Abstract
A more complete picture of the molecules that are critical for the organization of membrane compartments is beginning to emerge through the characterization of proteins in the vesicle-associated membrane protein (also called synaptobrevin) family of membrane trafficking proteins. To better understand the mechanisms of membrane trafficking within the endocytic pathway, we generated a series of monoclonal and polyclonal antibodies against the cytoplasmic domain of vesicle-associated membrane protein 7 (VAMP-7). The antibodies recognize a 25-kD membrane-associated protein in multiple tissues and cell lines. Immunohistochemical analysis reveals colocalization with a marker of late endosomes and lysosomes, lysosome-associated membrane protein 1 (LAMP-1), but not with other membrane markers, including p115 and transferrin receptor. Treatment with nocodozole or brefeldin A does not disrupt the colocalization of VAMP-7 and LAMP-1. Immunoelectron microscopy analysis shows that VAMP-7 is most concentrated in the trans-Golgi network region of the cell as well as late endosomes and transport vesicles that do not contain the mannose-6 phosphate receptor. In streptolysin- O–permeabilized cells, antibodies against VAMP-7 inhibit the breakdown of epidermal growth factor but not the recycling of transferrin. These data are consistent with a role for VAMP-7 in the vesicular transport of proteins from the early endosome to the lysosome.
Keywords: VAMP-7, SNARE, endosome, lysosome, membrane trafficking
Eucaryotic cells compartmentalize biological functions in a series of membrane-bound organelles. One critical and highly regulated trafficking decision made by all cells is whether to degrade a protein or transport it to a site of storage or function. The major degradative compartment in cells is the lysosome, an organelle filled with an array of enzymes that hydrolyze biological molecules to component metabolites (Kornfeld and Mellman 1989). Most trafficking into the lysosome is thought to originate from the highly dynamic early endosome (EE),1 or sorting endosome (Mellman 1996). The EE contains morphologically distinct domains, including a vacuolar domain that is continuous with a network of tubular extensions. In addition, an extended network of endosomal tubules and vesicles is often found dispersed throughout the cytoplasm (Mellman 1996).
Membrane proteins such as the transferrin receptor (TfR) are internalized from the cell surface through the formation of clathrin-coated vesicles (Pearse 1987; Pearse and Robinson 1990). Following endocytosis, proteins are transferred to the EE, where molecules that will recycle back to the plasma membrane accumulate within the tubular domain of the compartment (Mellman 1996; Robinson et al. 1996). From these tubular extensions of the EE, molecules can be rapidly trafficked back to the cell surface or to a centrally localized compartment known as the recycling endosome (RE) (Gruenberg and Maxfield 1995). These REs, also known as the peri-Golgi recycling compartment, have the ability to further sort proteins for various destinations within the cell (Hopkins 1983; Hopkins and Trowbridge 1983). Although some molecular markers appear to differentiate between EEs and REs, a distinct boundary between them is obscured due to the intricate network of tubules and vesicles that comprise the organelles (Hopkins 1983; Hopkins and Trowbridge 1983; Yamashiro et al. 1984; Gosh et al. 1994). Following endocytosis and transfer to the EE, proteins that are destined for degradation, such as EGF, remain largely within the vacuolar domain of the endosome (Hopkins et al. 1990). The vacuolar domain then either matures directly or shuttles proteins via vesicular trafficking to late endosomes (LEs), which further mature into lysosomes (Futter et al. 1996). Although the mechanisms of vesicle formation, sorting, and membrane fusion within the endosomal network are not understood, a large number of cargo proteins with well-characterized trafficking pathways are known and can be used to study the process.
The most intensively studied vesicle trafficking proteins are the low molecular weight GTPases, the Rabs (Novick and Zerial 1997), and membrane fusion proteins of the vesicle-associated membrane protein (VAMP), syntaxin and synaptosomal-associated protein of 25 kD (SNAP-25) families, the soluble N-ethylmaleimide–sensitive factor attachment protein (SNAP) receptors (SNAREs) (Bennett and Scheller 1994; Jahn and Südhof 1994; Rothman 1994). Approximately 40 Rabs are known to discretely localize throughout the secretory pathway. Rabs 4 and 5 (Bucci et al. 1992; Daro et al. 1996) are associated with the EE, Rab 11 (Ulrich et al. 1996) is believed to be specifically localized to the RE, and Rabs 7 and 9 (Chavrier et al. 1990; Lombardi et al. 1993) are associated with LEs. Rab proteins are likely to be important in early steps of the docking process by acting through a diverse set of effector proteins. Many different SNARE proteins have also been characterized. Formation of a very stable four-stranded helical bundle, comprised of SNAREs from opposing membranes, is thought to be the biochemical event that drives membrane fusion, although this issue is still of some debate (Lin and Scheller 1997; Poirier et al. 1998; Weber et al. 1998). The soluble factors αSNAP and N-ethylmaleimide (NEM)-sensitive factor (NSF) play an important role as molecular chaperones to dissociate SNARE protein complexes so that they are able to independently recycle and so that SNAREs can reform complexes to drive another round of membrane fusion (Sollner et al. 1993; Mayer et al. 1996). Although only three members of the SNAP-25 family (SNAP-23, -25, and -29) (Oyler et al. 1989; Bennett et al. 1993; Ravichandran et al. 1996; Steegmaier et al. 1998) are known, many more members of the VAMP and syntaxin families have been characterized. The SNAREs, particularly VAMPs and syntaxins, are specifically localized throughout the mammalian secretory pathway, including the ER, the Golgi apparatus, the TGN, the EE, synaptic vesicles, and the plasma membrane (Hay and Scheller 1997). Thus, while the localization of SNAREs to distinct membrane compartments is consistent with a function in regulating the specificity of membrane trafficking, different family members form complexes indiscriminately in vitro, leaving open the question of their roles in determining the fidelity of membrane fusion events (Yang et al. 1999).
Few SNAREs have been shown to specifically function within the endosomal pathway, and a subset of VAMPs and syntaxins do seem to localize to specific domains within these compartments. VAMP-3, VAMP-8, syntaxin 7, and syntaxin 13 are thought to localize to endosomes (Link et al. 1993; Wang et al. 1997; Advani et al. 1998; Wong et al. 1998). Syntaxin 13 is found in tubular EEs and REs where it colocalizes with TfR. Consistent with this localization, functional studies support a role for syntaxin 13 in transferrin (Tf) recycling in permeabilized PC12 cells. In neurons, syntaxin 13 forms SNARE complexes, likely with SNAP-25 and VAMP-2/3, and these complexes are dissociated by αSNAP and NSF (Prekeris et al. 1998).
If each distinct trafficking step requires specific SNAREs or a particular subset of SNAREs, many other VAMPs and syntaxins are likely to function within the endosomal network. In particular, little is known about the SNARE proteins responsible for trafficking from EEs to LEs on to the lysosome. To better understand membrane trafficking within the endosomal pathway, we have characterized the localization and function of VAMP-7, also known as TI-VAMP (Galli et al. 1998). This toxin-resistant SNARE is likely to function along the pathway from EEs to LEs or from LEs to lysosomes.
Materials and Methods
Antibodies
Full-length recombinant VAMP-7 protein lacking its COOH-terminal hydrophobic domain was used to immunize both a rabbit and a mouse. Affinity-purified polyclonal antibodies against VAMP-7 were then prepared from rabbit antisera as described previously (Bock et al. 1996).
mAbs against VAMP-7 were obtained from the corresponding hybridoma cell lines produced by fusion of NS-1 mouse myeloma cells with spleen cells from an immunized BALB/c mouse as described previously (Bock et al. 1997). The mAbs were isotyped using an ELISA system (Boehringer Mannheim). Ascites fluid was prepared by Joseph Beirao (Josman Laboratories, Napa Valley, CA). The mAb epitopes were mapped by Western blotting against three glutathione S-transferase (GST)–fusion protein constructs consisting of various portions of the cytoplasmic domain of VAMP-7: 1–23 amino acids (aa), 1–123 aa, or 1–182 aa.
Rat anti–lysosome-associated membrane protein 1 (LAMP-1) mAbs were purchased from PharMingen. Mouse anti-TfR mAbs were purchased from Zymed Laboratories, Inc. Mouse anti-p115 antibodies have been described previously (Waters et al. 1992). Texas red–labeled anti–rabbit IgG, FITC-labeled anti–mouse IgG, and FITC-labeled anti–rat IgG antibodies were obtained from Jackson ImmunoResearch Laboratories. Rabbit antibody lym3 against the cytosolic tail of lgp120 was kindly provided by Dr. I. Mellman (Yale University, New Haven, CT). Rabbit antibody MSCI against the cytosolic part of the cation-dependent mannose 6-phosphate receptor (CD-MPR) was a gift from Dr. A. Hille (Georg-August University, Gottingen, Germany). In some single-labeled sections, a swine anti–rabbit IgG (SWAR) antibody was used (Nordic Immunological Laboratories) after the incubation step with VAMP-7 antibody to enhance the density of immunostaining.
Western blotting was performed using ECL (Amersham Pharmacia Biotech).
Immunofluorescence Microscopy
Indirect immunofluorescence localization was performed on NIH-3T3 cells as described previously (Hay et al. 1996). Antisera were used at the following dilutions: anti–VAMP-7 polyclonal antibody at 2 μg/ml; anti–LAMP-1 mAb at 0.05 μg/ml; anti-TfR mAb at 1 μg/ml; and Texas red–labeled anti–rabbit IgG, FITC-labeled anti–mouse IgG, and FITC-labeled anti–rat IgG antibodies at 7.5 μg/ml.
Immunogold Labeling of Ultrathin Cryosections
PC12 cells were prepared for ultrathin cryosections, and (double)-immunogold labeling according to the protein A–gold method was carried out as described previously (Slot et al. 1991). For a quantitative analysis of the subcellular distribution of VAMP-7, well-preserved areas of two different grids single-immunogold labeled for VAMP-7 were selected, and at a magnification of 25,000 in the electron microscope, all gold particles were counted and subscribed to the compartment over which they were located. The definition of the distinct compartments was based on their morphology. The Golgi complex was recognized by its characteristic appearance of stacked cisternae, and the array of tubulovesicular membranes that were located at the trans-side of the Golgi was considered the TGN. EEs were defined as elongated, sometimes curved, electron-lucent vacuoles with few internal vesicles. LEs were defined as globular shaped compartments with numerous internal vesicles. Connected to or in close association with these endosomal vacuoles, many tubulovesicular membrane profiles were present that we indicated as endosome-associated vesicles. Electron-dense compartments without or with degraded internal membranes were categorized as lysosomes. These definitions are based on the description of the endocytic compartments in a variety of cell types (Griffiths et al. 1989; Klumperman et al. 1993; Kleijmeer et al. 1997). In PC12 cells, the compartments that by these definitions were defined as LEs and lysosomes stained positive for the LE/lysosomal marker protein lgp120 (see Fig. 6), whereas TfR localized to the compartments defined as EEs (data not shown). Finally, when the endocytic tracer BSA-gold was added to PC12 cells, it appeared in EEs, LEs, and lysosomes after 1-, 10-, and 60-min uptakes, respectively (Klumperman, J., manuscript in preparation).
Figure 6.

VAMP-7 colocalizes with lgp120 in LEs and is present on MPR-negative transport vesicles. PC12 cells double-immunogold–labeled for (A) the CD-MPR (MPR, 10 nm gold) and VAMP-7 (15 nm gold), and (B) VAMP-7 (10 nm gold) and lgp120 (15 nm gold). (A) The small arrowheads point to small-sized, electron-dense transport vesicles that contain the MPR but not VAMP-7. VAMP-7 is found on larger vesicles (large arrowheads) with a less electron-dense content (see also Fig. 5 C). (B) VAMP-7 colocalizes with lgp120 to the limiting membrane of an LE. The arrow points to a connection between a VAMP-7–positive vesicle that is in continuity with the vacuolar region of the LE. G, Golgi complex; L, lysosome; M, mitochondrion. Bars, 200 nm.
Membrane Extraction Studies
Membrane extraction studies of VAMP-7 were carried out on NIH-3T3 cells as described previously (Hay et al. 1996; Advani et al. 1998).
EGF and Tf Trafficking in Permeabilized HeLa Cells
To measure EGF and Tf trafficking, we adopted with slight modifications the streptolysin-O (SLO)-permeabilized cell system (Prekeris et al. 1998). In brief, HeLa cells were loaded with either 125I-EGF or 125I-Tf at 18°C for 1 h and then extensively washed to remove unbound EGF/Tf. Cells were then permeabilized with SLO as described previously (Prekeris et al. 1998). Where indicated, cells were incubated at 37°C for varying periods of time. To remove cytosol, cells were incubated for 1 h on ice with KTM buffer (115 mM potassium acetate, 25 mM Hepes, pH 7.4, 2.5 mM magnesium acetate, and 1 mg/ml BSA) and then washed extensively with ice-cold KTM buffer. Under these conditions, ∼95% of the cytosol can be removed from the permeabilized cells (Prekeris et al. 1998). Permeabilized cells were then resuspended in 0.5 ml of KTM buffer with or without 3 mg/ml rat brain cytosol. In all cases, KTM buffer was supplemented with 0.5 mM ATP and an ATP regeneration system (80 mM creatine phosphate, 9 U/ml creatine kinase). EGF/Tf trafficking was induced by incubating samples at 37°C for specified amounts of time. Where indicated, permeabilized HeLa cells were preincubated for 1 h with 100 μg/ml IgG, affinity-purified rabbit polyclonal anti–VAMP-7 antibodies, mouse monoclonal 22G2 and 1D9 anti–VAMP-7 antibodies, or anti–syntaxin 6 antibodies. Where indicated, affinity-purified rabbit polyclonal anti–VAMP-7 antibody was preincubated with 150 μg/ml GST–VAMP-7 or GST. After incubation at 37°C, KTM buffer was removed and cells were solubilized with 0.5 ml KTM buffer containing 2% Triton X-100 to determine the amount of EGF/Tf remaining in the cell. The removed buffer represents released EGF/Tf. To determine the amount of degraded EGF, the removed buffer was extracted with 10% TCA and the precipitate containing recycled intact EGF (Futter et al. 1996) was sedimented by centrifugation at 10,000 g for 15 min. Remaining soluble 125I represents degraded EGF (Futter et al. 1996). Similar TCA extraction of the buffer containing released 125I-Tf resulted in the complete sedimentation of all 125I (data not shown), in agreement with the data that Tf does not get targeted to the lysosomes for degradation. The amounts of 125I-EGF degraded and 125I-Tf recycled were determined by scintillation counting and expressed as the percentage of total EGF/Tf (the sum of released and intracellular Tf/EGF for each sample).
The rat brain cytosol for EGF degradation and Tf recycling assays was prepared as described previously (Prekeris et al. 1998). In brief, fresh rat brains were homogenized in 25 mM Hepes, pH 7.4, containing 115 mM potassium acetate, 2.5 mM magnesium acetate, 0.1 mM EGTA, 2 mM DTT, 4 μg/ml aprotinin, and 0.8 μg/ml pepstatin. This homogenate was then centrifuged at 10,000 g for 20 min, followed by 100,000 g for 45 min. Cytosol was then flash-frozen in liquid nitrogen and stored at −80°C. The protein concentration was determined by the Bradford assay according to the manufacturer's instructions (BioRad). Where indicated, cytosol was pretreated with 0.2 mM NEM for 30 min on ice.
Results
VAMP-7 Is Broadly Expressed
To further investigate the function of VAMP-7, we generated one rabbit polyclonal and five mouse mAbs using the full-length VAMP-7 protein lacking its COOH-terminal hydrophobic region as the immunogen. All six antibodies recognized a single band of the expected molecular mass, 25 kD, on Western blots of rat kidney postnuclear supernatant (PNS) (Fig. 1 A). To define more precisely the region of VAMP-7 recognized by the mAbs, each antibody was tested for binding to three different GST–fusion protein constructs of VAMP-7 (Fig. 1 B). mAbs 8B7 and 23B8 bind to aa 23–123. 1D9, 22G2, and 24C3 bind in the region encompassed by aa 123–182, the approximate region of the helical domain important in forming the core fusion complex. The rabbit polyclonal antibody used in these studies recognizes epitopes in both these domains.
Figure 1.
Polyclonal antibodies and mAbs specifically recognize VAMP-7. (A) 20 μg of PNS from rat kidney was probed for VAMP-7 with an affinity-purified polyclonal and five different mAbs. A 25-kD band is recognized by all antibodies. (B) The domain of VAMP-7 recognized by the mAbs was mapped. The transmembrane (TM) region corresponds to the predicted membrane anchor. The coil domain is recognized by three antibodies and a more NH2-terminal domain by two antibodies.
A previously reported Northern blot analysis indicates that VAMP-7 mRNA is present in brain, kidney, spleen, thymus, and liver while no RNA was detected in heart (Advani et al. 1998). To analyze the protein expression pattern of VAMP-7, affinity-purified polyclonal antibodies were used to detect VAMP-7 protein in several tissues. In agreement with the Northern blot results, VAMP-7 protein was expressed in all tissues tested except heart and muscle (Fig. 2 A). In liver, in addition to the 25-kD band, an additional immunoreactive species of ∼27 kD was detected. This band could represent either a posttranslational modification, an alternatively spliced isoform, or a cross-reactive protein distinct from VAMP-7 that is present only in liver. We used several cell lines for further investigation of VAMP-7 function and localization, including PC12, NIH-3T3, and HeLa cells. The antibodies detect a single band of 25 kD in these cell lines as exemplified by the pattern seen in PC12 cells (Fig. 2 A). Based on the broad mRNA and protein distribution of VAMP-7, it appears that VAMP-7 is involved in a trafficking pathway that is common to most cell types.
Figure 2.
VAMP-7 is broadly expressed and associates with membranes. (A) Seven tissues and PC12 cells were probed with the polyclonal antibody for VAMP-7 expression as described in the text and Materials and Methods. 20 μg of PNS was used for each tissue. (B) PNS was fractionated into cytosolic and membrane fractions. The membrane pellet was extracted with 1.5 M NaCl, pH 11.0, or 2% Triton X-100 and centrifuged into supernatant (S) or pellet (P) fractions.
VAMP-7 contains a hydrophobic COOH-terminal sequence thought to be a membrane anchor (Advani et al. 1998). To determine if VAMP-7 behaves as a membrane protein, NIH-3T3 cells were homogenized and the PNS was fractionated into cytosolic and membrane fractions. The membranes were then extracted under various conditions and fractionated into supernatant and pellet fractions. Even when extracted with 1.5 M NaCl or pH 11.0, VAMP-7 was recovered in the pellet fraction. Only when the membranes were solubilized with 2% Triton X-100 did the protein redistribute to the supernatant, consistent with VAMP-7 being an integral membrane protein (Fig. 2 B).
VAMP-7 Localizes to Endosomes
Understanding the localization of a SNARE within a cell is critical information for understanding the membrane trafficking step mediated by that protein. We reported previously that epitope-tagged, full-length VAMP-7 displayed a perinuclear punctate staining pattern, and showed significant overlap with the late endosomal/lysosomal protein lgp120 (Advani et al. 1998). To ascertain the localization of endogenous VAMP-7, we stained NIH-3T3 cells with anti–VAMP-7 mAb 1D9, anti–VAMP-7 mAb 24C3, and anti–VAMP-7 affinity-purified polyclonal antibodies. In double labeling experiments, each mAb exhibited a staining pattern identical to that of the polyclonal antibody, and addition of VAMP-7 cytoplasmic domain blocked the immunostaining. These control experiments demonstrate that the localization is due to specific recognition of VAMP-7 (data not shown).
To begin a more detailed study of the intracellular localization of endogenous VAMP-7, we compared its immunostaining pattern with those of well-characterized markers of Golgi, endosomal, and lysosomal compartments. VAMP-7 showed little if any colocalization with TfR, a marker of EEs, and p115, a marker of the Golgi (Fig. 3, D–I). However, endogenous VAMP-7, like the transfected protein, is localized in a pattern that overlapped considerably with LAMP-1, a marker of LEs and lysosomes (Fig. 3, A–C). In NIH-3T3 cells, EEs, LEs, and lysosomes are all clustered around the Golgi stacks making it difficult, at the level of light microscopy, to resolve these organelles in this region of the cell. However, it is possible to differentiate these membrane compartments in the periphery of the cells where the organelles are more dispersed. In the cell periphery, the precise colocalization of VAMP-7 and LAMP-1 in many puncta becomes most evident. The intensity of VAMP-7 and LAMP-1 staining for the individual puncta varies: some show more intense VAMP-7 immunoreactivity, whereas others show more intense LAMP-1 immunoreactivity.
Figure 3.
VAMP-7 partially colocalizes with an LE and lysosomal marker in NIH-3T3 cells. VAMP-7 immunoreactivity (Texas red; A, D, and G) is compared with immunofluorescence (FITC) of LAMP-1 (B), p115 (E), or TfR (H). C, F, and I are the merged images. Arrowheads in A–C point to puncta that are immunoreactive for both VAMP-7 and LAMP-1. Bar, 10 μm.
The effect of membrane trafficking perturbants such as nocodozole and brefeldin A (BFA) on vesicle trafficking proteins can unveil features of their native localization and cycling patterns. The microtubule-depolymerizing agent, nocodozole, has been shown to cause vesiculation and dispersal of both Golgi cisternae and early endosomal compartments (Turner and Tartakoff 1989). Treatment of NIH-3T3 cells with 10 μM nocodozole did cause TfR-positive EEs to scatter throughout the cytoplasm (Fig. 4, D–F). However, both LAMP-1–positive and VAMP-7–positive organelles were relatively unaffected by nocodozole treatment (Fig. 4, A–C).
Figure 4.
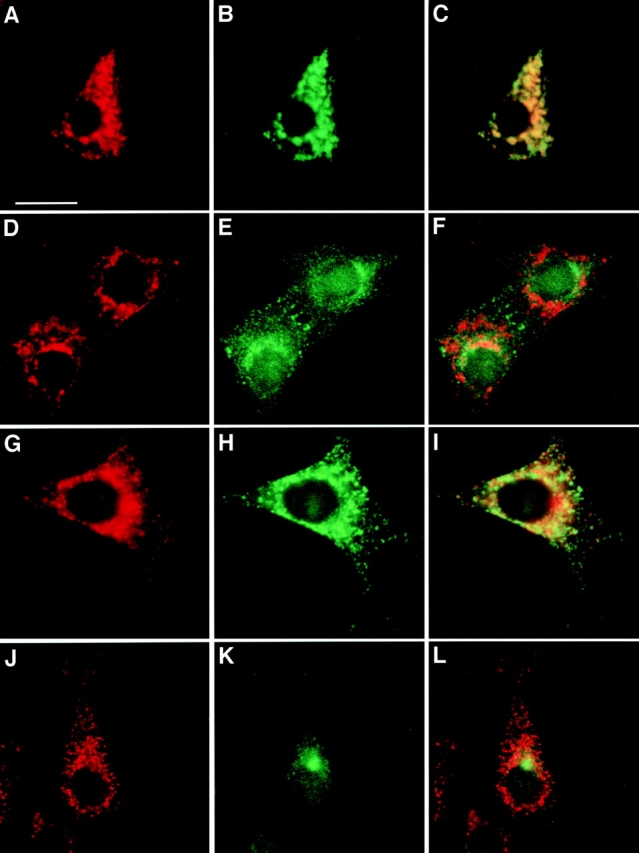
Distribution of VAMP-7 immunoreactivity in nocodozole- and BFA-treated NIH-3T3 cells. (A–F) Nocodozole-treated cells stained for VAMP-7 (A and D), LAMP-1 (B), or TfR (E). (G–L) BFA-treated cells stained for VAMP-7 (G and J), LAMP-1 (H), or TfR (K). Bar, 10 μm.
BFA treatment results in a block in anterograde ER-to-Golgi traffic, and due to the fact that the drug does not disrupt retrograde trafficking, Golgi proteins redistribute to the ER (Robinson and Kreis 1992). Additionally, in certain cells, BFA treatment results in the collapse of the TGN and certain classes of endosomes onto the microtubule-organizing center (Lippincott-Schwartz et al. 1991; Reaves and Banting 1992). Again, BFA seems to have little effect on the distribution of organelles containing either VAMP-7 or LAMP-1 (Fig. 4, G–I). In contrast, TfR staining collapses into the microtubule-organizing center (Fig. 4, J–L). These results are consistent with the localization of VAMP-7 on LEs and/or lysosomes.
VAMP-7 Is Enriched in the TGN Region and LEs
To gain a more precise understanding of the subcellular distribution of VAMP-7, ultrathin cryosections were prepared from PC12 cells and immunogold labeled with affinity-purified polyclonal anti–VAMP-7 antibody. The amount of labeling on various membranes was quantified by defining the distribution of 372 gold particles. A discussion of the criteria used for morphological definition of the membrane compartments is provided in Materials and Methods. Approximately 8% of the labeling was found to be within the Golgi stacks, whereas 30% was found within the TGN region (Fig. 5 A). The plasma membrane was virtually devoid of label. Previously, we localized two other SNARE proteins, syntaxin 6 and VAMP-4, in the TGN region of PC12 cells, and found that these SNAREs were incorporated into clathrin-coated vesicles and immature secretory granules (Bock et al. 1997; Steegmaier et al. 1999). Unlike these SNAREs, VAMP-7 did not significantly label clathrin-coated vesicles or immature secretory granules in the TGN. Within the endosomal compartments, 5% of the VAMP-7–representing gold particles were found on EEs, 22% on LEs, and 30% on LE-associated vesicles. 5% of the gold particles were found on lysosomes (Fig. 5B and Fig. C). On LEs, VAMP-7 colocalized with lgp120 (Fig. 6 B). 200 endocytic compartments were categorized into EEs, LEs, or lysosomes to quantify the number of these organelles in PC12 cells. According to the criteria we used to define these organelles (see Materials and Methods), 19% of the structures were EEs, 25% LEs, and 57% lysosomes. Thus, we conclude that VAMP-7 is enriched in the LE compartment. We further studied the sublocalization of VAMP-7 within the endosomes. Within EEs (89 particles), VAMP-7 was found at the limiting membrane of the vacuole (39%) and on associated vesicles (60%), whereas there was virtually no labeling of internal vesicles (1%). Within the LE compartment (195 particles), 44% of the gold particles were found on the vacuole membrane, 41% on associated vesicles, and 15% on internal vesicles.
Figure 5.

VAMP-7 is localized to the TGN, LEs, and transport vesicles. Ultrathin cryosections of PC12 cells immunogold-labeled (10 nm gold) for VAMP-7. In A and B, the signal for VAMP-7 was enhanced by using an intermediate swine anti–rabbit IgG antibody. (A) Overview of the perinuclear area. VAMP-7 is present in the Golgi complex (indicated by G) and many of the tubulovesicular membranes of the TGN. The open star indicates an immature secretory granule, as judged by the electron-dense cytosolic clathrin coat. The filled stars indicate mature secretory granules. No significant label is found over these secretory compartments. (B) VAMP-7 is present on the limiting membrane of an LE. (C) LE-associated vesicles. L, lysosome; M, mitochondrion. Bars, 200 nm.
Vesicles that label for VAMP-7 do not generally contain the MPR, suggesting VAMP-7 functions in a trafficking pathway distinct from TGN-to-endosome transport (Fig. 6 A). In addition, the vesicles that contain VAMP-7 were generally large and less electron-dense than the typical MPR-carrying vesicles (Fig. 6 A) (Klumperman et al. 1993). The light and EM immunohistochemical studies suggest that VAMP-7 plays a role in transport within the endosomal pathway, perhaps from EEs to LEs or from LEs to the lysosome. Although we are not able to precisely define the compartment labeled within the TGN, the morphological data are also consistent with a role in TGN trafficking.
VAMP-7 Functions in EE-to-Lysosome Trafficking
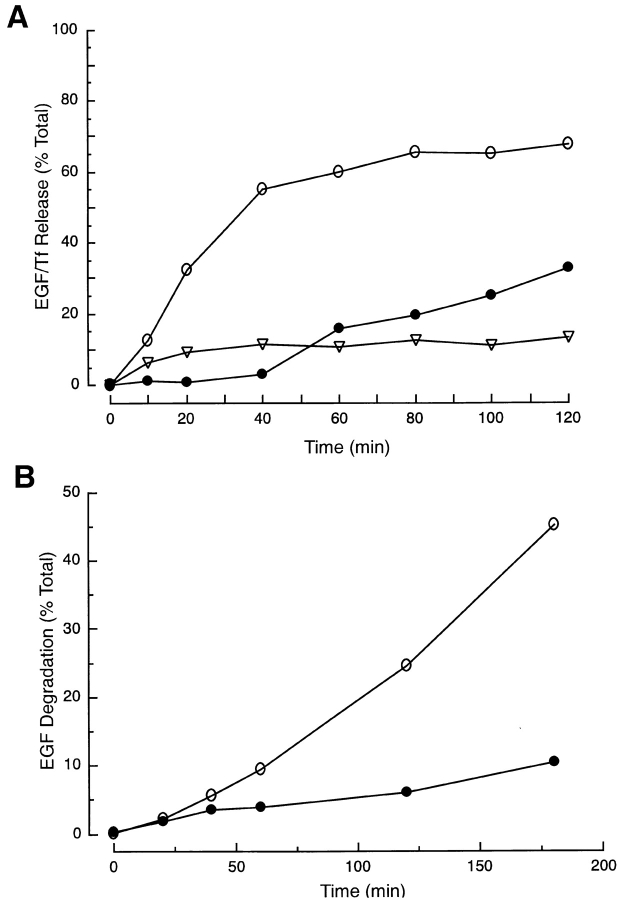
To investigate the role of VAMP-7 in Tf recycling and EGF breakdown, we use a modified SLO-permeabilized HeLa cell system (Prekeris et al. 1998). HeLa cells are incubated for 1 h at 18°C with either 125I-EGF or 125I-Tf and then washed to remove the unbound ligand. In intact cells, recycling of TfR to the plasma membrane results in the release of Tf, a process that occurs over a period of ∼80 min (Fig. 7 A). Similarly, ∼10% of the EGF is rapidly released from cells and recovered as intact protein. The level of released, intact EGF plateaus at ∼20 min. Degraded EGF, defined as EGF that is not precipitated by TCA, begins to increase after ∼40 min and continues out to 120 min when we ended the measurements. We further studied the release of intact and degraded EGF in SLO-permeabilized cells (Fig. 7 B). Release of the two forms of EGF occurs similarly in SLO-permeabilized cells as in intact cells, increasing to 45% of the total EGF released in degraded form after 180 min (Fig. 7 B). The remainder of the EGF is still present within the cell, suggesting that longer chase periods would be required for its breakdown.
Figure 7.
HeLa cells recycle Tf and break down EGF. (A) 125I-EGF or -Tf was loaded into cells and then chased into the supernatant. Tf was recovered in a TCA-precipitable form with a half-life of ∼20 min (open circles). EGF is recovered in two forms, TCA precipitated or intact (open triangles) and non-TCA precipitated or degraded (filled circles). The intact form of EGF rapidly appears in the cell media while the degraded form begins to appear after ∼40 min and continues to increase throughout the 120-min period. (B) Cells loaded with 125I-EGF were permeabilized with SLO. 125I-EGF degradation was measured in the presence (open circles) and absence (filled circles) of cytosol. EGF degradation proceeds with similar kinetics in permeabilized and intact cells.
The cellular processes required for recycling of Tf and breakdown of EGF are retained when cells are permeabilized with SLO. We assayed effects of various antibodies on the trafficking of the two ligands 2 h after incubation at 37°C. In the absence of cytosol or in the presence of NEM, EGF degradation is largely blocked (Fig. 8 A). Furthermore, addition of IgG or anti–syntaxin 6 mAb to the permeabilized cells has no effect on EGF breakdown. However, when anti–VAMP-7 mAbs (1D9 or 24C3) are added to the permeabilized cells, a significant reduction in EGF breakdown is observed. While the reduction is only ∼25%, it is statistically significant, reproducible, and not observed with control antibodies (Fig. 8 A). Addition of anti–VAMP-7 polyclonal antibodies to EGF-loaded, permeabilized cells resulted in a similar inhibition of EGF breakdown. To further control for the specificity of the antibody inhibition, we demonstrated that addition of VAMP-7 protein, but not GST, to the antibody reversed the inhibition of EGF degradation (Fig. 8 B). The inhibition of EGF breakdown is dependent on the concentration of anti–VAMP-7 antibody used in the assay, further confirming the specificity of the inhibition (Fig. 8 C). Since lysosomal degradation of EGF is likely to depend on several trafficking steps, we wondered if it would be possible to chase the EGF past the point at which the anti–VAMP-7 antibodies block. After EGF loading at 18°C, we incubated the cells for 0, 10, 20, or 40 min before permeabilization. Increasing the incubation time resulted in a progressive decrease in the level of EGF breakdown (Fig. 8 D). These data suggest that we are able to chase the EGF into a compartment, past the EE, which no longer depends on VAMP-7 for further trafficking to the lysosome. Finally, we further investigated the effect of anti–VAMP-7 antibodies on the recycling of Tf; however, we did not observe any effects on this process (Fig. 8 E). We interpret these data as demonstrating that antibody binding to VAMP-7 inhibits interactions with other proteins. Since inhibition of these protein interactions blocks EGF breakdown, we suggest that VAMP-7 is necessary for a vesicular trafficking step needed for EGF breakdown.
Figure 8.

VAMP-7 antibodies block EGF breakdown. (A) NEM treatment blocks the degradation of EGF, but addition of IgG or anti–syntaxin 6 antibodies has no effect on the process. Addition of anti–VAMP-7 antibodies partially blocks degradation of EGF. Measurements were made three times and the standard deviation is indicated. An F-test indicated that the effect observed with anti–VAMP-7 antibodies was statistically significant compared with control antibodies (P < 0.01). (B) Anti–VAMP-7 affinity-purified polyclonal antibody also inhibits EGF breakdown. Including VAMP-7 protein but not GST reverses the inhibition of EGF degradation produced by the anti–VAMP-7 polyclonal antibody. VAMP-7 protein and GST alone have no effect in the assay. Measurements were made two times and the average is plotted. (C) Increasing amounts of VAMP-7 polyclonal or monoclonal antibodies result in an increased inhibition of EGF breakdown. Maximum inhibition is achieved at a concentration of 100 μg/ml VAMP-7 antibodies. Filled circles indicate polyclonal antibodies; open circles indicate mAbs. (D) EGF-loaded cells were chased for varying amounts of time at 37°C followed by SLO-permeabilization. Cells were then incubated with antibody and EGF degradation measured as described above. (E) Tf recycling is also reconstituted by addition of cytoplasm to SLO-permeabilized cells. Whereas NEM inhibits the recycling, neither IgG nor anti–VAMP-7 antibodies affect the process.
Discussion
The formation of helical bundles of SNARE proteins from opposite membranes likely drives intracellular membrane fusion. The discovery of syntaxin 1A, a SNARE largely localized to the plasma membrane, and Sed5p (syntaxin 5 in mammalian cells) (Hardwick and Pelham 1992; Hay and Scheller 1997), a syntaxin isoform largely localized to the cis-Golgi complex and ER-Golgi intermediate compartment, led to the idea that intracellular membrane trafficking and membrane fusion will be mediated by sets of SNARE proteins. Furthermore, if SNARE proteins pair specifically, part of the specificity of membrane trafficking may be mediated by the formation of specific sets of core fusion complexes. These ideas have become known as the SNARE hypothesis (Bennett et al. 1993; Sollner et al. 1993). For this hypothesis to be correct, several criteria must be met. First, there need to be enough SNARE proteins so that specific SNAREs, or at least a specific combination of SNAREs, could direct each vesicular trafficking step. In mammalian cells, there are in fact many different SNARE proteins, and they do have specific patterns of localization within cells. For example, syntaxin 5, rsec22b, rbet1, and membrin are important in antero- and/or retrograde trafficking between the ER and the cis-Golgi apparatus (Hay et al. 1998). In contrast, syntaxin 1A, VAMP-1, SNAP-25, and their closely related homologues are required for exocytosis (Hay and Scheller 1997).
In this report we define a function for VAMP-7. The protein is localized to LEs, where our functional data suggest that it mediates trafficking to the lysosomes. The histochemical data are supported by both polyclonal and multiple mAbs, which strengthens the contention that the immunolocalization is specific. However, the EGF breakdown monitored in our assay is only partially inhibited by antibodies against VAMP-7. Complete inhibition of a transport step has never been observed in these types of assays. The partial inhibition may be explained in several ways. The antibodies may have access to only some of the active VAMP-7, or the antibody affinity may not be sufficient to block all SNARE interactions effectively. The antibodies used for the transport inhibition studies recognize the coil domain of VAMP-7 involved in SNARE complex formation. Although this is a domain of critical functional importance, and therefore an excellent site for function-blocking antibodies, the high stability of SNARE complexes may be such that the antibody can be displaced from VAMP-7 during complex formation. Alternatively, only a portion of the EGF may travel to a degradative compartment via a VAMP-7–mediated process. Since VAMP-7 is not present on vesicles that contain the MPR, we conclude that this SNARE is not likely to be involved in trafficking of enzymes to the lysosome.
VAMP-7 that is present on the internal vesicles of LEs is likely to be degraded. This may partially explain why only low levels of VAMP-7 are found at steady state in lysosomes. In addition, we assign a significant amount of the VAMP-7 immunoreactivity to the TGN although we have not defined the subregion of the TGN that is immunoreactive for VAMP-7. The morphological definition of the TGN may include vesicles or tubules that are more appropriately considered part of the endosomal system. Since nocodozole and BFA do not affect the localization of VAMP-7, VAMP-7–positive organelles in the TGN region are more likely to be related to endosomal compartments. Consistent with the TGN localization, some VAMP-7 may be retrieved from LEs to participate in another round of membrane transport. Our data do not rule out the possibility that VAMP-7 also has a role in a distinct TGN transport process and is not used for a single trafficking step. For example, previous studies of VAMP-7 suggest a role in transport to the apical membranes in MDCK cells (Lafont et al. 1999). In general, the morphological and functional data we present support the idea that different trafficking steps are mediated by distinct SNARE proteins, consistent with the SNARE hypothesis.
A second criteria necessary for the SNARE hypothesis to be correct is that the SNAREs pair specifically. The full spectrum of SNAREs important in trafficking through the endosomal system to the lysosomes, including those that might pair with VAMP-7, is not yet fully defined. Recent experiments have shown that many combinations of SNAREs pair with VAMP-7 to form stable complexes in vitro (Yang et al. 1999). Syntaxin 1A/SNAP-25, syntaxin 4/SNAP-23, and syntaxin 13/SNAP-29 all form SDS-resistant complexes with VAMP-7; the midpoint temperatures (T m) of the unfolding transitions for these complexes are 92°C, 88°C, and 85°C, respectively (Yang et al. 1999). However, relatively small differences in the thermal stabilities of SNARE complexes can result in dramatic differences in the abilities of the complexes to mediate membrane fusion. Therefore, the simple formation of a complex cannot be taken as an indication of a functional complex, nor can one easily draw conclusions regarding specificity based on complex formation in vitro. Given these caveats, it is the case that syntaxin 1A and SNAP-25 form the most stable complex with VAMP-7 in studies so far. The 92°C unfolding transition for this complex is more stable than that for the unfolding transition of syntaxin 1A/SNAP-25 with their known SNARE partner, VAMP-2 (T m, 90°C) (Yang et al. 1999). Therefore, it is unlikely that information for the specificity of membrane trafficking is contained within the core complex-forming helical domain of VAMP-7.
If the core complex formation is not the specificity-determining event, then why have such a large number of SNAREs, particularly within the endosomal pathways? Many SNAREs, including VAMP-7, are considerably larger than the 70 aa required for fusion complex formation. In addition, these non–core complex–forming regions are more highly divergent in aa sequence between the SNAREs expressed within a species than those sequences involved in SNARE complex formation. For example, the cytoplasmic domain of VAMP-7 is 180 aa, and the function of the NH2-terminal 120 residues of the protein is not known. Perhaps non–core complex–forming regions of SNAREs are important for interactions with proteins that regulate aspects of vesicle targeting specificity. These interactions are likely to involve interactions with Rab proteins and their effectors, and perhaps members of the sec1p (Hata et al. 1993; Garcia et al. 1994; Pevsner et al. 1994) family of syntaxin-binding proteins.
The mechanisms whereby SNAREs are localized to distinct compartments are an additional critical issue for understanding the organization of membrane compartments. The aa sequence motifs that direct SNARE localization are not well understood. It is also not clear for most SNAREs if particular coat or adaptor proteins are important in localizing the proteins to specific vesicles or matching the SNAREs to particular sets of cargo proteins. It is intriguing that VAMP-7 contains a potential adaptor protein binding motif, D/EXXXLL (aa 162–167), within the SNARE coil domain. Perhaps each of the SNAREs will contain specific sequences that direct binding to particular adaptor proteins. The small number of aa that are so far defined to be important for adaptor binding interactions makes it difficult to understand their significance simply by inspection of the aa sequences of the SNAREs. Further experiments are needed to clarify these issues.
Only when we have a complete understanding of the localizations and protein interactions of all of the membrane fusion proteins of the VAMP, syntaxin, and SNAP-25 families will we be in a position to fully consider their roles in specificity. In addition, a full definition of the complexity of SNARE protein function and organization will lead to a more precise definition of complex membrane trafficking pathways within cells.
Acknowledgments
We would like to thank M. Steegmaier (Stanford University, Stanford, CA) for his assistance, especially in hybridoma preparation; J. Bock, E. Fung, J. Hay, R. Lin, K. Peterson, and S.C. Hsu for their assistance; V. Oorschot (University of Utrecht, Utrecht, The Netherlands) for the preparation of ultrathin cryosections; and M. Niekerk, T. van Rijin, and R. Scriwaneck (University of Utrecht) for handling the electron micrographs.
Footnotes
1.used in this paper: aa, amino acid(s); BFA, brefeldin A; EE, early endosome; GST, glutathione S-transferase; LAMP-1, lysosome-associated membrane protein 1; LE, late endosome; MPR, mannose-6 phosphate receptor; NEM, N-ethylmaleimide; NSF, NEM-sensitive factor; PNS, postnuclear supernatant; RE, recycling endosome; SLO, streptolysin-O; SNAP, soluble NSF attachment protein; SNAP-25, synaptosomal-associated protein of 25 kD; SNARE, SNAP receptor; Tf, transferrin; TfR, Tf receptor; VAMP, vesicle-associated membrane protein
References
- Advani R.J., Bae H.R., Bock J.B., Chao D.S., Doung Y.C., Prekeris R., Yoo J.S., Scheller R.H. Seven novel mammalian SNARE proteins localize to distinct membrane compartments. J. Biol. Chem. 1998;273:10317–10324. doi: 10.1074/jbc.273.17.10317. [DOI] [PubMed] [Google Scholar]
- Bennett M.K., Scheller R.H. A molecular description of synaptic vesicle membrane trafficking. Annu. Rev. Biochem. 1994;63:63–100. doi: 10.1146/annurev.bi.63.070194.000431. [DOI] [PubMed] [Google Scholar]
- Bennett M.K., Garcia-Arraras J.E., Elferink L.A., Peterson K., Fleming A.M., Hazuka C.D., Scheller R.H. The syntaxin family of vesicular transport receptors. Cell. 1993;74:863–873. doi: 10.1016/0092-8674(93)90466-4. [DOI] [PubMed] [Google Scholar]
- Bock J.B., Lin R.C., Scheller R.H. A new syntaxin family member implicated in targeting of intracellular transport vesicles. J. Biol. Chem. 1996;271:17961–17965. doi: 10.1074/jbc.271.30.17961. [DOI] [PubMed] [Google Scholar]
- Bock J.B., Klumperman J., Davanger S., Scheller R.H. Syntaxin 6 functions in trans-Golgi network vesicle trafficking. Mol. Biol. Cell. 1997;8:1261–1271. doi: 10.1091/mbc.8.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci C., Parton R.G., Mather I.H., Stunnenberg H., Simons K., Hoflack B., Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- Chavrier P., Parton R.G., Hauri H.P., Simons K., Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990;62:317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- Daro E., van der Sliujis P., Galli T., Mellman I. Rab4 and cellubrevin define distinct populations of endosomes on the pathway of transferrin recycling. Proc. Natl. Acad. Sci. USA. 1996;93:9559–9564. doi: 10.1073/pnas.93.18.9559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futter C.E., Pearse A., Hewlett L.J., Hopkins C.R. Multivesicular endosomes containing internalized EGF-EGF receptor complexes mature and then fuse directly with lysosomes. J. Cell Biol. 1996;132:1011–1023. doi: 10.1083/jcb.132.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli T., Zahraoui A., Vaidyanathan V.V., Raposo G., Tian J.M., Karin M., Niemann H., Louvard D. A novel tetanus neurotoxin-insensitive vesicle-associated membrane protein in SNARE complexes of the apical plasma membrane of epithelial cells. Mol. Biol. Cell. 1998;9:1437–1448. doi: 10.1091/mbc.9.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia E.P., Gatti E., Butler M., Burton J., De Camilli P. A rat brain Sec1 homologue related to Rop and UNC18 interacts with syntaxin. Proc. Natl. Acad. Sci. USA. 1994;91:2003–2007. doi: 10.1073/pnas.91.6.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosh R.N., Gelman D.L., Maxfield F.R. Quantification of low density lipoproteins and transferrin endocytic sorting in HEp2 cells using confocal microscopy. J. Cell Sci. 1994;107:2177–2189. doi: 10.1242/jcs.107.8.2177. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Back R., Marsh M. A quantitative analysis of the endocytic pathway in baby hamster kidney cells. J. Cell Biol. 1989;109:2703–2720. doi: 10.1083/jcb.109.6.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J., Maxfield F.R. Membrane transport in the endocytic pathway. Curr. Opin. Cell Biol. 1995;7:552–563. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- Hardwick K.G., Pelham H.R. SED5 encodes a 39-kD integral membrane protein required for vesicular transport between the ER and the Golgi complex. J. Cell Biol. 1992;119:513–521. doi: 10.1083/jcb.119.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata Y., Slaughter C.A., Sudhof T.C. Synaptic vesicle fusion complex contains unc-18 homologue bound to syntaxin. Nature. 1993;366:347–351. doi: 10.1038/366347a0. [DOI] [PubMed] [Google Scholar]
- Hay J.C., Scheller R.H. SNAREs and NSF in targeted membrane fusion. Curr. Opin. Cell Biol. 1997;9:505–512. doi: 10.1016/s0955-0674(97)80026-9. [DOI] [PubMed] [Google Scholar]
- Hay J.C., Hirling H., Scheller R.H. Mammalian vesicle trafficking proteins of the endoplasmic reticulum and Golgi apparatus. J. Biol. Chem. 1996;271:5671–5679. doi: 10.1074/jbc.271.10.5671. [DOI] [PubMed] [Google Scholar]
- Hay J.C., Klumperman J., Oorschot V., Steegmaier M., Kuo C.S., Scheller R.H. Localization, dynamics, and protein interactions reveal distinct roles for ER and Golgi SNAREs [published erratum appears in J. Cell Biol. 1998. 142(3):following 881] J. Cell Biol. 1998;141:1489–1502. doi: 10.1083/jcb.141.7.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins C.R. Intracellular routing of transferrin receptors in epidermoid carcinoma A431 cells. Cell. 1983;35:321–330. doi: 10.1016/0092-8674(83)90235-0. [DOI] [PubMed] [Google Scholar]
- Hopkins C.R., Trowbridge I.S. Internalization and processing of transferrin and transferrin receptor in human carcinoma A431 cells. J. Cell Biol. 1983;97:508–521. doi: 10.1083/jcb.97.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins C.R., Gibson A., Shipman M., Miller K. Movement of internalized ligand-receptor complexes along a continuous endosomal reticulum. Nature. 1990;346:335–339. doi: 10.1038/346335a0. [DOI] [PubMed] [Google Scholar]
- Jahn R., Südhof T.C. Synaptic vesicles and exocytosis. Annu. Rev. Neurosci. 1994;17:219–246. doi: 10.1146/annurev.ne.17.030194.001251. [DOI] [PubMed] [Google Scholar]
- Kleijmeer M.J., Morkowski S., Griffith J.M., Rudensky A.Y., Geuze H.J. Major histocompatibility complex class II compartments in human and mouse B lymphoblasts represent conventional endocytic compartments. J. Cell Biol. 1997;139:639–649. doi: 10.1083/jcb.139.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumperman J., Hille A., Veenendaal T., Oorschot V., Stoorvogel W., von Figura K., Geuze H.J. Differences in the endosomal distributions of the two mannose 6-phosphate receptors. J. Cell Biol. 1993;121:997–1010. doi: 10.1083/jcb.121.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S., Mellman I. The biogenesis of lysosomes. Annu. Rev. Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- Lafont F., Verkade P., Galli T., Wimmer C., Louvard D., Simons K. Raft association of SNAP receptors acting in apical trafficking in Madin-Darby canine kidney cells. Proc. Natl. Acad. Sci. USA. 1999;96:3734–3738. doi: 10.1073/pnas.96.7.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R.C., Scheller R.H. Structural organization of the synaptic exocytosis core complex. Neuron. 1997;19:1087–1094. doi: 10.1016/s0896-6273(00)80399-2. [DOI] [PubMed] [Google Scholar]
- Link E., McMahon H., Fisher von Mollard G., Yamasaki S., Niemann H., Sudhof T.C., Jahn R. Cleavage of cellubrevin by tetanus toxin does not affect fusion of early endosomes. J. Biol. Chem. 1993;268:18423–18426. [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L.C., Tipper C., Amherdt M., Orci L., Klausner R. Brefeldin A's effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell. 1991;67:601–616. doi: 10.1016/0092-8674(91)90534-6. [DOI] [PubMed] [Google Scholar]
- Lombardi D., Soldati T., Riederer M.A., Goda Y., Zerial M., Pfeffer S.R. Rab9 functions in transport between late endosomes and the trans Golgi network. EMBO (Eur. Mol. Biol. Organ.) J. 1993;12:677–682. doi: 10.1002/j.1460-2075.1993.tb05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A., Wickner W., Haas A. Sec18p (NSF)-driven release of Sec17p (alpha-SNAP) can precede docking and fusion of yeast vacuoles. Cell. 1996;85:83–94. doi: 10.1016/s0092-8674(00)81084-3. [DOI] [PubMed] [Google Scholar]
- Mellman I. Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- Novick P., Zerial M. The diversity of Rab proteins in vesicle transport. Curr. Opin. Cell Biol. 1997;9:496–504. doi: 10.1016/s0955-0674(97)80025-7. [DOI] [PubMed] [Google Scholar]
- Oyler G.A., Higgins G.A., Hart R.A., Battenberg E., Billingsley M., Bloom F.E., Wilson M.C. The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J. Cell Biol. 1989;109:3039–3052. doi: 10.1083/jcb.109.6.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse B.M. Clathrin and coated vesicles. EMBO (Eur. Mol. Biol. Organ.) J. 1987;6:2507–2512. doi: 10.1002/j.1460-2075.1987.tb02536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse B.M., Robinson M.S. Clathrin, adaptors, and sorting. Annu. Rev. Cell Biol. 1990;6:151–171. doi: 10.1146/annurev.cb.06.110190.001055. [DOI] [PubMed] [Google Scholar]
- Pevsner J., Hsu S.C., Scheller R.H. n-Sec1a neural-specific syntaxin-binding protein. Proc. Natl. Acad. Sci. USA. 1994;91:1445–1449. doi: 10.1073/pnas.91.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier M.A., Xiao W., Macosko J.C., Chan C., Shin Y.K., Bennett M.K. The synaptic SNARE complex is a parallel four-stranded helical bundle. Nat. Struct. Biol. 1998;5:765–769. doi: 10.1038/1799. [DOI] [PubMed] [Google Scholar]
- Prekeris R., Klumperman J., Chen Y.A., Scheller R.H. Syntaxin 13 mediates cycling of plasma membrane proteins via tubulovesicular recycling endosomes. J. Cell Biol. 1998;143:957–971. doi: 10.1083/jcb.143.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravichandran V., Chawla A., Roche P.A. Identification of a novel syntaxin- and synaptobrevin/VAMP-binding protein, SNAP-23, expressed in non-neuronal tissues. J. Biol. Chem. 1996;271:13300–13303. doi: 10.1074/jbc.271.23.13300. [DOI] [PubMed] [Google Scholar]
- Reaves B., Banting G. Perturbation of the morphology of the trans-Golgi network following Brefeldin A treatmentredistribution of a TGN-specific integral membrane protein, TGN38. J. Cell Biol. 1992;116:85–94. doi: 10.1083/jcb.116.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M.S., Kreis T.E. Recruitment of coat proteins onto Golgi membranes in intact and permeabilized cellseffect of brefeldin A and G protein activators. Cell. 1992;69:129–138. doi: 10.1016/0092-8674(92)90124-u. [DOI] [PubMed] [Google Scholar]
- Robinson M.S., Watts C., Zerial C. Membrane dynamics in endocytosis. Cell. 1996;84:13–21. doi: 10.1016/s0092-8674(00)80988-5. [DOI] [PubMed] [Google Scholar]
- Rothman J.E. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Slot J.W., Geuze H.J., Gigengack S., Lienhard G.E., James D.E. Immunolocalization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J. Cell Biol. 1991;113:123–135. doi: 10.1083/jcb.113.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollner T., Whiteheart S.W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J.E. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Steegmaier M., Yang B., Yoo J.S., Huang B., Shen M., Yu S., Luo Y., Scheller R.H. Three novel proteins of the syntaxin/SNAP-25 family. J. Biol. Chem. 1998;273:34171–34179. doi: 10.1074/jbc.273.51.34171. [DOI] [PubMed] [Google Scholar]
- Steegmaier M., Klumperman J., Foletti D.L., Yoo J., Scheller R.H. Vesicle-associated membrane protein 4 is implicated in trans-Golgi network vesicle trafficking. Mol. Biol. Cell. 1999;10:1957–1972. doi: 10.1091/mbc.10.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J.R., Tartakoff A.M. The response of the Golgi complex to microtubule alterationsthe roles of metabolic energy and membrane traffic in Golgi complex organization. J. Cell Biol. 1989;109:2081–2088. doi: 10.1083/jcb.109.5.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich O., Reinsch S., Urbe S., Zerial M., Parton R.G. Rab11 regulates recycling through the pericentriolar recycling endosome. J. Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Frelin L., Pevsner J. Human syntaxin 7a Pep12p/Vps6p homologue implicated in vesicle trafficking to lysosomes. Gene. 1997;199:39–48. doi: 10.1016/s0378-1119(97)00343-0. [DOI] [PubMed] [Google Scholar]
- Waters M.G., Clary D.O., Rothman J.E. A novel 115-kD peripheral membrane protein is required for intercisternal transport in the Golgi stack. J. Cell Biol. 1992;118:1015–1026. doi: 10.1083/jcb.118.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T., Zemelman B.V., McNew J.A., Westermann B., Gmachl M., Parlati F., Sollner T.H., Rothman J.E. SNAREpinsminimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- Wong S.H., Xu Y., Zhang T., Hong W. Syntaxin 7, a novel syntaxin member associated with the early endosomal compartment. J. Biol. Chem. 1998;273:375–380. doi: 10.1074/jbc.273.1.375. [DOI] [PubMed] [Google Scholar]
- Yamashiro D., Tycko B., Fluss S., Maxfield F.R. Segregation of transferrin to a mildly acidic (pH 6.5) para-Golgi compartment in the recycling pathway. Cell. 1984;37:789–800. doi: 10.1016/0092-8674(84)90414-8. [DOI] [PubMed] [Google Scholar]
- Yang B., Gonzalez L., Jr., Prekeris R., Steegmaier M., Advani R.J., Scheller R.H. SNARE interactions are not selective. Implications for membrane fusion specificity. J. Biol. Chem. 1999;274:5649–5653. doi: 10.1074/jbc.274.9.5649. [DOI] [PubMed] [Google Scholar]