiac, endocrine and hepatobiliary disorders were reported in more than one patient.
Deaths
Two patients died during the follow-up period: one patient of the PEG-IFN plus RBV 24-week group who suffered from psychotic disorder committed suicide 19 months post-treatment. One patient of the PEG-IFN plus RBV 48-week group, who was prematurely withdrawn because of leucopenia, thrombopenia, elevated ALT levels and signs of cholestasis, developed sepsis and died during the follow-up period.
Efficacy
An SVR was achieved in 151 of the 309 patients (48.9%). The SVR rate was 64.4% (58/90 patients) in the PEG-IFN plus RBV 24-week group and 42.5% (93/219 patients) in the PEG-IFN plus RBV 48-week group.
Considering treatment history, there is no difference in response between patients whose HCV infection was never treated before and patients who had been previously treated without sustained success. Response rates were alike in treatment-naïve patients and in relapsers to previous IFN treatment. No significant difference has been observed in non-cirrhotic and cirrhotic patients with regard to the SVR rates. However, only 21 patients did present F4 fibrosis at baseline.
The baseline viral load data show relevant differences only in the 48-week treatment group, which is mainly composed of HCV genotype 1 patients. In these patients, a low viral load appears to correspond to an increased probability of achieve an SVR (see Table 3). There was no marked difference in response rates when stratifying for gender, weight and body mass index.
Table 3.
Sustained virological response by treatment group
| PEG-IFN + RBV – 24 weeks (n = 90), n (%) | PEG-IFN + RBV – 48 weeks (n = 219), n (%) | Total (n = 309), n (%) | |
|---|---|---|---|
| All patients | 58 (64.4) | 93 (42.5) | 151 (48.9) |
| Treatment status at baseline | |||
| Treatment-naïve | 52/80 (65.0) | 78/184 (42.4) | 130/264 (49.3) |
| Relapser | 6/10 (60.0) | 15/33 (45.5) | 21/43 (48.8) |
| Baseline viral load (IU/mL) | |||
| 0–400 000 | 29/38 (76.3) | 49/87 (56.3) | 78/125 (62.4) |
| >400 000 | 29/52 (55.8) | 44/132 (33.3) | 73/184 (39.7) |
| 0–800 000 | 35/55 (63.6) | 71/151 (47.0) | 106/206 (51.5) |
| >800 000 | 23/35 (65.7) | 22/68 (32.4) | 45/103 (43.7) |
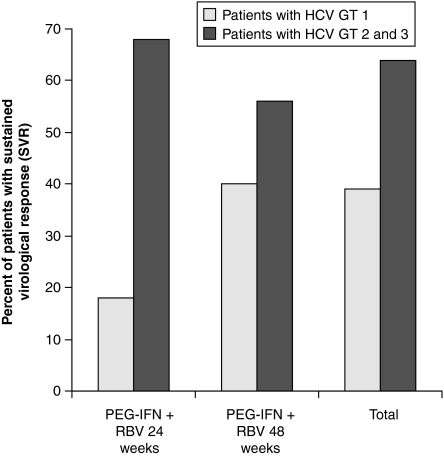
In patients with genotype 2 or 3, a longer duration of treatment (48 vs 24 weeks) did not result in any additional benefit. In patients with genotype 1, the SVR was higher in patients being treated for 48 weeks (40%vs 17%; see Table 4 and Fig. 3).
Table 4.
Sustained virological response by genotype
| Genotype 1, SVR | Genotype 2 or 3, SVR | Other genotypes, SVR | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| PEG-IFN + RBV – 24 weeks | 1/6 | 16.7 | 57/84 | 67.9 | 0 | |
| PEG-IFN + RBV – 48 weeks | 75/188 | 39.9 | 10/18 | 55.6 | 7/12 | 58.3 |
| Total | 76/194 | 39.2 | 67/102 | 65.7 | 7/12 | 58.3 |
Fig. 3.
Sustained virological response (SVR) and HCV genotype
Multiple logistic regression
Three independent factors predictive for increased SVR were identified using multiple logistic regression analysis: genotype non-1 (P = 0.003), a pre-treatment HCV-RNA ≤ 400 000 IU/mL (P = 0.002) and lower age (P = 0,0053). All other baseline factors and the intended treatment duration did not enter the final model.
The odds for SVR was about 2.5 times higher for genotype non-1 compared with those patients with genotype 1 and for patients with HCV-RNA ≤ 400 000 IU/mL compared with those patients with HCV-RNA ≥ 400,000 IU/mL. The odd for SVR was increased by a factor of 1.35 for patients who are 10 years younger than their counterparts.
Discussion
The aim of this study was to evaluate the safety and efficacy of PEG-IFN alpha-2a in a setting as close to everyday clinical practice as possible. Therefore, the design of the study was as realistic as possible: Patients were not randomized but assigned to one of the treatment groups at the investigator's discretion reflecting everyday clinical practice according to current guidelines. Participating centres were hospitals and office-based physicians who were regularly involved in the care of chronic hepatitis C patients, but did not have had any experience with PEG-IFN alpha-2a.
Eventhough the number of recruiting centres seems to be high, this refers to the rising number of physicians taking care of HCV patients after PEG-IFN has been introduced to the German market and became standard of care quite rapidly. Even in large international multi-centre trials (e.g. phase II and III) the numbers of investigational sites are high while the number of recruited patients per site is low. Therefore, our collected data did not account for homogenous distribution, as in well-balanced randomized studies, either with regard to patients or to physicians or to treatment modalities.
The treatment groups included PEG-IFN alpha-2a (Pegasys®) in combination with RBV for 24 or 48 weeks. Most of the patients (70.9%, 219/309) were assigned to the 48- week combination group. According to recommentations, a reduction of treatment duration was intended for patients with genotype non-1. This was true for 29.1% (90/309) of the patients being assigned to the 24-week combination group and as expected, 93% of these patients presented with genotype 2 or 3, whereas patients with genotype 1 were mostly in the 48-week treatment group (86%).
The clinical practice data of the current study revealed no surprises in safety results. No unexpected safety concerns were observed. The safety profile of the treatment with PEG-IFN alpha-2a (Pegasys®) plus RBV was in accordance with the safety profile known from previous studies [10,13,14]. The incidence of depression was reported to be highest during the first 12 weeks of treatment. This might be due to an increased awareness of the incidence of depressive symptoms being known from previous studies. As expected, safety concerns present as a problem for almost all patients with no organ class, demonstrating a clearly increased incidence in one of the groups. However, most of the problems appear in the initial weeks of treatment. They appear to be manageable because the rate of therapy withdrawals due to adverse events of 7.1% of all patients is relatively low. This rate corresponds to the rate found by Fried et al. [14] – 3% for laboratory abnormalities and 7% for other adverse events. However, a successful treatment needs an active handling of tolerability problems. This means a close contact with the patients and development of individual strategies to overcome possible adverse events particularly in the initials weeks of treatment.
As expected, treatment with PEG-IFN alpha-2a (Pegasys®) plus RBV for 24 weeks was better tolerated than for 48 weeks. The incidence of serious adverse events was distinctly lower in the 24-week group (4.4%vs 10.5%) as well as the incidence of dose reductions and withdrawals. These results are in accordance with studies comparing 24 and 48 weeks of treatment. So, Hadziyannis et al. [19] reported incidence rates of serious adverse events of 3–7% for the 24-week groups and 9–10% for the 48-week groups. Individual adverse events were equally frequent in both the 24- and 48-week group, with the exception of anaemia, cough and bronchitis being more frequent in the 48 week group.
An SVR was achieved in 48.9% of the patients. General SVR rates depend on the composition of population. Thus, it makes more sense to stratify the SVR rates for HCV genotype: for patients with genotype 2 or 3 an SVR rate of 65.7% was documented. For genotype 2 or 3 patients, being treated according to recommendations for 24 weeks, the SVR was 67.9%. Patients with genotype 1 achieved an SVR of 39.1%, whereas genotype 1 patients with a 48-week combination treatment achieved an SVR of 39.9%. Compared with previous study results, the current SVR rates are slightly lower: Bacon [21] reports SVR rates between 54% and 61% for all patients and SVR rates between 42% and 48% for genotype 1 patients. The reason for the slightly reduced SVR rates in this study may be associated with the open practice character of the study. As per definition, the calculation of SVR rates includes patients with missing HCV-RNA post-treatment values as non-responders. As this study includes a rate of 26% of these patients, looking at the SVR rates might possibly give the wrong picture of the real effect of the study medication more than in pivotal trials, in which patients may be easier available for follow-up visits.
Of great interest for the prognosis of treatment success are baseline characteristics of the patients which may correspond to the efficacy of treatment. As explained, one of these criteria is the HCV genotype of the patients. On top of this, the viral load at baseline is discussed as an important parameter [22]. Current results confirm this fact for the 48-week treatment group being dominated by genotype 1 patients. In this group, a low viral load (up to 400 000 IU/mL) is associated with a higher SVR rate (56.3%), whereas the SVR for patients with a high viral load (>400 000 IU/mL) was 33.3%. Data presented at the last AASLD in Boston by Berg et al. [23] and Zehnter et al. [24] confirmed our findings that, choosing a lower viral cut-off of 400 000 IU/mL prior to treatment is a significant predictive marker for SVR in patients with genotype 1. For the 24-week treatment group being dominated by genotype 2 and 3 patients, there was no relevant difference between patients with low and high viral loads. This result is in line with data shown by Hadziyannis et al. [19] and Fried et al. [25].
The well-known insufficient response rates of interferon monotherapy or combination therapy lead to the important question if patients without sustained response after IFN pretreatment will also show a higher rate of non-response to following PEG-IFN treatment. According to the results presented in Refs [18,26], reduced response rates for patients who failed to respond to a previous antiviral treatment are expected. However, the results of the current data set – including 43 IFN pretreatments out of 309 patients show the contrary results: 21 of the 43 pretreated patients (48.8%) achieved an SVR compared with 48.9% in the total population. Thus, for patients without sustained response after IFN standard therapy, treatment with PEG-IFN may be a promising treatment option. As far as our results have shown, their chance to respond to treatment is similar to treatment-naïve patients.
In summary, the results of the current study have shown that the well-known risk–benefit ratio of the treatment of chronic hepatitis C with PEG-IFN alpha-2a (Pegasys®) plus RBV found in clinical trial settings could also be confirmed in a clinical practice setting. The safety profile is similar and shows the highest incidence of adverse events in the first 12 weeks of treatment. Although almost all patients (95%) were affected by drug-related adverse events, these were manageable in most cases: Only in 7.1% of the patients was treatment terminated prematurely because of adverse events. As recommended, the duration of treatment was adapted to HCV genotype in most cases: patients with genotype 2 or 3 were treated for 24 weeks and patients with genotype 1 for 48 weeks. As expected, patients with genotype 2 or 3 being treated for 48 weeks did not benefit from the prolonged treatment. Replicating the results of clinical trials in a clinical practice setting, the recommended therapy regimens for these two populations were highly efficacious in daily routine practice and confirm the reasonable risk–benefit ratio for the treatment with PEG-IFN alpha-2a plus RBV.
References
- 1.McHutchinson JG. Understanding hepatitis C. Am J Manag Care. 2004;10:S21–S29. [PubMed] [Google Scholar]
- 2.Choo QL, et al. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 3.Kuo G, et al. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989;244:362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47:1–39. [PubMed] [Google Scholar]
- 5.Alter MJ, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–562. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 6.Fried M. Therapy of hepatitis C. Semin Liver Dis. 1995;15(1):82–91. doi: 10.1055/s-2007-1007265. [DOI] [PubMed] [Google Scholar]
- 7.Poynard T, et al. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT) Lancet. 1998;352(9138):1426–1432. doi: 10.1016/s0140-6736(98)07124-4. [DOI] [PubMed] [Google Scholar]
- 8.McHutchinson JG, et al. and the Hepatitis Interventional Therapy Group Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N Engl J Med. 1998;339:1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 9.Lam NP. Dose-dependent acute clearance of hepatitis C genotype 1 virus with interferon alfa. Hepatology. 1997;26:226–231. doi: 10.1002/hep.510260130. [DOI] [PubMed] [Google Scholar]
- 10.Zeuzem S, et al. Peginterferon alfa-2a in patients with chronic hepatitis C. N Engl J Med. 2000;343(23):1666–1672. doi: 10.1056/NEJM200012073432301. [DOI] [PubMed] [Google Scholar]
- 11.Nieforth KA. Use of an indirect pharmacodynamic stimulation model of MX protein induction to compare in vivo activity of interferon alfa-2a and a polyethylene glycol-modified derivative in healthy subjects. Clin Pharmacol Ther. 1996;59:636–646. doi: 10.1016/S0009-9236(96)90003-X. [DOI] [PubMed] [Google Scholar]
- 12.Reddy KR, et al. Efficacy and safety of pegylated (40-kd) interferon alpha-2a compared with interferon alpha-2a in noncirrhotic patients with chronic hepatitis C. Hepatology. 2001;33(2):433–438. doi: 10.1053/jhep.2001.21747. [DOI] [PubMed] [Google Scholar]
- 13.Heathcote EJ, et al. De Pamphilis J. Peginterferon alfa-2a in patients with chronic hepatitis C and cirrhosis. N Engl J Med. 2000;343(23):1673–1680. doi: 10.1056/NEJM200012073432302. [DOI] [PubMed] [Google Scholar]
- 14.Fried MW, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 15.National Institutes of Diabetes and Digestive and Kidney Disorders, National Institutes of health. Chronic Hepatitis C: Current Disease Management. [14 November 2003]. NIH Publication No. 02-4230, February 2003. http://digestive.niddk.nih.gov/ddiseases/pubs/chronichepc/chronichepc.pdf. [Google Scholar]
- 16.National Institutes of Health Consensus Development Conference Statement: Management of hepatitis C: 2003–June 10–12, 2002. Hepatology. 2002;36:S1–S20. [Google Scholar]
- 17.Strader DB. American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39(4):1147–1171. doi: 10.1002/hep.20119. [DOI] [PubMed] [Google Scholar]
- 18.Ferenci P. Current treatment for chronic hepatitis C. Curr Treat Options Gastroenterol. 2004;7(6):491–499. doi: 10.1007/s11938-004-0008-2. [DOI] [PubMed] [Google Scholar]
- 19.Hadziyannis SJ, et al. PEGASYS International Study Group. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140(5):346–355. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]
- 20.Ishak K, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22(6):696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 21.Bacon BR. Managing hepatitis C. Am J Manag Care. 2004;10(2 Suppl):S30–S40. [PubMed] [Google Scholar]
- 22.Lee SS, et al. Prognostic factors and early predictability of sustained viral response with peginterferon alfa-2a (40 KD) J Hepatol. 2002;37(4):500–506. doi: 10.1016/s0168-8278(02)00211-8. [DOI] [PubMed] [Google Scholar]
- 23.Berg T, et al. Definition of a pre-treatment viral load cut-off for an optimized prediction of treatment outcome in patients with genotype 1 infection receiving either 48 or 72 weeks of peginterferon alfa-2a plus ribavirin. Hepatology. 2006;44(4, Suppl. 1):A350. [Google Scholar]
- 24.Zehnter E, et al. Better prediction of SVR in patients with HCV genotype 1 (GT1) with Peginterferon alfa-2a (Pegasys) plus ribavirin: improving differentiation between low (LVL) and high baseline viral load (HVL) Hepatology. 2006;44(4, Suppl. 1):0A368. [Google Scholar]
- 25.Fried M. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36:S237–S244. doi: 10.1053/jhep.2002.36810. [DOI] [PubMed] [Google Scholar]
- 26.Shiffman ML, et al. Hepatitis C antiviral long-term treatment against cirrhosis trial group. Peginterferon alfa-2a and ribavirin in patients with chronic hepatitis C who have failed prior treatment. Gastroenterology. 2004;126(4):1015–1023. doi: 10.1053/j.gastro.2004.01.014. [DOI] [PubMed] [Google Scholar]