Abstract
CD44, the major cell surface receptor for hyaluronic acid (HA), was shown to localize to detergent-resistant cholesterol-rich microdomains, called lipid rafts, in fibroblasts and blood cells. Here, we have investigated the molecular environment of CD44 within the plane of the basolateral membrane of polarized mammary epithelial cells. We show that CD44 partitions into lipid rafts that contain annexin II at their cytoplasmic face. Both CD44 and annexin II were released from these lipid rafts by sequestration of plasma membrane cholesterol. Partition of annexin II and CD44 to the same type of lipid rafts was demonstrated by cross-linking experiments in living cells. First, when CD44 was clustered at the cell surface by anti-CD44 antibodies, annexin II was recruited into the cytoplasmic leaflet of CD44 clusters. Second, the formation of intracellular, submembranous annexin II–p11 aggregates caused by expression of a trans-dominant mutant of annexin II resulted in coclustering of CD44. Moreover, a frequent redirection of actin bundles to these clusters was observed. These basolateral CD44/annexin II–lipid raft complexes were stabilized by addition of GTPγS or phalloidin in a semipermeabilized and cholesterol-depleted cell system. The low lateral mobility of CD44 in the plasma membrane, as assessed with fluorescent recovery after photobleaching (FRAP), was dependent on the presence of plasma membrane cholesterol and an intact actin cytoskeleton. Disruption of the actin cytoskeleton dramatically increased the fraction of CD44 which could be recovered from the light detergent-insoluble membrane fraction. Taken together, our data indicate that in mammary epithelial cells the vast majority of CD44 interacts with annexin II in lipid rafts in a cholesterol-dependent manner. These CD44-containing lipid microdomains interact with the underlying actin cytoskeleton.
Keywords: CD44, annexin II, epithelial cell line, lipid rafts, cytoskeleton
Lipids and lipid derivatives participate in signal transduction events occurring on the plasma membrane, and regulate plasma membrane–cytoskeleton interactions (Chong et al. 1994; Sun et al. 1995; Hirao et al. 1996). Moreover, recent developments put forward a concept that certain lipids can organize plasma membrane microdomains, called lipid rafts, representing high-order structures. These microdomains are thought to exist as liquid-ordered phases, characterized by a high degree of acyl chain order, which contain sphingolipids with their predominantly saturated hydrocarbon tails and cholesterol (see reviews Brown and London 1998; Ikonen and Simons 1998; Brown 1998). The intervening regions are envisioned to be in liquid-disordered membrane phase occupied by unsaturated phosphatidyl-choline molecules. Sphingolipid-cholesterol rafts are relatively insoluble in nonionic detergents (e.g., Triton X-100) on ice, and can be recovered together with certain membrane proteins as insoluble complexes called detergent-insoluble glycolipid-enriched domains (DIGs)1 (Parton and Simons 1995). DIGs can be biochemically distinguished from detergent-insoluble cytoskeleton-associated material by density gradient centrifugation, where, due to their high lipid content, they float at a low density, while detergent-insoluble cytoskeletal assemblies remain at higher densities.
Cholesterol-rich microdomains differ in size, dynamics, and protein composition. One type are caveolae, morphologically defined as flask-shaped, noncoated invaginations of the plasma membrane (for reviews see Lisanti et al. 1995; Parton 1996; Shaul and Anderson 1998). They are stabilized by oligomers of the integral membrane protein caveolin/VIP21, which in roughly equimolar stoichiometry binds cholesterol (Parton and Simons 1995; Parton 1996; Scheiffele et al. 1998). There are several possible functions assigned to caveolae, including participation in signal transduction (Shenoy-Scaria et al. 1994; Mineo et al. 1996), lipid transport (Fielding and Fielding 1997; Thyberg et al. 1998; Uittenbogaard et al. 1998), potocytosis (Smart et al. 1996), and endocytosis/transcytosis (Benlimame et al. 1998; Orlandi and Fishman 1998; Schnitzer et al. 1994). Recent findings indicate that lipid rafts can also exist independently of caveolae (for reviews see Parton and Simons 1995; Harder and Simons 1997; Ikonen and Simons 1998). They are <70 nm in size and contain a restricted number of membrane proteins and lipids. Their existence in living cells was shown recently by assays using chemical cross-linking and measurement of energy transfer between fluorescently labeled membrane proteins (Friedrichson and Kurzchalia 1998; Varma and Mayor 1998). Partition of peripheral proteins and transmembrane receptors into cholesterol-rich domains such as lipid rafts or caveolae is in a dynamic equilibrium driven by raft cohesion and counteracted by entropy-driven dispersal. Lipid rafts can fuse to form larger and more stable complexes or move to preexisting caveolae (Harder and Simons 1997).
Proteins can associate with lipid rafts via at least three different modes, partitioning into the outer leaflet of lipid bilayer via glycosyl-phosphatidylinositol (GPI) anchors (Mayor et al. 1994), associating with the inner leaflet via acylation, palmitolyation, or direct interaction with cholesterol (Shenoy-Scaria et al. 1994; Parton et al., 1996; Mumby 1997), and/or, for transmembrane proteins such as the hemagglutinin of influenza virus, partitioning into lipid rafts due to the intrinsic properties of transmembrane domains, such as a stretch of hydrophobic residues in contact with the outer leaflet of the plasma membrane (Scheiffele et al. 1997).
The cell adhesion molecule CD44, the major cell surface receptor for hyaluronic acid, partitions into lipid rafts in fibroblasts (Neame et al. 1995; Perschl et al. 1995a). Moreover, CD44 associates with the activated protein tyrosine kinases Lck and Fyn in cholesterol-rich Triton X-100–insoluble plasma membrane domains of human peripheral blood lymphocytes (Ilangumaran et al. 1998). CD44 is involved in a variety of important biological events such as pattern formation in embryogenesis, hematopoiesis, lymphocyte homing and activation, inflammatory reactions and tumor dissemination (for reviews see Ruiz et al. 1995; Günthert 1996; Naot 1997; Ponta and Herrlich 1998; Günthert 1999). CD44 represents a large protein family with most likely variable functional properties. The isoforms are generated by alternative splicing of transcripts, which results in the addition of various stretches of amino acids in the extracellular part of this protein, and subsequent variable glycosylation. However, all CD44 isoforms studied so far contain common transmembrane and cytoplasmic domains.
We have addressed the following questions: (a) Which type of cholesterol-rich microdomains associates with CD44 in mammary epithelial cells? (b) Which other proteins are recruited into CD44-containing microdomains? (c) Do CD44-containing microdomains interact with the cytoskeleton and how is such an interaction mediated?
Materials and Methods
Cells, Antibodies, and Reagents
Media, tissue culture reagents, and FCS were purchased from GIBCO BRL and Boehringer Mannheim. As a model system, we used EpH4 cells, a spontaneously immortalized mouse mammary epithelial cell line which displays a fully polarized epithelial cell phenotype (Fialka et al. 1996, Fialka et al. 1997). EpH4 cells were cultured on permeable filter supports at 37°C, 5% CO2, and 98% humidity in Eagle's medium, supplemented with 10 mM Hepes and KOH (pH 7.3), 50 IU/ml penicillin, 50 mg/ml streptomycin, and 5% FCS. For some of the immunolocalization experiments, Transwell™ filters (no. 3450; Corning Costar Corporation), 24-mm-diam, 0.4-μm pore size, were used. Transepithelial electrical resistance (TER) of filter-grown EpH4 monolayers was measured using a Millicell Electrical Resistance System (Millipore Corporation) equipped with an Endohm-24-electrode (World Precision Instruments) according to standard procedures.
Hybridoma clone IM7.8.1 (TIB-235; American Type Culture Collection) (producing rat anti–human/mouse pan CD44 antibodies) was propagated in medium with Ig-depleted FCS and secreted antibody were purified on protein G–Sepharose columns (Pharmacia). The anti–human annexin II antibody HH7 is a mouse monoclonal antibody recognizing murine annexin II (Thiel et al. 1992). Anti-p11 antibody H21 reactive with a trans-dominant mutant of annexin II was previously described (Harder and Gerke 1993). Mouse monoclonal antibody cross-reacting with mouse annexin II (A14020) and rabbit polyclonal antibody against caveolin (C13630) were from Transduction Laboratories and mouse monoclonal antibody against actin (mAb 1501) as well as rat monoclonal against ZO-1 (mAb 1520) were purchased from Chemikon. Mouse monoclonal antibody H68.4 recognizing mouse transferrin receptor was a generous gift from Dr. J. Trowbridge. Secondary antibodies used for immunofluorescence were from Jackson ImmunoResearch Laboratories: Cy3-goat anti–rat (712-165-150), Cy3-goat anti–mouse (715-160-150), FITC-goat anti–mouse (115-095-100); and from Molecular Probes: Alexa488-goat anti–rabbit (A-11008), Alexa488-goat anti–rat (A-11006) and Alexa488-goat anti–mouse (A-11001). Protease inhibitors aprotinin, pepstatin, leupeptin, and Pefabloc SC were from Boehringer Mannheim. LipofectAMINE PLUS™ Reagent was from GIBCO BRL. Reagents for cholesterol depletion included digitonin (D-1407) and methyl-β-cyclodextrin (M-β-CD, C-4555) both from Sigma-Aldrich. Rhodamine-phalloidin was purchased from Molecular Probes (R-415). Latrunculin A was a kind gift from Dr. J. Knoblich (IMP, Vienna).
Isolation of Glycosphingolipid-rich Triton X-100–insoluble Membrane Fractions
Confluent cell monolayers were washed twice with cold PBS, scraped in PBS and spun down at 2,000 rpm at 4°C. Cells were lysed in 200 μl of TN (25 mM Tris HCl, pH 7.5, 150 mM NaCl, 1 mM DTT, cocktail of protease inhibitors, 10% sucrose, and 1% Triton X-100) on ice, and incubated for 30 min on ice. Samples were mixed with 400 μl of cold Optiprep™, transferred into SW60 centrifuge tubes and overlaid with 600 μl of each 35%, 30%, 25%, 20%, and 0% Optiprep™ in TN. The gradients were spun at 35,000 rpm in SW60 rotor for 12 h at 4°C. Fractions were collected from top to bottom of centrifuge tubes, proteins were precipitated according to Wessel and Flügge 1984 followed by Western blotting analysis. Only in case of the anti-panCD44 antibody (IM7.8.1) nonreducing SDS-PAGE was applied.
Sequestration of Membrane Cholesterol and Cholesterol Measurements
The cholesterol sequestration in vivo was performed with M-β-CD (Klein et al. 1995) in confluent cultures. Monolayers were washed once with PBS, then the prewarmed medium, containing 5 mM of M-β-CD, was added and cells were incubated for 15 min at 37°C. Cholesterol sequestration in isolated membrane fractions was achieved by treatment of pelleted membranes with varying concentrations of digitonin for 30 min on ice. The cholesterol content of pelleted membranes before and after treatment was determined by an enzymatic-fluorometric assay (Heider and Boyett 1978). Values were normalized to total membrane protein.
Clustering of CD44 by Antibody and Expression of the Trans-dominant Annexin II Mutant
Sparsely grown cells were incubated with anti-panCD44 antibody IM7.8.1 (10 μg/ml) diluted in normal Eagles's medium for 10 min at 37°C, followed by three washes in PBS, containing 1 mM CaCl2, 1 mM MgCl2, and 0.5% gelatin. Control cells were fixed in acetone and methanol before treatment with secondary Cy3-labeled goat polyclonal anti–rat antibodies to visualize CD44 staining. Clustering of CD44 was induced in the remaining cell samples by incubation with Cy3-labeled goat polyclonal anti–rat antibodies (20 μg/ml) in the normal Eagle's medium for 20 min at 37°C; thereafter, the cells were rinsed and fixed as above. All cell samples were then double stained with antibodies against annexin II or VIP21 and caveolin.
The construction of the trans-dominant mutant of annexin II was described previously (Harder and Gerke 1993). Transient transfections of sparse EpH4 cells were performed using LipofectAMINE PLUS™ Reagent according to the manufacture's recommendations.
Permeabilized EpH4 Cell System, GTPγS, and Phalloidin-TRITC Treatment
Cells were permeabilized as described in Mackay et al. 1997. In brief, confluent, filter-grown EpH4 cells were permeabilized at 37°C for 10–20 min using 0.003% digitonin in DK buffer (150 mM potassium glutamate, 10 mM Hepes and KOH, pH 7.3, 5 mM glucose, 2 mM MgCl2, and 0.4 mM EGTA), supplemented with an ATP-regenerating system (1 mM ATP, 5 mM creatine phosphate, and 10 μg/ml creatine phosphokinase), DTT as an antioxidizing agent and a cocktail of protease inhibitors. 50 μM of GTPγS or 0.05–0.1 μM of rhodamine-phalloidin was added to the permeabilized cells where indicated.
Immunofluorescence and Laser Confocal Microscopy
Cells were washed twice in PBS, fixed with acetone and methanol (1/1) at −20°C for 2 min, and air-dried. The cells were then washed with PBS and incubated in blocking buffer (0.5% gelatin in PBS containing 1 mM CaCl2, and 1 mM MgCl2) at room temperature for 30 min in order to decrease nonspecific antibody binding. Subsequently, cells were incubated for 1 h at room temperature with primary antibodies diluted in blocking buffer, followed by three washes in blocking buffer. Secondary antibodies were applied in the same way. The samples were mounted in Moviol and processed for microscopy. All confocal microscopy images were obtained using a Leica TCS NT confocal microscope (Leitz). Images were processed using an Octane Workstation (Silicon Graphics, Inc.) using the Huygens (Scientific Volume Imaging), Imaris, and Colocalization (Zitplane AG) software packages.
Fluorescence Recovery after Photobleaching
The sparse EpH4 cells grown on coverslips were washed briefly with fresh medium and incubated with FITC-labeled antistandard CD44 antibody IM7.8.1 (10 μg/ml) diluted in normal Eagles's medium for 20 min at room temperature. For the labeling of the transferrin receptor K+-depleted EpH4 cells (Hansen et al. 1993) were incubated with FITC-labeled transferrin (50 μg/ml) for 20 min on ice. The cells were rinsed with medium to remove nonbound antibody and the coverslips were assembled into the perfusion chamber (H. Saur Laborbedarf, Reutlingen, Germany) and connected to the peristaltic pump, allowing the exchange of the medium and maintaining cells at 37°C. Initial imaging, photobleaching of the selected regions of the plasma membrane, and recording of the fluorescent recovery were performed in a Leica TCS NT confocal microscope system (Leitz). The bleached areas had a surface area of ∼40 μm2. The fluorescence recovery was recorded immediately using a time-lapse option of the system. The data obtained were analyzed using TCS NT quantification software. The intensities of the bleached and recovered regions were normalized in regard to the fluorescent intensity of the independent region in the same samples. The percentage of recovery was calculated as Ir /Ii, multiplied by 100%, where Ir is the intensity of the photobleached region after recovery and Ii is the intensity of the selected region before photobleaching.
Results
CD44 and Annexin II Partition into Lipid Rafts and Colocalize in the Basolateral Plasma Membrane of Mouse Mammary Epithelial Cells
In fibroblasts, ≤50% of CD44 is resistant to Triton X-100 extraction (Lacy and Underhill 1987; Neame et al. 1995; Perschl et al. 1995a). However, in Madin-Darby canine (MDCK) and bovine (MDBK) kidney epithelial cells CD44 was completely soluble in Triton X-100 and no association of CD44 with the cytoskeleton could be demonstrated (Neame and Isacke 1993; Neame et al. 1995). We used the Triton X-100 solubility assay to explore a possible cytoskeletal association of CD44 in the EpH4 mammary epithelial cell line. The majority of CD44 remained in the insoluble fraction after Triton X-100 extraction at concentrations up to 1% (data not shown).
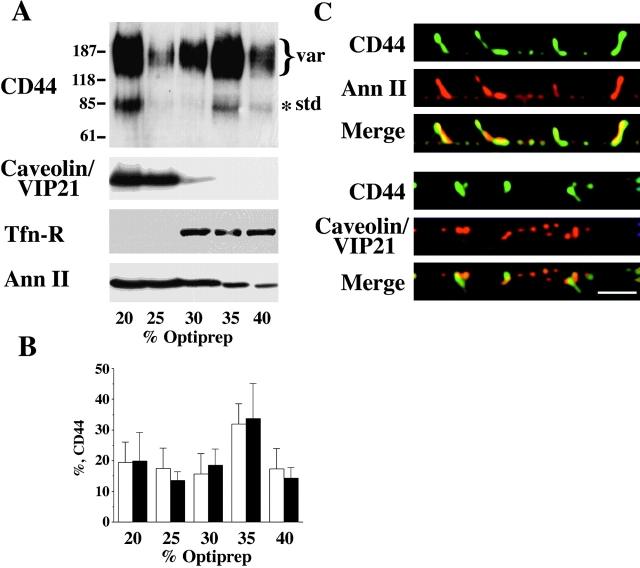
We tested whether Triton X-100 insolubility of CD44 could be a consequence of its localization in Triton X-100–insoluble membrane domains (Neame et al. 1995; Perschl et al. 1995a; Ilangumaran et al. 1998). Therefore, we performed floatation experiments in Optiprep™ gradients in the presence of Triton X-100. In this gradient, detergent-insoluble, glycosphingolipid-rich membrane fractions representing the lipid rafts will float to the interphase between the 0% and 20% Optiprep™ layers. Solubilized proteins or cytoskeleton-associated, detergent-insoluble proteins remain at the bottom of the gradient. In EpH4 cells, a fraction of CD44 isoforms floated to low density fractions, together with the detergent-resistant glycosphingolipid-rich membranes (Fig. 1 A). There were several bands detected by anti-panCD44 antibody (IM7.8.1) by Western blot, due to the fact that there are several CD44 isoforms expressed in EpH4 cells, as revealed by RT-PCR (data not shown). However, all isoforms partitioned into the lipid raft fractions with the same efficiency. The total amount of CD44 that could be recovered from lipid raft fractions under experimental conditions described above was determined as a result of three independent experiments, indicating that in a steady state ∼35% of CD44 partitions into lipid rafts as assessed by Optiprep™ gradient floatation, followed by Western blot (Fig. 1 A). Caveolin/VIP21, a known cholesterol-binding protein, was found in the same lipid raft fractions. A second lipid-binding protein, annexin II, also efficiently partitioned to this fraction (Fig. 1 A). However, addition of the Ca2+-chelating agent EDTA to the homogenization buffer reduced this efficiency to <10%, while CD44 distribution over the gradient did not change (data not shown). This suggested a Ca2+-dependent association of annexin II with cholesterol-rich membranes. In contrast, the transferrin receptor, a transmembrane protein localized to clathrin-coated pits, was always found to be associated with heavier fractions and never occurred in lipid rafts, confirming the correct partitioning of different membrane proteins in the gradient (Fig. 1 A).
Figure 1.
CD44 and annexin II are recovered in the lipid raft fractions and colocalize in the basolateral plasma membrane of EpH4 cells. Cells were lysed in buffer containing 1% Triton X-100 on ice in the absence of Ca2+-chelating agents. Floatation was performed in Optiprep™ gradients and fractions were collected, precipitated, and then equal amounts of total proteins were analyzed by Western blotting using antibodies to CD44, caveolin/VIP21, transferrin receptor (Tfn-R), and annexin II (A). Anti-panCD44 antibody IM 7.8.1 recognizes a standard 85-kD isoform and a number of high molecular mass variant isoforms. CD44 and caveolin/VIP21 are localized to the floated raft fractions. As expected, the control protein transferrin receptor could not be floated to the lipid raft fraction. The bulk of a lipid-binding protein annexin II can be found in the lipid raft fraction, too. Molecular mass markers are given in kilodaltons. When fractions from the Optiprep™ gradients were loaded by yield, the total efficiency of CD44 partition into the lipid rafts was determined as a result of three independent experiments (B). The mean percentages of standard (empty bars) and variant (full bars) CD44 isoforms with standard deviations are given. Confluent filter-grown monolayers of EpH4 were fixed in acetone and methanol and subjected to indirect immunofluorescence using anti-CD44 plus anti–annexin II or anti-CD44 plus anti-caveolin/VIP21 antibodies, respectively. Vertical (x-z axis) section images taken by confocal microscopy and image processing (see Materials and Methods) are shown. CD44 colocalized with annexin II in the basolateral plasma membrane (C, upper panel). However, CD44 virtually did not colocalize with caveolin/VIP21 (C, lower panel). Bar, 10 μm.
Our biochemical analysis suggested that CD44 occurred in detergent-insoluble lipid rafts, together with caveolin/VIP21 and annexin II. Both proteins are located basolaterally in EpH4 cells (Fig. 1 C). However, they may be incorporated into different subtypes of basolateral lipid rafts. Caveolin/VIP21 is a structural component of caveolae (Parton 1996), but a minor fraction is localized within the entire plasma membrane (Wary et al. 1998). In EpH4 cells, CD44 isoforms mostly failed to colocalize with caveolin/VIP21, indicating that CD44-containing lipid rafts represent structures distinct from caveolae (see lower part of Fig. 1 C for x-z axis visualization). However, CD44 was found to colocalize with annexin II along the basolateral plasma membrane domain (Fig. 1 C, upper part).
Colocalization of annexin II with CD44 in the lateral membrane of EpH4 cells was dependent on an intact lipid environment. To reduce the plasma membrane cholesterol concentration, we treated cells with the cholesterol-sequestering drug, M-β-CD (Klein et al. 1995), before fixation. M-β-CD forms stable inclusion complexes with cholesterol by incorporating it into its hydrophobic cavity. Typically, treatment of confluent EpH4 monolayers reduced cholesterol from 25 to 9.5 μg cholesterol/mg membrane protein as revealed by an enzymatic fluorometric assay (Heider and Boyett 1978). Upon depletion of membrane cholesterol by M-β-CD, both CD44 and annexin II redistributed to the entire plasma membrane, including the apical domain and were no longer colocalized (data not shown).
Annexin II Can Be Recruited into Antibody-induced CD44 Clusters
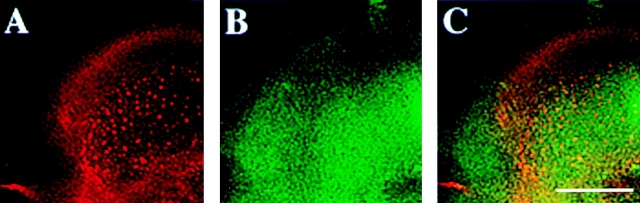
One of the possibilities to selectively analyze microdomains enriched in CD44 is to use in vivo antibody labeling of CD44 followed by cross-linking of CD44–antibody complexes with secondary antibodies in living cells. In sparsely grown EpH4 cells labeled with primary anti-CD44 antibodies followed by immediate fixation, both CD44 and annexin II were diffusely distributed all over the plasma membrane (Fig. 2, A–C). After cross-linking of CD44 in intact cells with anti-CD44 plus secondary antibodies, we observed that most of CD44 accumulated in big patches on the plasma membrane. Interestingly, these patches also contained annexin II, apparently recruited into these complexes at the cytosolic face of the plasma membrane (Fig. 2, D–F). In contrast, no significant colocalization of CD44 and caveolin/VIP21 was observed using anti-caveolin/VIP21 antibodies, either before (Fig. 2, G–I) or after antibody cross-linking of CD44 (Fig. 2, J–L). These results were consistent with our hypothesis that CD44-containing lipid rafts were distinct from the caveolae submembranous compartment.
Figure 2.
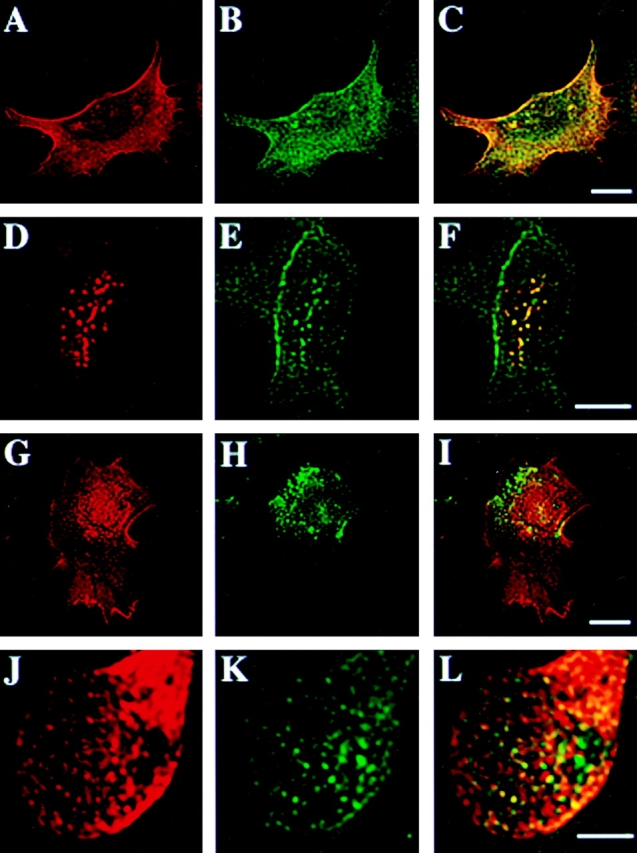
Annexin II is efficiently recruited to patches of antibody cross-linked CD44 microdomains. EpH4 cells grown at low cell density on coverslips were labeled with anti-CD44 antibody. Control samples (A–C and G–I) were fixed and then stained with secondary goat anti–rat antibody, while experimental samples were treated with secondary anti–rat IgG before fixation, causing in vivo cross-linking of CD44 on the cell surface (D–F and J–L). Control and experimental samples were then counterstained with anti-annexin II antibody (A–F) or anti-caveolin/VIP21 antibody (G–L). In control cells, CD44 (A) and annexin II (B, see merged images in C) were evenly distributed over the entire plasma membrane. Upon antibody cross-linking CD44 as well as annexin II could be found in patch-like clusters on the surface of the cells (D, E, merge see F). In contrast, caveolin/VIP21 was located in larger spots most likely representing caveolae before or after antibody cross-linking of CD44 and failed to colocalize with CD44 in control- (G–I) or cross-linked (J–L) cells. Bar, 10 μM.
We previously found that the colocalization of CD44 and annexin II in lipid rafts was impaired after depletion of the cells from cholesterol by M-β-CD (data not shown). Therefore, it was of interest to analyze if M-β-CD would also impair the formation of patches after CD44 antibody cross-linking and prevent the inclusion of annexin II into these patches. Interestingly, the ability of CD44 to form clusters upon antibody cross-linking was significantly reduced by pretreatment of cells with 5 mM M-β-CD. Smaller clusters formed upon cross-linking failed to recruit annexin II (Fig. 3, A–C).
Figure 3.
CD44 clustering induced by antibody cross-linking is significantly impaired in cholesterol-depleted cells. Sparsely seeded EpH4 cells (see Fig. 2) were pretreated with M-β-CD and processed as described in the legend to Fig. 2, using only costaining with anti-annexin II antibody. In M-β-CD–treated cells, antibody cross-linking of CD44 produced much smaller clusters (A) than in untreated cells, (compare with Fig. 2 D) and annexin II (B, merge in C) was no longer recruited to these small patches. Bar, 10 μm.
Aggregation of a Trans-dominant Annexin II Mutant Induces Coclustering of CD44 and the Minor Rearrangements of the Actin Cytoskeleton
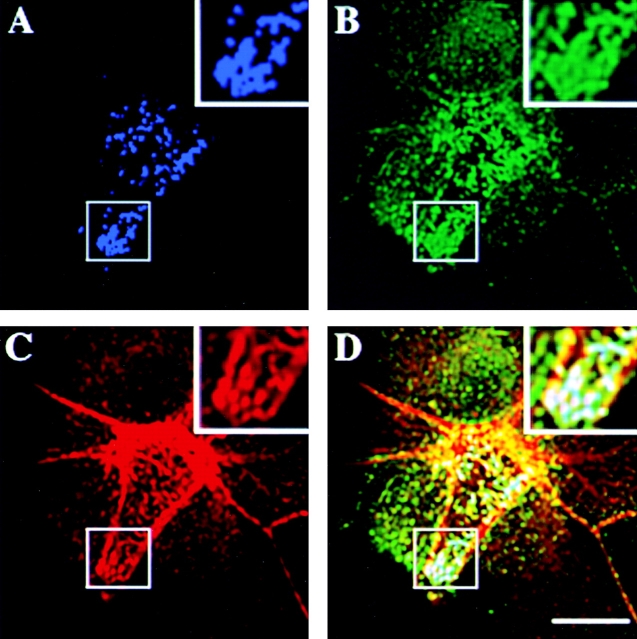
In most cell lines studied so far, annexin II exists as a heterotetramer bound to its ligand p11. The chimeric p11–annexin II protein inducing the aggregation of endogenous annexin II and p11 was previously described (Harder and Gerke 1993). In brief, it is a chimeric protein comprising the NH2-terminal domain of annexin II fused to the NH2 terminus of p11. As a result, this mutant has two binding sites for p11 and one for annexin II. p11 dimers bound to this chimera would accommodate additional sites for binding of chimera and/or annexin II–p11 complexes, leading to the formation of the large aggregates containing annexin II, p11, and the chimeric protein. In nonpolarized EpH4 cells transiently expressing the mutant, the appearance of large annexin II–p11-positive clusters underneath the plasma membrane was observed (Fig. 4 A). The extent of aggregate formation was dependent on the levels of expression. Most interestingly, CD44 was also redistributed in the plasma membrane of transfected cells, largely colocalizing with trans-dominant annexin II (Fig. 4 B). Actin cytoskeleton was rearranged in transfected cells, and the ends of the actin bundles were often found colocalizing with the clusters of trans-dominant mutant (Fig. 4 C, see D for the merge of three colors).
Figure 4.
Aggregation of trans-dominant mutant of annexin II causes coclustering of the plasma membrane CD44. Sparsely grown EpH4 cells were transiently transfected with expression construct pCMV XM driving the expression of trans-dominant mutant of annexin II. After ∼48 h the expression of this construct caused formation of the relatively large aggregates of mutant annexin II underneath the plasma membrane (A). As evident from comparison with surrounding nontransfected cells, CD44 in transfected cells was found to cocluster with annexin II–p11 aggregates, visualized by p11 antibody H21 (B). In some cases actin cytoskeleton was rearranged too and actin fibers were colocalizing with CD44–annexin II clusters (C). Merge image of this triple-labeling experiment is shown in D. There are zoomed-in images of the regions of the plasma membrane on the magnified insets in corresponding panels. Bar, 10 μm.
CD44/Annexin II–containing Lipid Rafts Can Be Stabilized in the Basolateral Plasma Membrane following the Increased Polymerization of the Actin Cytoskeleton
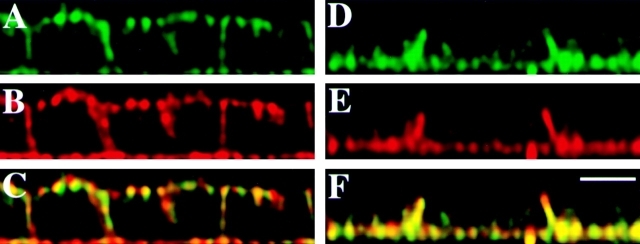
To further characterize CD44/annexin II–containing lipid rafts, we tested whether the stability of these complexes with respect to cholesterol depletion was dependent on direct or indirect interaction with the actin cytoskeleton. The first approach was to test if active GTP-binding proteins were required for CD44-containing lipid raft stability, using a nonhydrolyzable GTP analogue (GTPγS). To allow the use of GTPγS, we adapted a method to generate semipermeabilized cells described by Mackay et al. 1997 to our polarized, filter-grown EpH4 cells. For this, EpH4 cells were permeabilized by very low concentrations of digitonin (0.003%), a known cholesterol-sequestering detergent (Elias et al. 1978). This treatment depleted cholesterol from EpH4 cells twice as efficient than M-β-CD (from 25 to 3.9 μg cholesterol/mg membrane protein, see above). The permeabilized cells were supplemented with an ATP-regenerating system, a cocktail of protease inhibitors, and with DTT as antioxidant. Under these conditions we could observe the redistribution of CD44 and annexin II over the entire plasma membrane (Fig. 5, A–C). However, when nonhydrolyzable GTPγS was added to the cells during digitonin treatment no redistribution to the apical membrane occurred, and CD44 and annexin II continued to be colocalized in the basolateral plasma membrane domain (Fig. 5, D–F).
Figure 5.
Activation of GTP-binding proteins by GTPγS prevents redistribution of CD44 and annexin II upon depletion of plasma membrane cholesterol by digitonin. Confocal vertical (x-z) sections of digitonin-treated EpH4 cells are shown. In filter-grown cells subjected to cholesterol depletion and semipermeabilization with digitonin, both CD44 (A) and annexin II (B) could be found at the apical surface after fixation and respective antibody staining. However, when GTPγS was added to the permeabilization buffer, neither CD44 (D) nor annexin II (E) underwent redistribution, but remained colocalized at the basolateral cell membrane domain. Merged images are shown in C and F. Bar, 10 μm.
One obvious consequence of arresting small Rho-GTPases in their active state (as caused by GTPγS) is the stabilization of filamentous actin cables (Ridley and Hall 1992), as suggested by our observation that this treatment resulted in appearance of thicker bundles of stress fibers and a more prominent subcortical actin ring (not shown). Thus, a possibility to explain the above results was that GTPγS treatment stabilized CD44-containing lipid rafts in the basolateral plasma membrane through their enhanced interaction with stabilized, underlying actin cables under these experimental conditions. To test for this possibility, we chose an alternative way to stabilize F-actin filament bundles, using the fungal toxin phalloidin. The phalloidin-stabilized actin filaments still retain some of their functional properties; for example, they are able to move on solid-phase myosin substrates (Kinosita et al. 1991). This experiment was performed in the same digitonin-semipermeabilized EpH4 cell system as described above. While digitonin permeabilization caused redistribution of CD44 in the control cells (Fig. 6 A), treatment of the cells with phalloidin during digitonin-induced cholesterol depletion fully mimicked the activity of GTPγS. CD44 remained undetectable on the apical surface of phalloidin-treated cells after the depletion of plasma membrane cholesterol (Fig. 6 C). At the same time, the subcortical actin ring was stabilized (Fig. 6 D). These results suggest that the stability of lipid rafts containing CD44 also requires intact actin filament bundles and/or an interaction of the proteins present in the lipid rafts with the underlying cytoskeleton.
Figure 6.
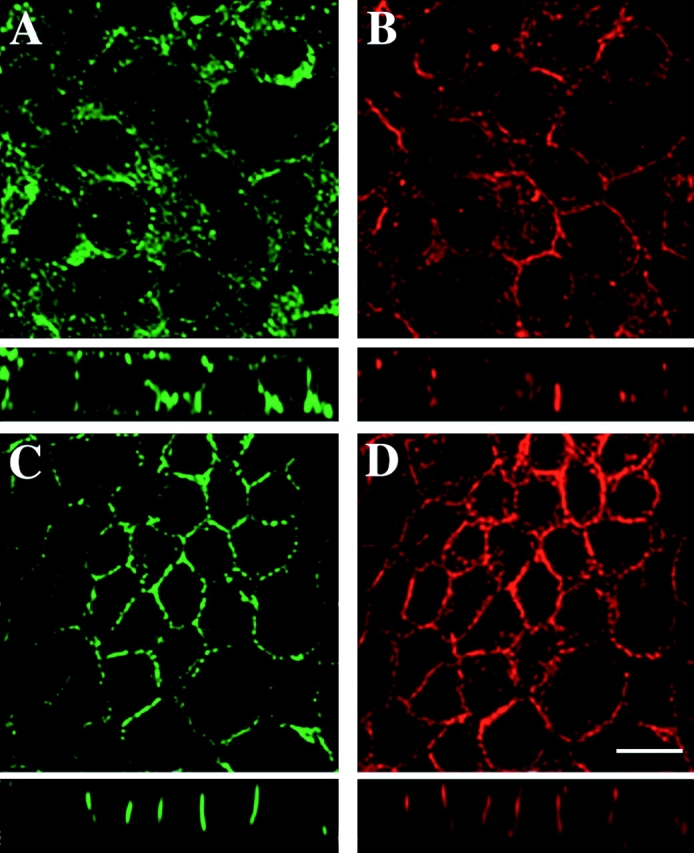
Stabilization of actin by phalloidin prevents redistribution of CD44 upon digitonin-induced depletion of plasma membrane cholesterol. Extended focus images and vertical (x-z) sections are shown. In digitonin-permeabilized EpH4 cells, CD44 stained by the respective antibody was redistributed to the apical surface (A; similar to the experiment shown in Fig. 5). The actin cytoskeleton is visualized by phalloidin-rhodamine, failing to show an extensive subcortical actin ring in many cells (B). When phalloidin was added to the permeabilization buffer (see Fig. 5), CD44 remained precisely localized to the basolateral plasma membrane domain (C). Phalloidin-rhodamine showed enhanced staining of filamentous actin and stabilization of the subcortical actin ring upon treatment with phalloidin (D). Bar, 10 μm.
CD44-containing Lipid Rafts Interact with the Underlying Actin Cytoskeleton
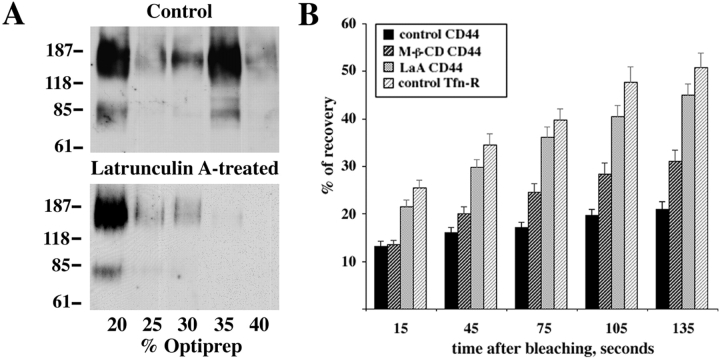
Looking for the biochemical evidence that CD44-containing lipid rafts interact with the actin cytoskeleton, we decided to treat EpH4 cells with the actin cytoskeleton-disrupting drug latrunculin A before the lipid rafts floatation experiment. To our surprise, the proportion of CD44 in the detergent-insoluble, glycosphingolipid-rich membrane fractions increased dramatically (Fig. 7 A, lower panel), as compared with the mock-treated control (Fig. 7 A, upper panel). These results indicate that a significant bulk of CD44-containing lipid rafts is not capable of floating up to the light lipid rafts fractions due to its interaction with the heavy cytoskeleton elements. This experiment further implied that the vast majority of CD44 molecules in EpH4 cells is localized to the lipid rafts.
Figure 7.
CD44-containing lipid rafts interact with the actin cytoskeleton. To further assess the possibility of the direct interaction of CD44-containing lipid rafts with the actin cytoskeleton, the Optiprep™ gradient floatation following the disruption of the actin cytoskeleton by latrunculin A was performed. A vast majority of CD44 was now found in the floating lipid rafts–rich fraction (A, lower panel) in comparison with control cells (A, upper panel). Molecular mass markers are given in kilodaltons. The measurements of FRAP of CD44 in the plasma membrane of EpH4 cells were made as described in Materials and Methods and percentages of recovery of labeled proteins are shown (B). The lateral mobility of CD44 was dependent on the intact actin cytoskeleton and the presence of the plasma membrane cholesterol. The transmembrane protein of clathrin-coated pits, the transferrin receptor, was used as a control. Mean ± SE values are shown.
To obtain complimentary evidence that CD44-containing lipid rafts do indeed interact with the underlying actin cytoskeleton, we employed a technique known as fluorescence recovery after photobleaching (FRAP). In brief, CD44 molecules on the surface of living cells were labeled with FITC-labeled anti-panCD44 antibody IM7.8.1. The selected regions of the plasma membrane were bleached and the subsequent recovery of the bleached regions was recorded. The absolute values obtained were normalized by comparison with the fluorescent intensities in an independent region of identical size. These lateral diffusion measurements indicated that CD44 molecules were virtually immobilized in the plasma membrane of control cells, as compared with the transferrin receptor, a transmembrane protein of clathrin-coated pits (Fig. 7 B) or free lipid dye BODIPY-FL-sphingomyelin (data not shown). The FRAP measurements of the lateral mobility of the transferrin receptor were performed in K+-depleted cells in order to prevent receptor internalization (Hansen et al. 1993). However, the lateral mobility of CD44 was notably improved in the cells pretreated with either M-β-CD or actin disrupting drug latrunculin A (Fig. 7 B). For instance, the percentage of recovery of the photobleached region in control cells after 105 s was ∼19%, in comparison with 28% and 40% in M-β-CD–treated and latrunculin A–treated cells, respectively. This finding indicates that lateral mobility of CD44 molecules in the plasma membrane is dependent on the presence of the plasma membrane cholesterol and an intact actin cytoskeleton, again suggesting the direct interaction of lipid raft–associated CD44 with underlying cytoskeleton.
Discussion
Association of CD44 and Annexin II with the Same Type of Lipid Rafts
In unpolarized, sparsely seeded epithelial cells, CD44 distributes over the entire plasma membrane, including microvilli, and areas of cell-to-cell contacts. As epithelial cells polarize CD44 redistributes to the basolateral surface of the plasma membrane and all isoforms looked at so far localize exclusively within this plasma membrane domain (Isacke 1994). In MDCK cells the localization and restriction of the standard form of CD44 (CD44s) to the lateral cell surface depends on a critical dileucine motif in the cytoplasmic tail of the protein (Neame and Isacke 1993). This motif serves as a dominant localization signal since it is also able to redirect the normally apical protein placental alkaline phosphatase (PLAP) to the basolateral plasma membrane (Sheikh and Isacke 1996).
In polarized EpH4 mammary epithelial cells, the standard and several variant CD44 isoforms are expressed endogenously and all localize to the basolateral plasma membrane domain. Previous studies in polarized MDCK cells showed that CD44 was completely Triton X-100 soluble (Neame and Isacke 1993; Isacke 1994; Neame et al. 1995). However, we found in EpH4 cells that a majority of CD44 could not be extracted by Triton X-100 at 4°C. Other authors reported previously that CD44 insolubility can be attributed to its partition into Triton X-100–insoluble lipid microdomains in certain cell types (Neame et al. 1995; Ilangumaran et al. 1998). To test this hypothesis, we floated Triton X-100–insoluble material on Optiprep™ density gradients and were able to show that in EpH4 cells a significant portion of all CD44 isoforms was present in lipid rafts.
How could CD44 partition into basolateral lipid rafts in epithelial cells? CD44 might first partition into basolateral TGN-derived vesicles, due to its active basolateral sorting signal. Upon arrival at the basolateral membrane domain, CD44 might then partition into lipid rafts due to the intrinsic properties of its transmembrane domain. Alternatively, CD44 might partition into cholesterol-rich membranes already in the TGN, causing these vesicles to be sorted basolaterally instead of apically, due to the dihydrophobic sorting motif Leu331/Val332. This scenario would result not only in proper basolateral sorting of CD44, but would also recruit cholesterol-rich membranes to the basolateral surface, allowing CD44 to serve as an assembly anchor for basolateral lipid rafts.
One of the proteins expected to localize to cholesterol-rich membrane domains was caveolin/VIP21. However, the bulk of CD44 did not colocalize with caveolin/VIP21 in EpH4 cells and the observed minor colocalization did not occur in deep plasma membrane invaginations indicative of caveolae. A considerable degree of overlap has been previously observed between CD44 and annexin II in Triton X-100–extracted fibroblasts, as revealed by immunofluorescence analysis (Neame and Isacke 1993). We observed a colocalization between CD44 and annexin II in EpH4 cells. In addition, the depletion of membrane cholesterol by M-β-CD abolished this colocalization, causing redistribution of both CD44 and annexin II over the entire cell surface, including the apical plasma membrane. This drug preferentially extracts cholesterol from the outer leaflet of the plasma membrane and partially liberates transmembrane and GPI-anchored proteins from lipid rafts, leaving caveolae virtually intact (Ilangumaran and Hoessli 1998). Together, these observations suggest that CD44 partitions into lipid rafts but not into caveolae.
Annexin II is a Ca2+-binding protein present in a wide variety of cells and tissues which can associate with actin filaments and membranes. Within cells, annexin II occurs either as a 36-kD monomer (p36) or as a heterotetrameric complex (p90) coupled with the S-100–related protein, p11 (Kube et al. 1992; Harder and Gerke 1994). Annexin II has been suggested to be involved in membrane transport, both the regulated exocytic pathway (Creutz 1992) and the endocytic pathway (Emans et al. 1993; Harder and Gerke 1993). In addition, annexin II is a major cellular substrate for the src-family of protein kinases (Hubaishy et al. 1995) which partition into lipid rafts in various cell types (Rodgers et al. 1994; Shenoy-Scaria et al. 1994). Also it has been implicated that annexin II might interact with the submembranous actin network with this activity depending on its ability to form the heterotetrameric annexin II–p11 complex and on the presence of intact Ca2+-binding sites (Thiel et al. 1992). It has been shown previously that interaction of the annexin II–p11 heterotetramer with the plasma membrane, but not with the submembranous cytoskeleton, induces the tyrosine phosphorylation of annexin II, presumably exposing the tyrosine-phosphorylation site as a consequence of induced conformational changes (Bellagamba et al. 1997). Moreover, recent work indicated that approximately half of the cellular annexin II Ca2+ independently binds to BHK membranes where it may be involved in organizing lipid microdomains (Harder et al. 1997). Interestingly, this pool of annexin II is released upon cholesterol sequestration. In EpH4 cells only a minor fraction of annexin II was associated with detergent-insoluble membranes in a Ca2+-independent manner (data not shown), while the bulk of cellular annexin II was bound to rafts in the presence of Ca2+. Our findings also clearly agree with the earlier observation that the annexin II– p11 tetramer is significantly enriched in the Triton X-100–insoluble fraction of MDCK cells in the presence of Ca2+ (Harder and Gerke 1994). It is tempting to speculate, that the interaction of annexin II with lipid rafts in living cells may be regulated by elevated intracellular Ca2+ concentrations and/or phosphorylation. Interestingly, another member of the annexin family, annexin XIIIb was also found in the Triton X-100–insoluble fraction of MDCK cells. Annexin XIIIb is associated with the apical plasma membrane, enriched in apical transport vesicles and is involved in apical delivery of TGN-derived transport vesicles (Lafont et al. 1998). Thus, annexin II and annexin XIIIb associate with Triton X-100–insoluble fractions from different cellular locations. These data suggest that, at least in MDCK and EpH4 cells, different annexins might organize rafts and/or modulate raft function in distinct locations; e.g., by stabilizing inner leaflets of sphingolipid-cholesterol rafts (Harder and Simons 1997).
Artificial clustering of the smallest 85-kD isoform of CD44 (CD44s) has previously been shown to promote binding of the protein to soluble hyaluronic acid (HA) (Lesley et al. 1993; Perschl et al. 1995b). In rat pancreatic carcinoma cells, CD44 splice variants, but not the standard CD44, form molecular aggregates within the plasma membrane. It was postulated that the regulation of clustering, mediated by either the presence of variant exons and/or glycosylation, allows cells in turn to regulate the binding properties of CD44 to HA (Sleeman et al. 1996). It was also shown that Cys286 within the transmembrane domain of CD44 was required for TPA-induced covalent dimerization of CD44 and enhanced HA binding (Liu and Sy 1997).
These observations prompted us to cluster CD44 on the cell surface by antibodies. This clustering of CD44 induced efficient coclustering with annexin II. Significant amounts of annexin II at the cytoplasmic face of the plasma membrane were recruited into these clusters. Most importantly, both clustering of CD44 by antibodies and recruitment of annexin II to these clusters were dependent on the presence of membrane cholesterol. The fact that the antibody-induced patches were practically devoid of caveolin/VIP21 again confirmed that CD44–annexin II lipid rafts were distinct from caveolae. Neither did CD44 colocalize with the transferrin receptor (not shown), an integral membrane protein of clathrin-coated pits which is virtually excluded from lipid rafts (Harder et al. 1998).
The possibility of artificial clustering of annexin II was previously described (Harder and Gerke 1993). Expression of an annexin II–p11 chimera, acting as a trans-dominant mutant, causes aggregation of annexin II–p11 tetramers. This therefore interferes with the normal distribution of annexin II, and may also alter annexin II–dependent proteins and/or structures. We observed the formation of annexin II–positive clusters underneath the plasma membrane of transiently transfected cells. These complexes also contained CD44 and, interestingly, the actin cytoskeleton was rearranged. Actin bundles were either surrounding or coclustering with annexin II aggregates. In previous work using the same mutant it was reported that in MDCK cells the formation of annexin II–p11 aggregates did not affect the actin-based cell cortex, but translocated early endosomes (Harder and Gerke 1993). The difference between aggregates formed in MDCK and EpH4 cells and their morphological consequences can be attributed to the different cell types used and distinct experimental techniques. Our observation that annexin II aggregation caused both coclustering of CD44 and rearrangements of the actin cytoskeleton strongly suggested that annexin II might participate in a functionally important clustering of CD44-containing lipid rafts in the plasma membrane and their coupling to the actin cytoskeleton.
CD44-containing Lipid Rafts Interact with the Actin Cytoskeleton
In fibroblasts, CD44 was previously found to interact with proteins of the ERM (ezrin-radixin-moesin) family, which are peripheral actin cross-linkers (Hirao et al. 1996). In turn, ERM proteins directly interact with Rho GDP dissociation inhibitor (RhoGDI). This reduces RhoGDI activity and thus activates Rho subfamily members (Hirao et al. 1996; Takahashi et al. 1997). These results suggest that members of the ERM family as well as RhoGDI and RhoGDP/GTP exchange factor are involved in the activation of the Rho subfamily members, which in turn regulate reorganization of actin filaments underneath the plasma membrane. For instance, RhoA appears to regulate actin stress fibers at the basal membrane representing a ground state from which epithelial structures are built, whereas Rac1 activity appears to be required to assemble circumferential actin structures at the lateral membrane which affect the distribution of actin-associated membrane proteins (Jou and Nelson 1998). In line with this, members of the ERM protein family could reconstitute stress fiber assembly, cortical actin polymerization and focal complex formation in response to activation of Rho and Rac in digitonin-permeabilized fibroblasts (Mackay et al. 1997). CD44 interacting with ERM can provide the necessary link to the plasma membrane (Hirao et al. 1996). We performed immunoprecipitation analysis with anti-CD44 antibodies in EpH4 cells, but we were unable to detect the presence of ERM proteins or ankyrin/fodrin (Bourguignon et al. 1998) in immunoprecipitates with anti-panCD44 antibodies (data not shown). However, the rearrangement of the actin cytoskeleton after expression of trans-dominant annexin II mutant suggested the possible involvement of annexin II in linking of CD44-containing lipid rafts to the cytoskeleton.
This prompted us to ask if changes in actin cytoskeleton structure or stability might alter the integrity and localization of CD44–annexin II lipid rafts. Therefore, EpH4 cells were treated with digitonin to deplete them from cholesterol and at the same time render them permeable to GTPγS, a nonhydrolyzable analogue of GTP, which locks GTP-binding proteins in their active state. GTPγS is a pleiotropic reagent affecting many different classes of GTP-binding proteins. However, one of the major consequences of treating the cells with GTPγS is an increased actin polymerization. Adapting the method described by Mackay et al. 1997 to our fully polarized EpH4 cells, disruption of the association between CD44 and annexin II as well as their redistribution to the apical cell surface could be completely prevented by GTPγS, maintaining colocalized CD44 and annexin II in the basolateral plasma membrane domain despite of the cholesterol depletion. GTPγS also enhanced the formation of stress fibers and the formation of a pronounced subcortical actin ring (data not shown). Stabilization of filamentous actin itself by phalloidin also protected CD44–annexin II membrane complexes from dissociation and redistribution by cholesterol depletion, suggesting a direct involvement of stabilized actin filaments in this process.
One of the direct possibilities to address the question whether CD44-containing lipid rafts interact with the actin cytoskeleton are the measurements of FRAP. Surprisingly, CD44 exhibited distinct recovery rates in different sites of the sparsely grown, nonpolarized EpH4 cells. The mobility rate was lower in the ventral and trailing regions of the cells than at the leading edge. This difference may reflect weaker coupling of CD44 to the underlying cytoskeleton in the dynamic leading edge region. Interestingly, our results are in line with the observations by Jacobson et al. 1984. The diffusion coefficient of an 80-kD glycoprotein that they studied and which was identified several years later as CD44, was threefold higher near the leading edge of motile cells compared with the trailing region. However, when the fluorescence recovery rates were measured in arbitrary regions of the cells, typically ventral or trailing, it was clear that the extent of the lateral mobility of CD44 was dependent on the state of the actin cytoskeleton and lipid composition of the plasma membrane. Thus, the lateral mobility of CD44 was significantly enhanced when either the actin cytoskeleton was disrupted by latrunculin A or the plasma membrane cholesterol was depleted by M-b-CD. This observation would support our hypothesis that CD44 interacts with the actin cytoskeleton in a cholesterol-dependent manner. In addition, there is significantly more CD44 floating to the lipid rafts fraction after the disruption of the actin cytoskeleton by latrunculin A, which again argues for the interaction of CD44 with actin taking place in the lipid rafts.
Annexin II could fulfill a cross-linker function in several ways. First, it might directly link CD44 with actin, which could be achieved by its interaction with CD44 on a protein–protein level. However, our experiments do not point in this direction. Alternatively, annexin II can either modify rafts, therefore, ensuring the possibility of their interaction with the cytoskeleton via different means, or, due to its high affinity to lipids, cross-link entire raft structures to the underlying actin cables. Clearly, more work will be required to resolve these exciting questions.
Acknowledgments
We are most grateful to Peter Steinlein for help with the FRAP experiments and stimulating discussions throughout all stages of this work. We also thank Michael Glotzer, Bianca Habermann, Thomas Bader, Jean Pieters, Winfried Wunderlich, Christian Wimmer, and Luca Bolliger for critically reading this manuscript and for providing helpful comments. S. Oliferenko would like to thank D. Balzac for continuous support and encouragement. We thank Hans Dieplinger (University of Innsbruck) for performing the fluorimetric cholesterol measurements.
This work was supported by the I.M.P. and by grants from the Austrian Industrial Research Promotion Fund (FFF, 3/11504), the Austrian Science Foundation (FWF, P11446-MED) as well as by a grant from the Johnson & Johnson focused giving program. The Basel Institute for Immunology was founded and is supported by F. Hoffmann La-Roche Inc. (Basel, Switzerland).
Footnotes
1.used in this paper: DIG, detergent-insoluble glycosphingolipid-enriched domains; EpH4, mouse mammary epithelial cell line; ERM, ezrin/radixin/moesin family of proteins; HA, hyaluronic acid; FRAP, fluorescent recovery by photobleaching; M-β-CD, methyl-β-cyclodextrin; PLAP, placental alkaline phosphatase; RhoGDI, Rho GDP dissociation inhibitor; TER, trans-epithelial resistance
References
- Bellagamba C., Hubaishy I., Bjorge J.D., Fitzpatrick S.L., Fujita D.J., Waisman D. Tyrosine phosphorylation of annexin II tetramer is stimulated by membrane binding. J. Biol. Chem. 1997;272:3195–3199. doi: 10.1074/jbc.272.6.3195. [DOI] [PubMed] [Google Scholar]
- Benlimame N., Le P.U., Nabi I.R. Localization of autocrine motility factor receptor to caveolae and clathrin-independent internalization of its ligand to smooth endoplasmic reticulum. Mol. Biol. Cell. 1998;9:1773–1786. doi: 10.1091/mbc.9.7.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon L., Zhu D., Zhu H. CD44 isoform-cytoskeleton interaction in oncogenic signaling and tumor progression. Front. Biosci. 1998;3:637–649. doi: 10.2741/a308. [DOI] [PubMed] [Google Scholar]
- Brown R. Sphingolipid organization in biomembraneswhat physical studies of model membranes reveal. J. Cell Sci. 1998;111:1–9. doi: 10.1242/jcs.111.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D.A., London E. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- Chong L.D., Traynor-Kaplan A., Bokoch G.M., Schwartz M.A. The small GTP-binding protein Rho regulates a phosphatidylinositol 4-phosphate 5-kinase in mammalian cells. Cell. 1994;79:507–513. doi: 10.1016/0092-8674(94)90259-3. [DOI] [PubMed] [Google Scholar]
- Creutz C.E. The annexins and exocytosis. Science. 1992;258:924–931. doi: 10.1126/science.1439804. [DOI] [PubMed] [Google Scholar]
- Elias P.M., Goerke J., Friend D.S., Brown B.E. Freeze-fracture identification of sterol-digitonin complexes in cell and liposome membranes. J. Cell Biol. 1978;78:577–596. doi: 10.1083/jcb.78.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emans N., Gorvel J.-P., Walter C., Gerke V., Kellner R., Griffiths G., Gruenberg J. Annexin II is a major component of fusogenic endosomal vesicles. J. Cell Biol. 1993;120:1357–1369. doi: 10.1083/jcb.120.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fialka I., Schwarz H., Reichmann E., Oft M., Busslinger M., Beug H. The estrogen-dependent c-JunER protein causes a reversible loss of mammary epithelial cell polarity involving a destabilization of adherens junctions. J. Cell Biol. 1996;132:1115–1132. doi: 10.1083/jcb.132.6.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fialka I., Oft M., Reichmann E., Huber L.A., Beug H. Three-dimensional organotypic growth of epithelial cells in reconstituted extracellular matrix. In: Celis J.E., editor. Cell BiologyA Laboratory Handbook. 2nd edition. Academic Press; San Diego, CA: 1997. pp. 1:107–112. [Google Scholar]
- Fielding C.J., Fielding P.E. Intracellular cholesterol transport. J. Lipid Res. 1997;38:1503–1521. [PubMed] [Google Scholar]
- Friedrichson T., Kurzchalia T.V. Microdomains of GPI-anchored proteins in living cells revealed by cross-linking. Nature. 1998;394:802–805. doi: 10.1038/29570. [DOI] [PubMed] [Google Scholar]
- Günthert U. CD44 in malignant disorders. Curr. Top. Microbiol. Immunol. 1996;213:271–285. [PubMed] [Google Scholar]
- Günthert, U. 1999. Importance of CD44 variant isoforms in mouse models for inflammatory bowel disease. CTMI 246: Mechanisms of B Cell Neoplasia 1998. F. Melchers and M. Potter, editors. 307–313. [DOI] [PubMed]
- Hansen S.H., Sandvig K., van Deurs B. Clathrin and HA2 adaptorseffects of potassium depletion, hypertonic medium, and cytosol acidification. J. Cell Biol. 1993;121:61–72. doi: 10.1083/jcb.121.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder T., Gerke V. The subcellular distribution of early endosomes is affected by the annexin II2p112 complex. J. Cell Biol. 1993;123:1119–1132. doi: 10.1083/jcb.123.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder T., Gerke V. The annexin II2 p112 complex is the major protein component of the triton-X100-insoluble low-density fraction prepared from MDCK cells in the presence of Ca2+ . Biochim. Biophys. Acta. 1994;1223:375–382. doi: 10.1016/0167-4889(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Harder T., Kellner R., Parton R.G., Gruenberg J. Specific release of membrane-bound annexin II and cortical cytoskeletal elements by sequestration of membrane cholesterol. Mol. Biol. Cell. 1997;8:533–545. doi: 10.1091/mbc.8.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder T., Simons K. Caveolae, DIGs, and the dynamics of sphingolipid-cholesterol microdomains. Curr. Opin. Cell Biol. 1997;9:534–542. doi: 10.1016/s0955-0674(97)80030-0. [DOI] [PubMed] [Google Scholar]
- Harder T., Scheiffele P., Verkade P., Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J. Cell Biol. 1998;141:929–942. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heider J.G., Boyett R.L. The picomole determination of free and total cholesterol in cells in culture. J. Lipid. Res. 1978;19:514–518. [PubMed] [Google Scholar]
- Hirao M., Sato N., Kondo T., Yonemura S., Monden M., Sasaki T., Takai Y., Tsukita S. Regulation mechanism of ERM (ezrin/radixin/moesin) protein/plasma membrane associationpossible involvement of phosphatidylinositol turnover and Rho-dependent signaling pathway. J. Cell Biol. 1996;135:37–51. doi: 10.1083/jcb.135.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubaishy I., Jones P.G., Bjorge J., Bellagamba C., Fitzpatrick S., Fujita D.J., Waisman D.M. Modulation of annexin II tetramer by tyrosine phosphorylation. Biochemistry. 1995;34:14527–14534. doi: 10.1021/bi00044a031. [DOI] [PubMed] [Google Scholar]
- Ikonen E., Simons K. Protein and lipid sorting from the trans-Golgi network to the plasma membrane in polarized cells. Semin. Cell Devel. Biol. 1998;9:503–509. doi: 10.1006/scdb.1998.0258. [DOI] [PubMed] [Google Scholar]
- Ilangumaran S., Briol A., Hoessli D. CD44 selectively associates with active Src family protein tyrosine kinases Lck and Fyn in glycosphingolipid-rich plasma membrane domains of human peripheral blood lymphocytes. Blood. 1998;91:3901–3908. [PubMed] [Google Scholar]
- Ilangumaran S., Hoessli D. Effects of cholesterol depletion by cyclodextrin on the sphingolipid microdomains of the plasma membrane. Biochem. J. 1998;335:433–440. doi: 10.1042/bj3350433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isacke C.M. The role of the cytoplasmic domain in regulating CD44 function. J. Cell Sci. 1994;107:2353–2359. doi: 10.1242/jcs.107.9.2353. [DOI] [PubMed] [Google Scholar]
- Jacobson K., O'Dell D., August J.T. Lateral diffusion of an 80,000-dalton glycoprotein in the plasma membrane of murine fibroblastsrelationships to cell structure and function. J. Cell Biol. 1984;99:1624–1633. doi: 10.1083/jcb.99.5.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou T.S., Nelson W.J. Effects of regulated expression of mutant RhoA and Rac1 small GTPases on the development of epithelial (MDCK) cell polarity. J. Cell Biol. 1998;142:85–100. doi: 10.1083/jcb.142.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinosita K., Itoh H., Ishiwata S. Dual-view microscopy with a single camerareal-time imaging of molecular orientations and calcium. J. Cell Biol. 1991;115:67–73. doi: 10.1083/jcb.115.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein U., Gimpl G., Fahrenholz F. Alteration of the myometrial plasma membrane cholesterol content with beta-cyclodextrin modulates the binding affinity of the oxytocin receptor. Biochemistry. 1995;34:13784–13793. doi: 10.1021/bi00042a009. [DOI] [PubMed] [Google Scholar]
- Kube E., Becker T., Weber K., Gerke V. Protein-protein interaction studied by site-directed mutagenesis. Characterization of the annexin II-binding site on p11, a member of the S100 protein family. J. Biol. Chem. 1992;267:14175–14182. [PubMed] [Google Scholar]
- Lacy B.E., Underhill C.B. The hyaluronate receptor is associated with actin filaments. J. Cell Biol. 1987;105:1395–1404. doi: 10.1083/jcb.105.3.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafont F., Lecat S., Verkade P., Simons K. Annexin XIIIb associates with lipid microdomains to function in apical delivery. J. Cell Biol. 1998;142:1413–1427. doi: 10.1083/jcb.142.6.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesley J., Kincade P.W., Hyman R. Antibody-induced activation of the hyaluronan receptor function of CD44 requires multivalent binding by antibody. Eur. J. Immunol. 1993;23:1902–1909. doi: 10.1002/eji.1830230826. [DOI] [PubMed] [Google Scholar]
- Lisanti M., Tang Z., Scherer P., Kubler E., Koleske A.J., Sargiacomo M. Caveolae, transmembrane signalling and cellular transformation. Mol. Membr. Biol. 1995;12:121–124. doi: 10.3109/09687689509038506. [DOI] [PubMed] [Google Scholar]
- Liu D., Sy M.S. Phorbol myristate acetate stimulates the dimerization of CD44 involving a cysteine in the transmembrane domain. J. Immunol. 1997;159:2702–2711. [PubMed] [Google Scholar]
- Mackay D.J., Esch F., Furthmayr H., Hall A. Rho- and rac-dependent assembly of focal adhesion complexes and actin filaments in permeabilized fibroblastsan essential role for ezrin/radixin/moesin proteins. J. Cell Biol. 1997;138:927–938. doi: 10.1083/jcb.138.4.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor S., Rothberg K., Maxfield F. Sequestration of GPI-anchored proteins in caveolae triggered by cross-linking. Science. 1994;264:1948–1951. doi: 10.1126/science.7516582. [DOI] [PubMed] [Google Scholar]
- Mineo C., James G.L., Smart E.J., Anderson R.G.W. Localization of epidermal growth factor-stimulated Ras/Raf-1 interaction to caveolae membrane. J. Biol. Chem. 1996;271:11930–11935. doi: 10.1074/jbc.271.20.11930. [DOI] [PubMed] [Google Scholar]
- Mumby S. Reversible palmitoylation of signaling proteins. Curr. Opin. Cell. Biol. 1997;9:148–154. doi: 10.1016/s0955-0674(97)80056-7. [DOI] [PubMed] [Google Scholar]
- Naot D. CD44structure, function and association with the malignant process. Adv. Cancer Res. 1997;71:241–319. doi: 10.1016/s0065-230x(08)60101-3. [DOI] [PubMed] [Google Scholar]
- Neame S.J., Isacke C.M. The cytoplasmic tail of CD44 is required for basolateral localization in epithelial MDCK cells but does not mediate association with the detergent-insoluble cytoskeleton of fibroblasts. J. Cell Biol. 1993;121:1299–1310. doi: 10.1083/jcb.121.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neame S.J., Uff C.R., Sheikh H., Wheatley S.C., Isacke C.M. CD44 exhibits a cell type dependent interaction with triton X-100 insoluble, lipid rich, plasma membrane domains. J. Cell Sci. 1995;108:3127–3135. doi: 10.1242/jcs.108.9.3127. [DOI] [PubMed] [Google Scholar]
- Orlandi P.A., Fishman P.H. Filipin-dependent inhibition of cholera toxinevidence for toxin internalization and activation through caveolae-like domains. J. Cell Biol. 1998;141:905–915. doi: 10.1083/jcb.141.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton R.G., Simons K. Digging into caveolae. Science. 1995;269:1398–1399. doi: 10.1126/science.7660120. [DOI] [PubMed] [Google Scholar]
- Parton R.G. Caveolae and caveolins. Curr. Opin. Cell Biol. 1996;8:542–548. doi: 10.1016/s0955-0674(96)80033-0. [DOI] [PubMed] [Google Scholar]
- Perschl A., Lesley J., English N., Hyman R., Trowbridge I. Transmembrane domain of CD44 is required for its detergent insolubility in fibroblasts J. Cell Sci. 108 1995. 1033 1041a [DOI] [PubMed] [Google Scholar]
- Perschl A., Lesley J., English N., Trowbridge I., Hyman R. Role of CD44 cytoplasmic domain in hyaluronan binding Eur. J. Immunol. 25 1995. 495 501b [DOI] [PubMed] [Google Scholar]
- Ponta H., Herrlich P. The CD44 protein familyroles in embryogenesis and tumor progression. Front. Biosci. 1998;3:650–656. doi: 10.2741/a309. [DOI] [PubMed] [Google Scholar]
- Ridley A.J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Rodgers W., Crise B., Rose J.K. Signals determining protein tyrosine kinase and glycosyl-phosphatidylinositol-anchored protein targeting to a glycolipid-enriched membrane fraction. Mol. Cell. Biol. 1994;14:5384–5391. doi: 10.1128/mcb.14.8.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz P., Schwärzler C., Günthert U. CD44 isoforms during differentiation and development. Bioessays. 1995;17:17–24. doi: 10.1002/bies.950170106. [DOI] [PubMed] [Google Scholar]
- Shaul P.W., Anderson R.G. Role of plasmalemmal caveolin in signal transduction. Am. J. Physiol. 1998;275:843–851. doi: 10.1152/ajplung.1998.275.5.L843. [DOI] [PubMed] [Google Scholar]
- Scheiffele P., Roth M.G., Simons K. Interaction of influenza virus haemagglutinin with sphingolipid-cholesterol membrane domains via its transmembrane domain. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:5501–5508. doi: 10.1093/emboj/16.18.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P., Verkade P., Fra M., Virta H., Simons K., Ikonen E. Caveolin-1 and -2 in the exocytic pathway of MDCK cells. J. Cell Biol. 1998;140:795–806. doi: 10.1083/jcb.140.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer J.E., Oh P., Pinney E., Allard J. Filipin-sensitive caveolae-mediated transport in endotheliumreduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J. Cell Biol. 1994;127:1217–1232. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh H., Isacke C.M. A di-hydrophobic Leu-Val motif regulates the basolateral localization of CD44 in polarized Madin-Darby canine kidney epithelial cells. J. Biol. Chem. 1996;271:12185–12190. doi: 10.1074/jbc.271.21.12185. [DOI] [PubMed] [Google Scholar]
- Shenoy-Scaria A.M., Dietzen D.J., Kwong J., Link D.C., Lublin D.M. Cysteine3 of Src family protein tyrosine kinase determines palmitoylation and localization in caveolae. J. Cell Biol. 1994;126:353–363. doi: 10.1083/jcb.126.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleeman W., Hofmann M., Moll J., Herrlich P., Ponta H. Regulated clustering of variant CD44 proteins increases their hyaluronate binding capacity. J. Cell Biol. 1996;135:1139–1150. doi: 10.1083/jcb.135.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart E.J., Mineo C., Anderson R.G. Clustered folate receptors deliver 5-methyltetrahydrofolate to cytoplasm of MA104 cells. J. Cell Biol. 1996;134:1169–1177. doi: 10.1083/jcb.134.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H.Q., Kwiatkowska K., Wooten D.C., Yin H.L. Effects of CapG overexpression on agonist-induced motility and second messenger generation. J. Cell Biol. 1995;129:147–156. doi: 10.1083/jcb.129.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Sasaki T., Mammoto A., Takaishi K., Kameyama T., Tsukita S., Takai Y. Direct interaction of the Rho GDP dissociation inhibitor with ezrin/radixin/moesin initiates the activation of the Rho small G protein. J. Biol. Chem. 1997;272:23371–23375. doi: 10.1074/jbc.272.37.23371. [DOI] [PubMed] [Google Scholar]
- Thiel C., Osborn M., Gerke V. The tight association of the tyrosine kinase substrate annexin II with the submembranous cytoskeleton depends on intact p11- and Ca(2+)-binding sites. J. Cell Sci. 1992;103:733–742. doi: 10.1242/jcs.103.3.733. [DOI] [PubMed] [Google Scholar]
- Thyberg J., Calara F., Dimayuga P., Nilsson J., Regnstrom J. Role of caveolae in cholesterol transport in arterial smooth muscle cells exposed to lipoproteins in vitro and in vivo. Lab. Invest. 1998;78:825–837. [PubMed] [Google Scholar]
- Uittenbogaard A., Ying Y., Smart E.J. Characterization of a cytosolic heat-shock protein-caveolin chaperone complex. Involvement in cholesterol trafficking. J. Biol. Chem. 1998;273:6525–6532. doi: 10.1074/jbc.273.11.6525. [DOI] [PubMed] [Google Scholar]
- Varma R., Mayor S. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature. 1998;394:798–801. doi: 10.1038/29563. [DOI] [PubMed] [Google Scholar]
- Wary K.K., Mariotti A., Zurzolo C., Giancotti F.G. A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell. 1998;94:625–634. doi: 10.1016/s0092-8674(00)81604-9. [DOI] [PubMed] [Google Scholar]
- Wessel D., Flügge U.I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 1984;138:141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]