It's not easy being a nuclear transport factor. During the G1, S, and G2 phases of the cell cycle, the nucleocytoplasmic transport machinery must tirelessly zigzag between the nucleus and cytoplasm while directing other proteins to the correct subcellular compartment. When M phase finally arrives, and the nucleus breaks down, do these proteins finally get to take a break? Apparently not. In the case of the Ran-GTPase and its associated nuclear transport factors, there is no rest for the weary. Instead, Ran and its associated proteins shift their focus from nuclear transport to the regulation of microtubule dynamics. Recent findings by several groups have shown that the GTP-bound form of Ran is necessary for the nucleation and organization of microtubule structures during M phase. Moreover, the implication that the active form of Ran is concentrated in the vicinity of chromosomes suggests that these findings may resolve the long-standing question of how chromosomes can influence spindle assembly in the absence of microtubule-nucleating organelles (i.e., as in plant mitosis or mammalian meiosis).
Ran and Nuclear Transport
Ran is an abundant GTP-binding protein that is required for the trafficking of proteins and RNA in and out of the nucleus (Moore and Blobel 1993). In general, it forms a complex with nuclear transport signal (NLS) receptors and their cargoes and directs their movement through nuclear pores. Like other small GTP-binding proteins (e.g., Ras), Ran's GTP hydrolysis activity and guanine nucleotide affinity are modulated by accessory factors. GTP hydrolysis is stimulated by a GTPase-activating protein known as Ran-GAP1 (Bischoff et al. 1994) and its accessory factor RanBP1 (Bischoff et al. 1995), while replacement of GDP with GTP is accomplished by the guanine nucleotide exchange factor (GEF) RCC1 (Bischoff and Ponstingl 1995; Ohtsubo et al. 1989). Together, these proteins comprise an enzymatic cycle by which Ran binds GTP, hydrolyzes it to GDP (due to the activity of Ran-GAP1), releases the GDP (due to RCC1 activity), and rebinds GTP (due to the presence of a relatively high GTP concentration in the cell).
Ran's function as a nuclear transport factor depends upon the nature of its bound guanine nucleotide. Specificity of binding (i.e., to import or export receptors) and direction of movement is determined by whether Ran is bound to GTP or GDP. Ran-GDP typically associates with nuclear import receptors (e.g., importin α/β) and directs their movement from the cytoplasm into the nucleus. Conversely, Ran-GTP generally associates with nuclear export receptors (e.g., CRM1) and directs movement of their cargoes from the cytoplasm into the nucleus (see Koepp and Silver 1996; Mattaj and Englmeier 1998).
According to this scheme, the Ran protein should be predominantly bound to GTP while in the nucleus and to GDP while in the cytoplasm. This is achieved by compartmentalization of Ran's GAP and GEF. Whereas Ran is localized throughout the cell, RCC1 is bound to chromatin in the nucleus (Ohtsubo et al. 1989), while Ran-GAP1 and RanBP1 are found exclusively in the cytoplasm (Matunis et al. 1996; Richards et al. 1996). Consequently, Ran-GDP is prevalent in the cytoplasm due to stimulation of GTPase activity by Ran-GAP1 and RanBP1, whereas Ran-GTP is prevalent in the nucleus due to the nucleotide exchange activity of RCC1 (Fig. 1 A) (Koepp and Silver 1996; Mattaj and Englmeier 1998). Thus, the localization of the GAP and GEF is paramount in regulating the proper function of Ran in nuclear transport.
Figure 1.
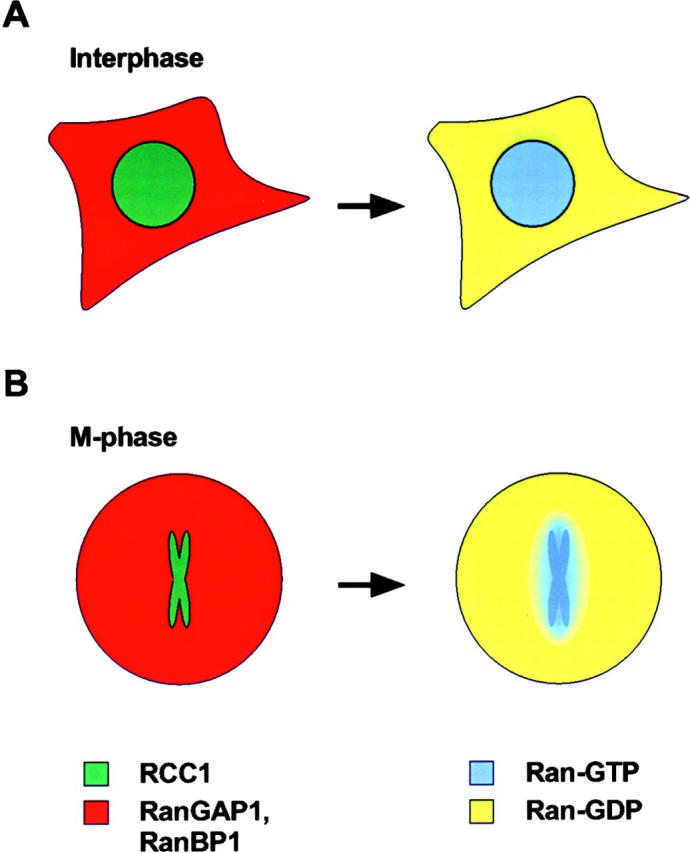
Localization of the Ran GAP and GEF leads to compartmentalization of Ran-GTP vs. Ran-GDP. (A) During interphase, nuclear localization of RCC1 (green) and cytoplasmic localization of RanGAP1 and RanBP1 (red) yield a distribution of Ran-GDP (yellow) in the cytoplasm and Ran-GTP (blue) in the nucleus. (B) During mitosis or meiosis, chromatin-associated RCC1 establishes a gradient of Ran-GTP that is concentrated at the surface of the chromosomes.
Microtubule Nucleation by Ran and Its Associated Factors
Whereas the nuclear transport activity of Ran has been extensively characterized, evidence for its role in microtubule regulation has, until recently, been largely circumstantial. An initial hint for Ran involvement in microtubule regulation was derived from the observation that, in the budding yeast Saccharomyces cerevisiae, overexpression of the RCC1 homologue Prp20 can suppress the toxic effects of certain hyperstable α-tubulin mutants (Kirkpatrick and Solomon 1994). Moreover, yeast strains harboring temperature-sensitive mutant alleles of the yeast Ran-binding protein Yrb1 exhibit spindle misorientation defects due to a lack of astral microtubules (Ouspenski 1998). Thus, at a phenotypic level, some mutant forms of Ran-associated proteins can have an effect on microtubule structure in vivo.
Well beyond this initial suggestion, a series of recent papers have converged upon the discovery that Ran and its cohorts are key components of aster formation and spindle assembly, especially for spindles assembled in the absence of centrosomes. An initial insight into this process came from the identification (Nakamura et al. 1998) of a novel mammalian Ran-binding protein, RanBPM, that could elicit microtubule polymerization. Isolated on the basis of its interaction with Ran-GTP in a two-hybrid assay, RanBPM has been shown to associate with centrosomes, the microtubule-nucleating centers of mammalian cells. Interestingly, it has been observed that overexpression of RanBPM can induce ectopic aster formation in transfected cells. Such asters are structurally similar to normal centrosomal asters in that they contain centrosomal proteins such as γ-tubulin at their foci. Moreover, inhibition of RanBPM or Ran activity can prevent in vitro aster formation from a mixture of purified centrosomes and tubulin. Taken together, these results suggest that Ran and RanBPM somehow act together to effect microtubule nucleation.
Manipulation of Ran-GTP Levels Leads to Alterations in Aster Formation in Xenopus Extracts
It has long been known that asters will readily grow from sperm centrioles in Xenopus egg extracts arrested in meiotic metaphase II. Surprisingly, this centriole-mediated process has now been found to be Ran-GTP–dependent. Reduction in the relative levels of Ran-GTP by immunodepletion of RCC1 (Ohba et al. 1999) or by addition of a mutant form of Ran that favors GDP binding (T24N; see Table ; Carazo-Salas et al. 1999; Kalab et al. 1999; Ohba et al. 1999; Wilde and Zheng 1999), severely inhibits aster formation from centrioles. Therefore, the GTP-bound form of Ran is necessary for centriole-dependent aster formation in these extracts.
Table 1.
Proteins Involved in Aster Formation and Nuclear Transport
| Protein | Functional property | Reference |
|---|---|---|
| Ran | Small GTPase required for nuclear transport. Localizes throughout the cell. | Moore and Blobel 1993 |
| RanGAP1 | GTPase activating protein for Ran. Localizes to the cytoplasm and nuclear pores during interphase as well as with mitotic spindle during mitosis. | Bischoff et al. 1994; Matunis et al. 1996 |
| RCC1 | Guanine nucleotide exchange factor for Ran. Associates with chromatin throughout the cell cycle. | Bischoff and Ponstingl 1991; Ohtsubo et al. 1987; Ohtsubo et al. 1989 |
| RanBP1 | Ran-GTP binding protein that increases the rate of GTP hydrolysis by Ran and RanGAP1. Localizes to the cytoplasm of nondividing cells. | Bischoff et al. 1995 |
| RanBPM | Centrosomal protein that interacts with the GTP-bound form of Ran. Overexpression elicits ectopic aster formation in transfected COS cells. | Nakamura et al. 1998 |
| Ran G19V, Ran Q69L | Mutant forms of Ran that are locked in the GTP-bound form due to lack of GTPase activity. | Bischoff et al. 1994; Lounsbury et al. 1996 |
| Ran L45E | Mutant form of Ran that is locked in GTP-bound form due to lack of GTPase activity. Mutation is in effector domain, so this mutant may interact differently with Ran-binding proteins. | Lounsbury et al. 1996 |
| Ran T24N | Mutant form of Ran with decreased affinity for guanine nucleotides. Interacts with RCC1 and inhibits its nucleotide exchange activity. | Klebe et al. 1995 |
Conversely, addition of high levels of purified Ran-GTP, GTP-locked forms of Ran (e.g. Ran-GTPγS; Ran G19V, Ran Q69L; RanL45E; see Table ), or high levels of RCC1 strongly stimulates centriole-associated aster growth in sperm-treated extracts as well as de novo aster formation in extracts lacking added chromatin or centrioles (Carazo-Salas et al. 1999; Kalab et al. 1999; Ohba et al. 1999; Wilde and Zheng 1999; Zhang et al. 1999). Like the asters formed by overexpression of RanBPM (Nakamura et al. 1998), the asters formed by artificially raising the levels of Ran-GTP include typical centrosome-associated proteins (e.g., γ-tubulin, NuMA, XGRIP109, and XMAP215) at their foci (Ohba et al. 1999; Wilde and Zheng 1999). Strikingly, centrosome-free asters formed by high levels of Ran L45E are capable of forming into bipolar spindle-like structures (Wilde and Zheng 1999). In contrast, asters formed without Ran (i.e., by chemically stimulating tubulin polymerization with agents such as dimethyl sulfoxide) do not require γ-tubulin or XMAP215 and do not assemble into higher-order structures (Wilde and Zheng 1999). In sum, the findings that loss of Ran function blocks aster formation while overexpression of Ran-GTP induces ectopic formation of microtubule structures clearly implicate the Ran-GTPase cycle in microtubule assembly in M phase.
How Does Ran-GTP Function in Microtubule Assembly?
Although it can induce aster assembly in Xenopus extracts, Ran-GTP does not stimulate microtubule polymerization from purified tubulin subunits (Wilde and Zheng 1999). Thus, there must exist additional factors that more directly affect polymerization properties. The most plausible candidate now known for such an effector is RanBPM, which both associates with centrosomes and Ran-GTP in mammalian cells. In the absence of centrosomes, the simplest view is that a soluble (i.e., non-centrosomal) portion of RanBPM (or an as yet undiscovered relative) may associate with Ran-GTP generated by chromosomal RCC1, thereby promoting microtubule polymerization adjacent to chromosomes.
Is there a role for GTP hydrolysis by Ran in microtubule assembly? It has been suggested that during nuclear transport, the nucleotide-bound state of Ran acts simply as a switch to delineate the direction of movement and that the energy of GTP hydrolysis is not strictly required (Richards et al. 1997; Schwoebel et al. 1998). The observation that forms of Ran that do not hydrolyze GTP (e.g. Ran L45E, Ran-GTPγS) can induce aster formation in Xenopus extracts (Carazo-Salas et al. 1999; Kalab et al. 1999; Ohba et al. 1999; Wilde and Zheng 1999), suggests that a similar situation exists for microtubule assembly. However, while GTPγS-bound Ran can induce aster formation, such asters are considerably smaller than those formed by Ran-GTP (Ohba et al. 1999). Thus, GTP hydrolysis by Ran may have some secondary role in the elongation of previously nucleated microtubules.
A Model for Ran-GTP–driven Spindle Formation
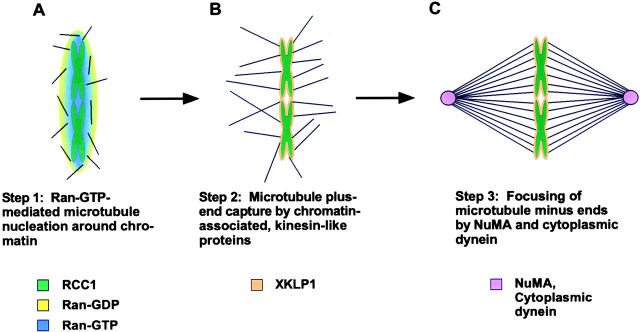
The recognition that Ran-GTP may be a key component of microtubule nucleation allows resolution of an old cell biological problem: how chromatin can drive spindle assembly, especially in the absence of centrosomes (Karsenti et al. 1984). What now seems likely is that in the absence of a nucleus, chromatin-bound RCC1 and cytoplasmic RanGAP1 produce a natural gradient of Ran-GTP that is most concentrated at the chromosome surface (Fig. 2 A). Consequently, a Ran-GTP–dependent factor such as RanBPM, would stimulate microtubule assembly adjacent to the chromosomes. Then, other cellular factors could act to organize these microtubules into spindles. For instance, it has been shown that the chromosome-associated, kinesin-like protein XKLP1 is required for the stable attachment of microtubules to chromatin (Vernos et al. 1995; Walczak et al. 1998). Such chromatin-bound, plus end–directed kinesins could, thus, attach to the newly formed microtubules and draw the rapidly growing plus ends to the surface of the chromosomes (Fig. 2 B). Finally, a complex of cytoplasmic dynein, dynactin and NuMA, previously shown to be required for maintenance of focused microtubule arrays both in the presence or absence of centrosomes (Yang and Snyder 1992; Merdes et al. 1996), could organize the chromosome-bound microtubules into spindles (Fig. 2 C). This would arise through the microtubule cross-linking activity of NuMA and the retrograde motility of dynein acting together to draw the microtubules into poles at their minus ends. Such a model could explain the inside-out assembly of spindles during vertebrate oogenesis and plant mitosis, both of which are accomplished without centrosomes.
Figure 2.
Three-step mechanism by which Ran-GTP initiates centrosome-free spindle assembly. (A) The high concentration of Ran-GTP produced by chromatin-bound RCC1 in the vicinity of chromatin stimulates microtubule assembly around chromosomes (see Carazo-Salas et al. 1999). (B) These randomly-oriented microtubules are then captured at their plus ends by chromosome-bound, microtubule-binding proteins (e.g., XKLP1). (C) Finally, the minus ends of microtubules are focused into poles by cytoplasmic dynein and its microtubule-cross-linking cargo NuMA.
Indeed, the works described herein offer some evidence in support of such a model. First there is evidence for the influence of chromatin-dependent components. While it had previously been shown that random segments of DNA attached to a solid-phase support (e.g., polystyrene beads) could initiate centrosome-free spindle assembly in CSF-arrested Xenopus extracts (Heald et al. 1996), Carazo-Salas et al. 1999 have now shown that this process requires generation of Ran-GTP by chromatin-associated RCC1. Second, concerning a role in centrosome-free pole formation, it has been demonstrated that microtubules formed by addition of purified Ran-GTP require the activity of cytoplasmic dynein for organization into aster-like structures (Ohba et al. 1999; Wilde and Zheng 1999).
Partitioning of Ran-GTP during Interphase and M Phase
The linkage of Ran-GTP to M phase microtubule nucleation reinforces a principle long understood from nuclear transport. Compartmentalization of the Ran GAP and GEF modulate the function of Ran itself. Because of its association with chromatin, RCC1 can establish a high concentration of Ran-GTP exclusively in the vicinity of chromosomes during M phase. This, in turn, can serve as the initial positional cue for spindle formation in the absence of centrosomes. Thus, the observation that the GTP-bound form of Ran can stimulate microtubule polymerization offers a significant insight into the process by which chromosomes drive spindle assembly, especially in the absence of microtubule-organizing centers.
References
- Bischoff F.R., Ponstingl H. Catalysis of guanine nucleotide exchange of Ran by RCC1 and stimulation of hydrolysis of Ran-bound GTP by Ran-GAP1. Methods Enzymol. 1995;257:135–144. doi: 10.1016/s0076-6879(95)57019-5. [DOI] [PubMed] [Google Scholar]
- Bischoff F.R., Klebe C., Kretschmer J., Wittinghofer A., Ponstingl H. RanGAP1 induces GTPase activity of nuclear Ras-related Ran. Proc. Natl. Acad. Sci. USA. 1994;91:2587–2591. doi: 10.1073/pnas.91.7.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff F.R., Krebber H., Smirnova E., Dong W., Ponstingl H. Co-activation of RanGTPase and inhibition of GTP dissociation by Ran-GTP binding protein RanBP1. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:705–715. doi: 10.1002/j.1460-2075.1995.tb07049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff F.R., Ponstingl H. Mitotic regulator protein RCC1 is complexed with a nuclear ras-related polypeptide. Proc. Natl. Acad. Sci. USA. 1991;88:10830–10834. doi: 10.1073/pnas.88.23.10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carazo-Salas R.E., Guarguaglini G., Gruss O.J., Segref A., Karsenti E., Mattaj I.W. Generation of GTP-bound Ran by RCC1 is required for chromatin-induced mitotic spindle formation. Nature. 1999;400:178–181. doi: 10.1038/22133. [DOI] [PubMed] [Google Scholar]
- Heald R., Tournebize R., Blank T., Sandaltzopoulos R., Becker P., Hyman A., Karsenti E. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature. 1996;382:420–425. doi: 10.1038/382420a0. [DOI] [PubMed] [Google Scholar]
- Kalab P., Pu R.T., Dasso M. The ran GTPase regulates mitotic spindle assembly. Curr. Biol. 1999;9:481–484. doi: 10.1016/s0960-9822(99)80213-9. [DOI] [PubMed] [Google Scholar]
- Karsenti E., Newport J., Kirschner M. Respective roles of centrosomes and chromatin in the conversion of microtubule arrays from interphase to metaphase. J. Cell Biol. 1984;99:47s–54s. doi: 10.1083/jcb.99.1.47s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick D., Solomon F. Overexpression of yeast homologs of the mammalian checkpoint gene RCC1 suppresses the class of alpha-tubulin mutations that arrest with excess microtubules. Genetics. 1994;137:381–392. doi: 10.1093/genetics/137.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebe C., Bischoff F.R., Ponstingl H., Wittinghofer A. Interaction of the nuclear GTP-binding protein Ran with its regulatory proteins RCC1 and RanGAP1. Biochemistry. 1995;34:639–647. doi: 10.1021/bi00002a031. [DOI] [PubMed] [Google Scholar]
- Koepp D.M., Silver P.A. A GTPase controlling nuclear traffickingrunning the right way or walking RANdomly? Cell. 1996;87:1–4. doi: 10.1016/s0092-8674(00)81315-x. [DOI] [PubMed] [Google Scholar]
- Lounsbury K.M., Richards S.A., Carey K.L., Macara I.G. Mutations within the Ran/TC4 GTPase. Effects on regulatory factor interactions and subcellular localization. J. Biol. Chem. 1996;271:32834–32841. doi: 10.1074/jbc.271.51.32834. [DOI] [PubMed] [Google Scholar]
- Mattaj I.W., Englmeier L. Nucleocytoplasmic transportthe soluble phase. Annu. Rev. Biochem. 1998;67:265–306. doi: 10.1146/annurev.biochem.67.1.265. [DOI] [PubMed] [Google Scholar]
- Matunis M.J., Coutavas E., Blobel G. A novel ubiquitin-like modification modulates the partitioning of the Ran-GTPase-activating protein RanGAP1 between the cytosol and the nuclear pore complex. J. Cell Biol. 1996;135:1457–1470. doi: 10.1083/jcb.135.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdes A., Ramyar K., Vechio J.D., Cleveland D.W. A complex of NuMA and cytoplasmic dynein is essential for mitotic spindle assembly. Cell. 1996;87:447–458. doi: 10.1016/s0092-8674(00)81365-3. [DOI] [PubMed] [Google Scholar]
- Moore M.S., Blobel G. The GTP-binding protein Ran/TC4 is required for protein import into the nucleus. Nature. 1993;365:661–663. doi: 10.1038/365661a0. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Masuda H., Horii J., Kuma K., Yokoyama N., Ohba T., Nishitani H., Miyata T., Tanaka M., Nishimoto T. When overexpressed, a novel centrosomal protein, RanBPM, causes ectopic microtubule nucleation similar to gamma-tubulin. J. Cell Biol. 1998;143:1041–1052. doi: 10.1083/jcb.143.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba T., Nakamura M., Nishitani H., Nishimoto T. Self-organization of microtubule asters induced in Xenopus egg extracts by GTP-bound Ran. Science. 1999;284:1356–1358. doi: 10.1126/science.284.5418.1356. [DOI] [PubMed] [Google Scholar]
- Ohtsubo M., Kai R., Furuno N., Sekiguchi T., Sekiguchi M., Hayashida H., Kuma K., Miyata T., Fukushige S., Murotsu T. Isolation and characterization of the active cDNA of the human cell cycle gene (RCC1) involved in the regulation of onset of chromosome condensation. Genes Dev. 1987;1:585–593. doi: 10.1101/gad.1.6.585. [DOI] [PubMed] [Google Scholar]
- Ohtsubo M., Okazaki H., Nishimoto T. The RCC1 protein, a regulator for the onset of chromosome condensation locates in the nucleus and binds to DNA. J. Cell Biol. 1989;109:1389–1397. doi: 10.1083/jcb.109.4.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouspenski I.I. A RanBP1 mutation which does not visibly affect nuclear import may reveal additional functions of the ran GTPase system. Exp. Cell Res. 1998;244:171–183. doi: 10.1006/excr.1998.4174. [DOI] [PubMed] [Google Scholar]
- Richards S.A., Lounsbury K.M., Carey K.L., Macara I.G. A nuclear export signal is essential for the cytosolic localization of the Ran binding protein, RanBP1. J. Cell Biol. 1996;134:1157–1168. doi: 10.1083/jcb.134.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards S.A., Carey K.L., Macara I.G. Requirement of guanosine triphosphate-bound ran for signal-mediated nuclear protein export. Science. 1997;276:1842–1844. doi: 10.1126/science.276.5320.1842. [DOI] [PubMed] [Google Scholar]
- Schwoebel E.D., Talcott B., Cushman I., Moore M.S. Ran-dependent signal-mediated nuclear import does not require GTP hydrolysis by Ran. J. Biol. Chem. 1998;273:35170–35175. doi: 10.1074/jbc.273.52.35170. [DOI] [PubMed] [Google Scholar]
- Vernos I., Raats J., Hirano T., Heasman J., Karsenti E., Wylie C. Xklp1, a chromosomal Xenopus kinesin-like protein essential for spindle organization and chromosome positioning. Cell. 1995;81:117–127. doi: 10.1016/0092-8674(95)90376-3. [DOI] [PubMed] [Google Scholar]
- Walczak C.E., Vernos I., Mitchison T.J., Karsenti E., Heald R. A model for the proposed roles of different microtubule-based motor proteins in establishing spindle bipolarity. Curr. Biol. 1998;8:903–913. doi: 10.1016/s0960-9822(07)00370-3. [DOI] [PubMed] [Google Scholar]
- Wilde A., Zheng Y. Stimulation of microtubule aster formation and spindle assembly by the small GTPase Ran. Science. 1999;284:1359–1362. doi: 10.1126/science.284.5418.1359. [DOI] [PubMed] [Google Scholar]
- Yang C.H., Snyder M. The nuclear-mitotic apparatus protein is important in the establishment and maintenance of the bipolar mitotic spindle apparatus. Mol. Biol. Cell. 1992;3:1259–1267. doi: 10.1091/mbc.3.11.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Hughes M., Clarke P.R. Ran-GTP stabilises microtubule asters and inhibits nuclear assembly in Xenopus egg extracts. J. Cell Sci. 1999;112:2453–2461. doi: 10.1242/jcs.112.14.2453. [DOI] [PubMed] [Google Scholar]