Abstract
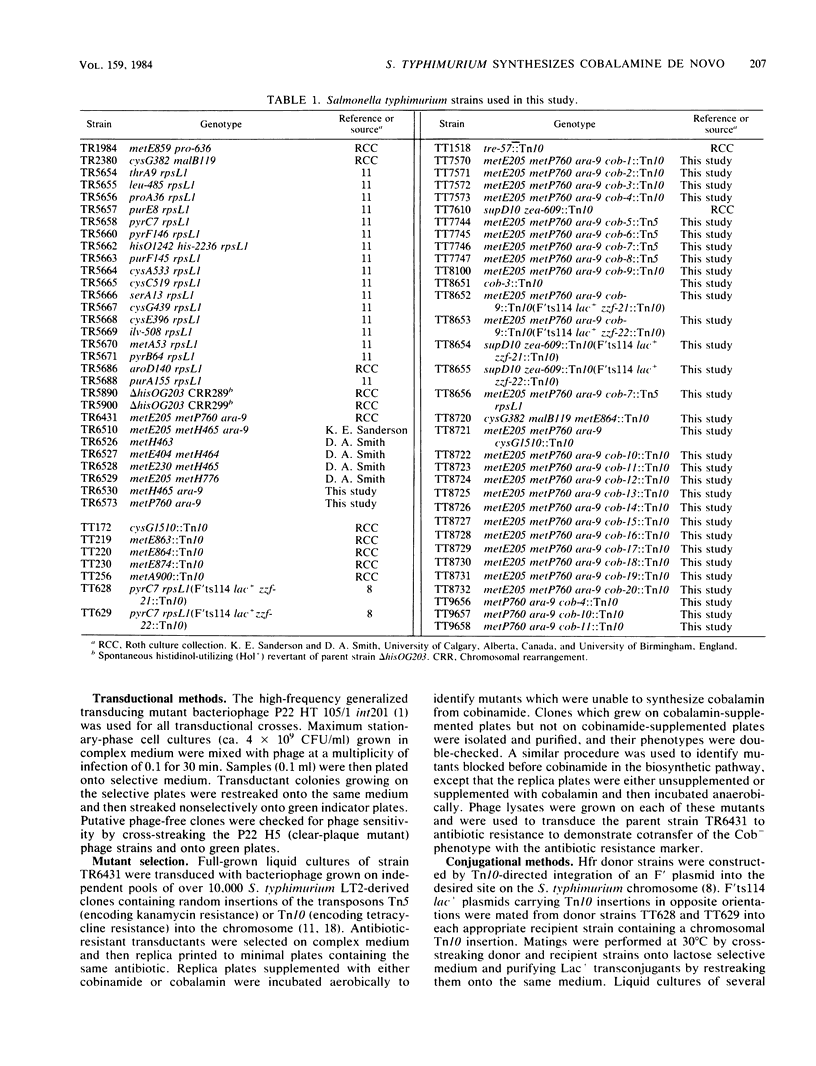
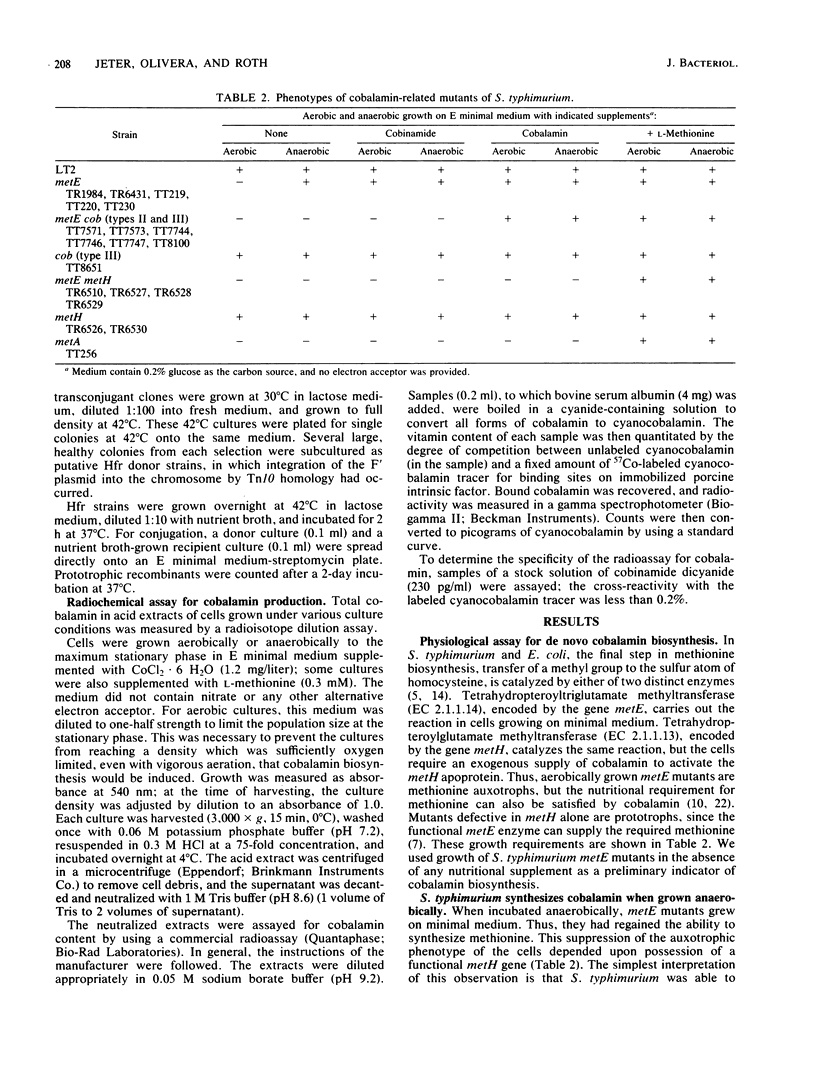
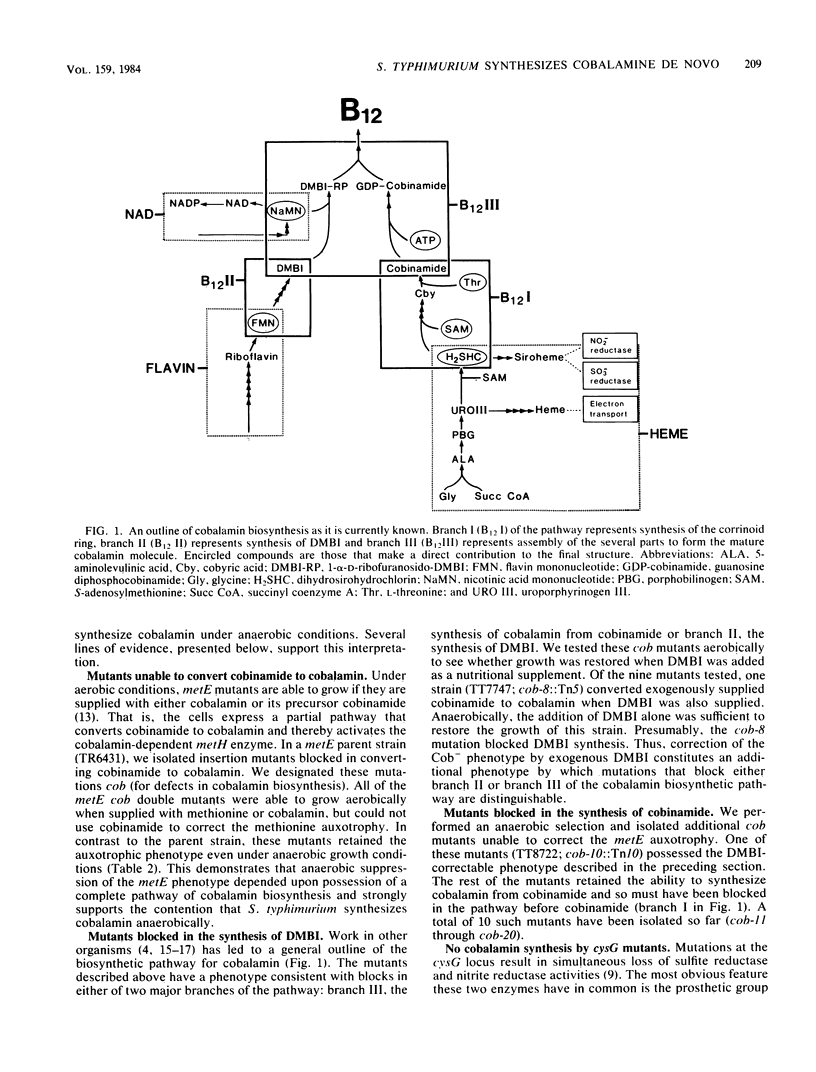
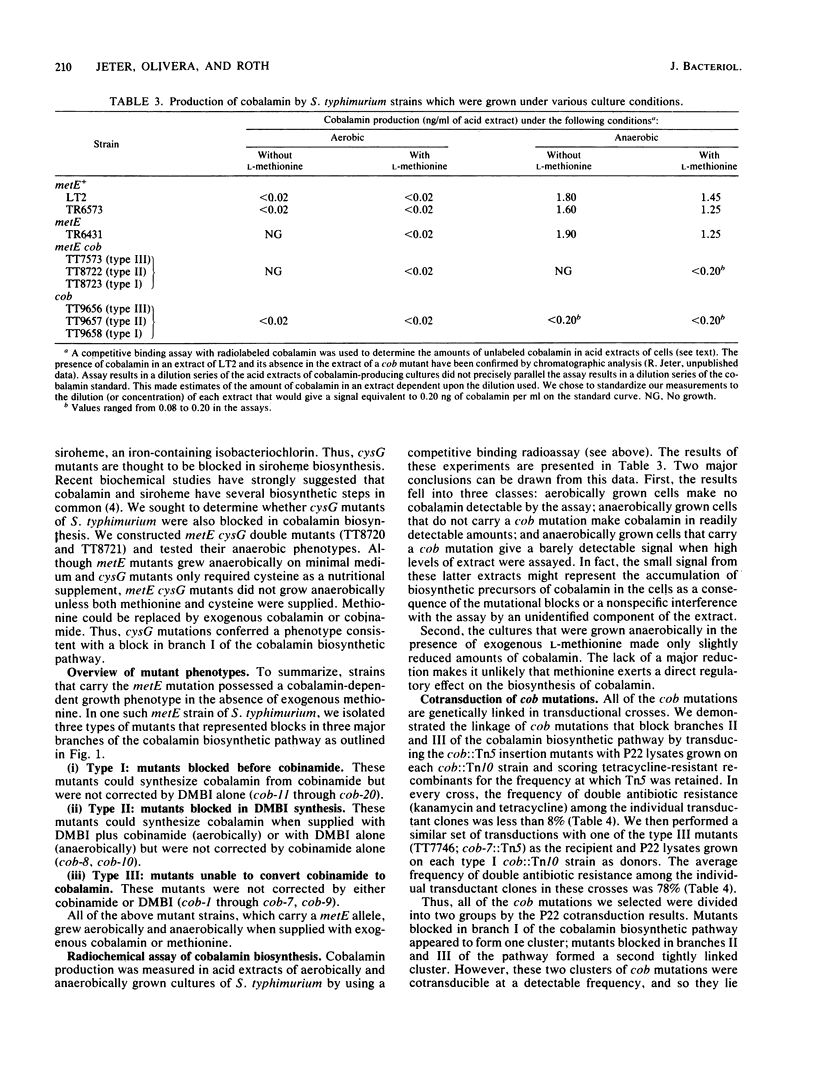
In this paper, we report that the enteric bacterium Salmonella typhimurium synthesized cobalamin de novo under anaerobic culture conditions. Aerobically, metE mutants of S. typhimurium need either methionine or cobalamin as a nutritional supplement for growth. The growth response to cobalamin depends upon a cobalamin-requiring enzyme, encoded by the gene metH, that catalyzes the same reaction as the metE enzyme. Anaerobically, metE mutants grew without any nutritional supplements; the metH enzyme functioned under these conditions due to the endogenous biosynthesis of cobalamin. This conclusion was confirmed by using a radiochemical assay to measure cobalamin production. Insertion mutants defective in cobalamin biosynthesis (designated cob) were isolated in the three major branches of the cobalamin biosynthetic pathway. Type I mutations blocked the synthesis of cobinamide, type II mutations blocked the synthesis of 5,6-dimethylbenzimidazole, and type III mutations blocked the synthesis of cobalamin from cobinamide and 5,6-dimethylbanzimidazole. Mutants that did not synthesize siroheme (cysG) were blocked in cobalamin synthesis. Genetic mapping experiments showed that the cob mutations are clustered in the region of the S. typhimurium chromosome between supD (40 map units) and his (42 map units). The discovery that S. typhimurium synthesizes cobalamin de novo only under anaerobic conditions raises the possibility that anaerobically grown cells possess a variety of enzymes which are dependent upon cobalamin as a cofactor.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. P., Roth J. R. Tandem chromosomal duplications in Salmonella typhimurium: fusion of histidine genes to novel promoters. J Mol Biol. 1978 Feb 15;119(1):147–166. doi: 10.1016/0022-2836(78)90274-7. [DOI] [PubMed] [Google Scholar]
- Barker H. A., Weissbach H., Smyth R. D. A COENZYME CONTAINING PSEUDOVITAMIN B(12). Proc Natl Acad Sci U S A. 1958 Nov 15;44(11):1093–1097. doi: 10.1073/pnas.44.11.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauthen S. E., Foster M. A., Woods D. D. Methionine synthesis by extracts of Salmonella typhimurium. Biochem J. 1966 Feb;98(2):630–635. doi: 10.1042/bj0980630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang G. W., Chang J. T. Evidence for the B12-dependent enzyme ethanolamine deaminase in Salmonella. Nature. 1975 Mar 13;254(5496):150–151. doi: 10.1038/254150a0. [DOI] [PubMed] [Google Scholar]
- Childs J. D., Smith D. A. New methionine structural gene in Salmonella typhimurium. J Bacteriol. 1969 Oct;100(1):377–382. doi: 10.1128/jb.100.1.377-382.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumley F. G., Menzel R., Roth J. R. Hfr formation directed by tn10. Genetics. 1979 Apr;91(4):639–655. doi: 10.1093/genetics/91.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J. A., Newman B. M., White P. Biochemical and genetic characterization of nirB mutants of Escherichia coli K 12 pleiotropically defective in nitrite and sulphite reduction. J Gen Microbiol. 1980 Oct;120(2):475–483. doi: 10.1099/00221287-120-2-475. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORD J. E., HOLDSWORTH E. S., KON S. K. The biosynthesis of vitamin B12-like compounds. Biochem J. 1955 Jan;59(1):86–93. [PMC free article] [PubMed] [Google Scholar]
- Foster M. A., Tejerina G., Guest J. R., Woods D. D. Two enzymic mechanisms for the methylation of homocysteine by extracts of Escherichia coli. Biochem J. 1964 Sep;92(3):476–488. doi: 10.1042/bj0920476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann H. C., Cagen L. M. Microbial biosynthesis of B12-like compounds. Annu Rev Microbiol. 1970;24:159–208. doi: 10.1146/annurev.mi.24.100170.001111. [DOI] [PubMed] [Google Scholar]
- Kleckner N., Roth J., Botstein D. Genetic engineering in vivo using translocatable drug-resistance elements. New methods in bacterial genetics. J Mol Biol. 1977 Oct 15;116(1):125–159. doi: 10.1016/0022-2836(77)90123-1. [DOI] [PubMed] [Google Scholar]
- Menon I. A., Shemin D. Concurrent decrease of enzymic activities concerned with the synthesis of coenzyme B 12 and of propionic acid in propionibacteria. Arch Biochem Biophys. 1967 Aug;121(2):304–310. doi: 10.1016/0003-9861(67)90080-x. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, Edition VI. Microbiol Rev. 1983 Sep;47(3):410–453. doi: 10.1128/mr.47.3.410-453.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sennett C., Rosenberg L. E., Mellman I. S. Transmembrane transport of cobalamin in prokaryotic and eukaryotic cells. Annu Rev Biochem. 1981;50:1053–1086. doi: 10.1146/annurev.bi.50.070181.005201. [DOI] [PubMed] [Google Scholar]
- Smith M. W., Neidhardt F. C. Proteins induced by aerobiosis in Escherichia coli. J Bacteriol. 1983 Apr;154(1):344–350. doi: 10.1128/jb.154.1.344-350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers A. O., Silver S. Microbial transformations of metals. Annu Rev Microbiol. 1978;32:637–672. doi: 10.1146/annurev.mi.32.100178.003225. [DOI] [PubMed] [Google Scholar]
- Taylor R. T., Hanna M. L. Escherichia coli B 5-methyltetrahydrofolate-homocysteine cobalamin methyltransferase: catalysis by a reconstituted methyl-14C-cobalamin holoenzyme and the function of S-adenosyl-l-methionine. Arch Biochem Biophys. 1970 Apr;137(2):453–459. doi: 10.1016/0003-9861(70)90462-5. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Weissbach H., Toohey J., Barker H. A. ISOLATION AND PROPERTIES OF B(12) COENZYMES CONTAINING BENZIMIDAZOLE OR DIMETHYLBENZIMIDAZOLE. Proc Natl Acad Sci U S A. 1959 Apr;45(4):521–525. doi: 10.1073/pnas.45.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield C. D., Steers E. J., Jr, Weissbach H. Purification and properties of 5-methyltetrahydropteroyltriglutamate-homocysteine transmethylase. J Biol Chem. 1970 Jan 25;245(2):390–401. [PubMed] [Google Scholar]



