Abstract
The purpose of this study was the economic evaluation of short-duration treatments of chronic hepatitis B (CHB) and longer duration antiviral treatment for up to 5 years. Two 10-health state Markov models were developed for hepatitis B e antigen (HBeAg)-positive and HBeAg-negative CHB patients respectively. The perspective of this economic evaluation was the Singapore healthcare system and CHB patient. The models followed cohorts of HBeAg-positive and HBeAg-negative CHB patients, respectively, over a period of 40 years, by which time the majority of the cohorts would have died if left untreated. Costs and benefits were discounted at 5% per annum. Annual rates of disease progression and the magnitude of treatment effects were obtained from the literature, with a focus on data obtained in Asian patients and meeting the criteria for therapy as described in internationally recognized management guidelines. Short-course therapy with α-interferon, or 1-year treatment with pegylated interferon α-2a, lamivudine or adefovir had limited impact on disease progression. In contrast, treatment of CHB with antiviral therapy for 5 years substantially decreased the rate of disease progression. Treatment with lamivudine for 1-year is highly cost-effective compared with no treatment of CHB but has limited effect on reducing the rate of disease progression. Compared with 1-year treatment with lamivudine, sequential antiviral therapies for up to 5 years (i.e. lamivudine plus adefovir on emergence of lamivudine resistance or adefovir plus lamivudine on emergence of adefovir resistance) are highly cost-effective by international standards. These conclusions are robust to uncertainties in model inputs and are consistent with the findings of other recently published studies.
Keywords: α-interferon, adefovir, chronic hepatitis B, cost-effectiveness, lamivudine, pegylated interferon α-2a
Introduction
Burden of chronic hepatitis B (CHB)
Worldwide more than 350 million people are chronically infected with hepatitis B virus (HBV) and at increased risk of death from cirrhosis of the liver and hepatocellular carcinoma (HCC) [1].
Chronic hepatitis B is characterized by elevated serum aminotransferase (ALT) levels, high or fluctuating levels of serum HBV-DNA and active liver disease on biopsy [2]. Persistently elevated serum HBV-DNA levels are associated with increased long-term risk of cirrhosis, HCC and liver failure [3–5].
Persons with CHB may have hepatitis B e antigen (HBeAg) or antibodies to HBeAg (anti-HBe) in their sera. HBeAg seroconversion is associated with clearance of HBeAg from serum and with the appearance of anti-HBe. In persons who are HBeAg-positive, spontaneous HBeAg seroconversion can occur, often accompanied by a transient elevation in ALT levels. After HBeAg seroconversion, most persons have persistently normal ALT levels and suppressed levels of HBV-DNA, usually <103 copies/mL. However, almost 20% of patients have active liver disease following spontaneous HBeAg-seroconversion, due to the development of mutations in the precore stop codon or core promoter region. HBeAg-negative, anti-HBe-positive type CHB is now recognized as an important and increasingly prevalent form of CHB, often associated with certain HBV genotypes (B, C and D but not A or G) [2]. More than 95% of HBV infections in Asia-Pacific countries are HBV genotypes B or C, explaining the high prevalence of HBeAg-negative CHB in this region.
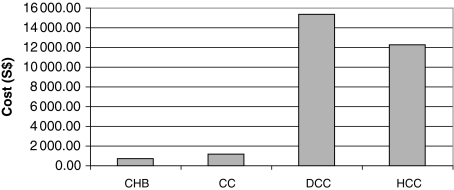
Chronic hepatitis B remains an important public health problem in Singapore. Although the incidence of HBV infection is decreasing, as a result of the compulsory vaccination of babies born to infected mothers, nevertheless, between 160 000 and 230 000 Singaporeans were chronically infected as of 2001 [6]. The annual healthcare costs for the year 2003 of managing CHB, compensated cirrhosis (CC), decompensated cirrhosis (DCC) and HCC, were estimated by Li et al., 2004 [6]. Direct healthcare costs were classified in terms of outpatient consultations, inpatient admissions, antiviral drug treatment, other medications, laboratory tests, imaging and procedures. Lost income and lost productivity from illness will be additional indirect costs of CHB for the population. The annual cost of treatment increases dramatically as the disease progresses from CC to DCC or HCC respectively (Fig. 1).
Fig. 1.
Average cost per patient per disease state.
Asian-Pacific guidelines for the management of CHB
The Asia-Pacific consensus guidelines (2005) [7] on the management of CHB state: ‘… sustained viral suppression is the key to the reduction or prevention of hepatic injury and disease progression. Therefore, the primary goal of treatment for CHB is to eliminate or permanently suppress HBV. The ultimate long-term goal of therapy is to prevent hepatic decompensation, to reduce or prevent progression to cirrhosis and/or HCC, and to prolong survival’.
Current treatment options
Conventional interferon-α has been used in the treatment of CHB for many years. Treatment is administered by subcutaneous injection, at a dosage of 5–10 MU three times a week for 4–6 months. Predictors of a nonresponse to interferon-α include Asian ethnicity, childhood infection, HBeAg-negative CHB, high serum HBV-DNA levels and low serum ALT levels [8].
Pegylated interferon α-2a is administered by subcutaneous injection (180 μg) once-weekly for 48 weeks. Although pegylated interferon has more antiviral efficacy than conventional interferon, the side-effect profile appears to be similar for both formulations [8].
For most patients with CHB, sustained viral suppression is only attainable through long-term nucleos(t)ide analogue therapy, as short-course therapies (4–12 months) only achieve sustained responses in a minority of patients [7].
The disadvantage of long-term antiviral monotherapy is the emergence of virological breakthrough secondary to the selection of drug resistant mutants [9]. If resistance develops in patients with compensated liver disease, an alternative antiviral drug should be used to control the disease. The patient can either have a second drug added or can be switched to another drug with the option of a 1- or 2-month overlap. If resistance emerges in patients with advanced liver disease, a second drug should be added to continued ongoing therapy with the first drug [9].
Lamivudine was the first nucleoside analogue to be licensed by the FDA for use in the chronic treatment of HBV infection in 1998. It is well tolerated and results in HBV suppression, ALT normalization and reduction of hepatic inflammation, although resistant strains emerge when lamivudine is used long term [10].
Adefovir dipivoxil is a new CHB antiviral treatment option that is well tolerated and long-term therapy results in incremental increase in rate of viral suppression below the level of detection, ALT normalization and histological improvement. In comparison with lamivudine, resistance to adefovir is delayed and less frequent [11].
Mechanisms of resistance to lamivudine and adefovir have distinct structural differences, and mutations conferring cross-resistance to both drugs have not been reported [9].
Economic evaluation of CHB treatments
A new treatment is usually regarded as cost-effective when it provides improved outcomes at an acceptable increased cost. Cost-utility is a form of cost-effectiveness analysis that involves the estimation of incremental cost per quality adjusted life year (QALY). QALYs take into consideration quality of life as well as quantity of life [12]. The ‘Q’ in QALY is a utility weight with values from 0 to 1. Death has a utility value of 0 and perfect health has a utility value of 1. Cost per QALY potentially allows for comparison between the cost-effectiveness of treatments in different disease areas with very different clinical outcome measures. This can facilitate healthcare resource allocation decisions [12].
The decision rule that payers apply to judging whether a new healthcare intervention is cost-effective is often not stated explicitly. However, US health economists generally agree that if an intervention can save 1 year of life for less than US$ 50 000, it is cost-effective. Moreover, the UK National Institute for Clinical Excellence recently stated that the cost-effectiveness threshold is between £20 000 and £30 000 (approximately US$ 36 000–54 000) [13]. As US$ 1 = approximately S$ 1.6, therefore a cost-effective threshold of $50 000 is equivalent to approximately S$ 80 000 in Singapore. However, adjusting for differences in purchasing power parity (PPP) based Gross National Income (GNI) per capita (2001) provides a cost-effectiveness threshold in Singapore of approximately S$ 53 000 ($80 000 × US$ 22 850/US$ 34 280) [14].
The purpose of this study was to establish the economic evaluation of short-duration treatments of CHB and long-duration antiviral treatments for up to 5 years. The context for the economic evaluation was the Singapore healthcare system and the healthcare needs of the CHB patient, in which only direct costs were included. As a result, indirect costs, such as lost productivity, from time off work due to illness or premature death were excluded.
Methods
Treatment scenarios
Three different treatment scenarios were investigated:
No treatment
Short-duration therapy with conventional interferon-α (IFN) or 1-year treatment with pegylated interferon-α 2a (Pegasys™), lamivudine or adefovir
Five-year antiviral treatment with adefovir or lamivudine monotherapy, with switch to the alternative antiviral agent as ‘rescue’ therapy upon emergence of resistance (i.e. sequential monotherapy with either lamivudine followed by adefovir as rescue for lamivudine-resistance or adefovir followed by lamivudine as rescue for adefovir-resistance). If resistance emerged in patients with more severe disease (Metavir stage 3 or 4 fibrosis), the alternative antiviral agent was added to rather than switched with the initial agent and combination therapy with both adefovir plus lamivudine was continued long term.
Patient populations and model design
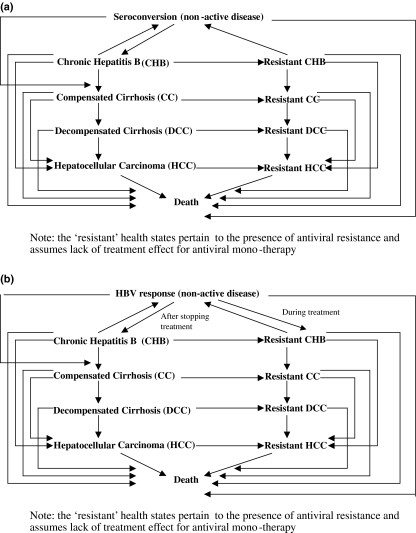
The pharmaco-economic models comprise two 10-health state Markov models, adapted from Crowley et al. (2000) [15]. The HBeAg-positive CHB model (Fig. 2a) included equal numbers of male and female patients from Singapore; the average patient age at start of treatment was 30 years [15,16]. HBeAg-seroconversion is the criterion for treatment cessation. The HBeAg-negative CHB model (Fig. 2b) included patients with an average age of 40 years at start of treatment, because of differences in age profiles between HBeAg-positive and HBeAg-negative populations studied in clinical trials (e.g. [17,18]). HBsAg-seroconversion is the only criterion for treatment cessation in HBeAg-negative CHB. Both models evaluate short-course treatments and longer duration treatments up to 5 years, with duration of follow-up of a total of 40 years, by which time the majority of the cohort will have died, if left untreated. This enabled long-term costs and outcomes to be determined.
Fig. 2.
Structure of (a) HBeAg-positive chronic hepatitis B (CHB) Economic Model; (b) HBeAg-negative CHB Economic Model.
Data sources
Disease progression rates in the absence of treatment were derived mainly from a recent systematic literature review of CHB disease progression in the Asia-Pacific region and Africa [19] and are given in Table 1.
Table 1.
Disease progression rates for CHB derived from the literature and the effect of antiviral treatment on reducing the rates of disease progression
| Model input | Treatment | Estimate | Source |
|---|---|---|---|
| Annual rate of progression from CHB to CC (HBeAg-positive) | 0.0260 | From Lin X et al. 2005 [19]* | |
| Annual rate of progression from CHB to CC (HBeAg-negative) | 0.0900 | From de Franchis et al. 2003 [23] | |
| % Decrease in annual rate of progression from CHB to CC in absence of resistance | LAM | 85.7% | From Crowley et al. 2002 [16] |
| IFN & PEG-IFN | 85.7% | From Crowley et al. 2002 [16] | |
| ADF | 85.7% | Assumption† | |
| % Decrease in annual rate of progression from CHB to CC in presence of resistance | LAM; ADF | 0.0000 | Conservative assumption |
| Annual rate of progression from CHB to HCC | 0.0066 | From Lin X et al. 2005 [19]* | |
| % Decrease in annual rate of progression from CHB to HCC in presence of resistance | LAM; ADF | 0.0000 | Conservative assumption |
| Annual rate of progression from CC to DCC | 0.0420 | From Lin X et al. 2005 [19]* | |
| % Decrease in annual rate of progression from CC to DCC in absence of resistance | LAM | 22.6% | From Liaw et al. 2004 [5]‡ |
| IFN & PEG-IFN | 22.6% | Assumption | |
| ADF | 22.6% | Assumption† | |
| % Decrease in annual rate of progression from CC to DCC in presence of resistance | LAM; ADF | 0.0000 | Conservative assumption |
| Annual rate of progression from CC to HCC | 0.0430 | From Lin X et al. 2005 [19]* | |
| % Decrease in annual rate of progression from CC to HCC in absence of resistance | LAM | 17.6% | Derived from Liaw et al. 2004 [5]§ |
| IFN & PEG-IFN | 17.6% | Assumption | |
| ADF | 17.6% | Assumption† | |
| % Decrease in annual rate of progression from CC to HCC in presence of resistance | LAM; ADF | 0.0000 | Conservative assumption |
| Annual rate of progression from DCC to HCC | 0.0710 | From Lin X et al. 2005 [19]* | |
| Annual rate of progression from Seroconversion or HBV response to death | Life table values | From age-specific life tables [28] | |
| Annual rate of progression from CHB to death | 0.0060 | From Lin X et al. 2005 [19]* | |
| Annual rate of progression from CC to death | 0.0540 | From Lin X et al. 2005 [19]* | |
| Annual rate of progression from DCC to death | 0.1630 | From Lin X et al. 2005 [19]* | |
| Annual rate of progression from HCC to death | 0.4300 | From Lin X et al. 2005 [19]* |
CHB, chronic hepatitis B; CC, compensated cirrhosis; HCC, hepatocellular carcinoma; DCC, decompensated cirrhosis; LAM, lamivudine; PEG-IFN, pegylated-interferon; ADF, adefovir; HBV, hepatitis B virus.
Weighted average derived from Lin X et al. 2005 [19] (base case estimate).
Control is assumed to be as good with adefovir as with lamivudine in those who do not develop resistance.
Derived from Liaw et al. 2004 [5] (annualized % decrease) from increase in Child-Pugh score.
Derived from Liaw et al. 2004 [5] (annualized % decrease).
Disease progression rates in the presence of treatment were derived from studies investigating the effect of lamivudine treatment on disease progression and are given in Table 1.
HBeAg-seroconversion rates in HBeAg-positive patients were obtained from the published literature and are given in Table 2a. HBV treatment response rates in HBeAg-negative patients were obtained from the published literature and are given in Table 2b.
Table 2.
(a) Annual HBeAg-seroconversion rates, relapse rates and resistance rates for different treatments of HBeAg-positive CHB derived from the literature; (b) Annual HBV response rates and lack of durability of response rates for different treatments of HBeAg-negative CHB for different treatments derived from the literature
| Model input | Treatment | Estimate | Comment |
|---|---|---|---|
| (a) | |||
| HBeAg-seroconversion rates for patients with ALT levels >2 × ULN in the absence of resistance | No treatment/placebo | 0.1020 | Derived from Perrillo et al. 2002 [29] |
| LAM | 0.3070 | Derived from Perillo et al. 2002 [29]* | |
| IFN | 0.2250 | Derived from Perillo et al. 2002 [29] | |
| PEG-IFN | 0.2660 | From Lau et al. 2005 [17] | |
| ADF | 0.1520 | Derived from Marcellin et al. 2002 [30]† | |
| HBeAg-seroconversion lack of durability (% who relapse back to CHB state) | LAM | 0.1020 | Derived from Dienstag et al. 2003 [26]‡ |
| No treatment; IFN | 0.1020 | From Crowley et al. 2002 [16] | |
| PEG-IFN | 0.0440 | Derived from Lau et al. 2005 [17]§ | |
| ADF | 0.0800 | From Chang et al. 2004 [27]¶ | |
| Emergence of resistance during treatment | LAM | 0.2570 | Derived from the cumulative rates given in Lai et al. 2003 [10]** |
| No treatment; IFN & PEG-IFN | 0.0000 | ||
| ADF | 0.0640 | Derived from the cumulative rates given in Locarnini 2005 [31] (Adefovir mono-therapy trials)†† | |
| (b) | |||
| HBV response rates for patients with ALT levels >2 × ULN in the absence of resistance | No treatment/placebo | 0.0000 | Hadziyannis et al. 2003 [32] |
| LAM | 0.8950 | From Marcellin et al. 2004 [18]‡‡ | |
| PEG-IFN | 0.6330 | Marcellin et al. 2004 [18] | |
| ADF | 0.5120 | Hadziyannis et al. 2003 [32]§§ | |
| Annual lack of durability of response (% who relapse back to CHB state) | LAM | 0.9030 | Derived from Marcellin et al. 2004 [18] & Hadziyannis et al. 2005 [33] |
| No treatment | 0.9030 | Derived from Marcellin et al. 2004 [18] & Hadziyannis et al. 2005 [33] | |
| PEG-IFN | 0.6960 | Marcellin et al. 2004 [18] | |
| ADF | 0.9030 | Derived from Marcellin et al. 2004 [18] & Hadziyannis et al. 2005 [33] | |
CHB, chronic hepatitis B; ALT, aminotransferase; LAM, lamivudine; PEG-IFN, pegylated-interferon; ADF, adefovir; HBV, hepatitis B virus.
Annual HBeAg-seroconversion rate = 25.4% based on all patients with ALT levels ≥2 × ULN, which is equivalent to an annual seroconversion rate = 30.7% in those without resistance and 10.2% in those with resistance. Assumption: These rates remain constant with time.
Annual HBeAg-seroconversion rate = 14.9% based on all patients with ALT levels ≥2 × ULN, which is equivalent to an annual seroconversion rate = 15.2% in those without resistance and 10.2% in those with resistance. Assumption: These rates remain constant with time.
Assumption: % relapse rate per year is average of that occurring over years 1–3 after stopping treatment.
The annual rate of loss of HBeAg seroconversion for LAM was 10.2% (derived from Dienstag et al. 2003) [26]. Therefore, the corresponding estimate for PEG-IFN would be 10.2% × (18.1/41.8) = 4.4%, where 18.1% and 41.8% were the 6-month seroreversion rates obtained for PEG-IFN and LAM, respectively (Lau et al. 2005) [17].
Baseline ALT levels unknown.
Assumption: Resistance rates for years 1–5 is the average of that observed over years 1–4.
Assumption: Resistance rate is zero for year 1. The annual resistance rate estimate (6.4%) for years 2–5 is the average of that observed over years 2–4.
The rate of HBV DNA suppression is 73.5% after 1-year treatment with LAM. In the same study YMDD variants occurred in 17.9% after 1 year. Therefore, the corresponding rate in the absence of resistance = [0.735/(1−0.179)] = 89.5%, assuming loss of suppression in those with resistance. Assumption: Annual response rate for years 2–5 in the absence of resistance is the same as for year 1.
Assumption: Annual response rate for ADF in the absence of resistance for years 2–5 is the same as for year 1.
Healthcare costs used are those applying in Singapore. The healthcare costs for managing CHB, CC, DCC, HCC, for the year 2003 were obtained from Li et al. (2004) [6] (Fig. 1). Drug acquisition costs for CHB treatment alternatives for the year 2005 are given in Table 3.
Table 3.
Annual drug acquisition costs (2005)
| Drugs | Annual acquisition costs (wholesale) of drugs ($S) |
|---|---|
| LAM | 2774.00 |
| ADF | 3606.20 |
| IFN* | 7329.75 |
| PEG-IFN† | 20 740.00 |
LAM, lamivudine; PEG-IFN, pegylated-interferon; ADF, adefovir.
IFN costs are the average for Roferon-A and Intron-A (range of doses) and 6–12 months treatment durations.
PEG-IFN costs are the average of those for Pegasys and Peg-Intron for a duration of 12 months.
Health state utilities were adapted from Crowley et al. (2000) [15] and are given in Table 4.
Table 4.
Disease health state utilities derived from the literature and the effect of antiviral resistance
| Health state utility | Estimate | Source |
|---|---|---|
| Seroconversion | 0.783 | From Crowley et al. 2002 [16] |
| CHB | Untreated = 0.692 LAM = 0.692* ADF = 0.692* IFN = 0.467 PEG-IFN = 0.5795† | Adapted from Crowley et al. 2002 [16] |
| CC | 0.561 | From Crowley et al. 2002 [16] |
| DCC | 0.150 | From Crowley et al. 2002 [16] |
| HCC | 0.118 | From Crowley et al. 2002 [16] |
| Resistant CHB | Untreated = 0.692 LAM = 0.692 ADF = 0.692 IFN = 0.467 PEG-IFN = 0.5795 | Adapted from Crowley et al. 2002 [16]‡ |
| Resistant CC | 0.561 | From Crowley et al. 2002 [16]‡ |
| Resistant DCC | 0.150 | From Crowley et al. 2002 [16]‡ |
| Resistant HCC | 0.118 | From Crowley et al. 2002 [16]‡ |
CHB, chronic hepatitis B; CC, compensated cirrhosis; DCC, decompensated cirrhosis; HCC, hepatocellular carcinoma; LAM, lamivudine; ADF, adefovir; PEG-IFN, pegylated-interferon.
Assumptions
LAM and ADF are well tolerated in CHB patients and have at least the same utility as untreated patients.
PEG-IFN is better tolerated than IFN but less well tolerated than LAM or ADF, resulting in a utility mid-way between that for IFN and LAM/ADF.
No effect of resistance per se on the health state utility.
Treatment effects incorporated into the model
The following aspects of treatment effects were incorporated into the model:
HBeAg-seroconversion or HBV response: In HBeAg-positive CHB, HBeAg-seroconversion was used as a treatment-stopping criterion. In HBeAg-negative CHB, HBV response occurred when suppression of HBV viral load occurred to non-detectable levels (<300–400 copies/mL). Patients who underwent HBeAg-seroconversion or HBV response were assumed to have a much reduced rate of disease progression (limited to a 1% rate of progression to CC per annum).
Seroreversion or loss of HBV response: Seroreversion (loss of HBeAg-seroconversion and reoccurrence of active CHB) could occur in HBeAg-positive CHB and loss of HBV response (reappearance of detectable levels of HBV and active CHB) could occur in HBeAg-negative CHB (Fig. 2a,b).
Antiviral resistance: Emergence of resistance could occur with time during antiviral therapies (Fig. 2a,b). The rates of emergence of resistance for each treatment alternative were assumed to be the same in HBeAg-positive and HBeAg-negative CHB. Antiviral treatments were assumed to be ineffective in patients who developed antiviral resistance, unless the appropriate salvage therapy was administered.
Direct effects of treatment on reducing the rates of disease progression: In addition to effects mediated by HBeAg-seroconversion or HBV response, direct effects of treatments on disease progression were factored in [16]. All short-duration treatments were assumed to have the same magnitude of direct effect on reducing the rates of disease progression as that achieved with lamivudine administered for 1 year. For long-duration therapies, the direct effects on disease progression lasted for the duration of therapy in those patients who did not develop antiviral resistance (Table 1).
Effect of treatment on HR QoL as a result of side-effects: The side-effects of interferon-α were assumed to have a negative effect on health-related quality of life (HR QoL) for the duration of treatment, reducing the CHB utility during treatment [15]. This was also assumed to be the case for pegylated interferon-α 2a but with an impact on CHB utility 50% less than that of interferon-α. Lamivudine and adefovir are well tolerated in CHB patients and were assumed to have at least the same utility as untreated CHB patients (Table 4).
Model outcomes
During annual cycles of the Markov models the proportion of patients remaining alive in each health state and their associated healthcare costs and QALYs were determined for each treatment scenario. The total healthcare costs, life years and QALYs over the 40-year time horizon of the model were calculated for each treatment scenario, in the presence and absence of discounting.
Model assumptions
There is a hierarchy of assumptions. The patient population pertains to those CHB patients with ALT levels ≥2 × ULN; all patients are in the CHB health-state at the outset and all are eligible for therapy. Where available, model inputs were obtained or derived from the literature for this population. However, for some model inputs the ALT status was either elevated or unknown.
A list of all model assumptions is given in Appendix I.
Discounting
Costs and benefits were discounted at 5% per annum, although in year 1 there was no discounting. The effect of discounting was investigated in the sensitivity analyses.
Sensitivity analyses
Sensitivity analyses were carried out for the longer duration CHB antiviral therapies (lamivudine plus adefovir as rescue medication or adefovir plus lamivudine as rescue medication) administered for up to 5 years. One-way sensitivity analyses were carried out by changing individual model inputs over ranges of values obtained from the literature or using pessimistic estimates for the magnitude of treatment effectiveness. In addition, sensitivity analyses were also carried out in which several model inputs were varied simultaneously.
Results
Disease progression in the presence of treatment
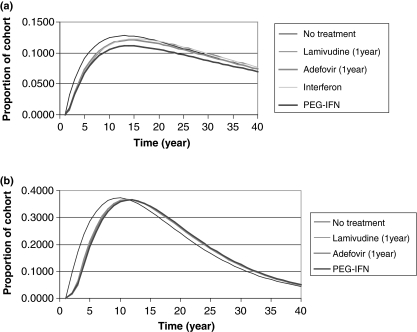
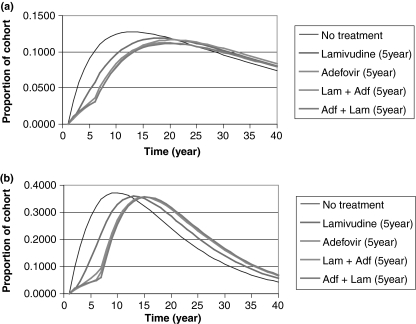
Short-course therapy with α-interferon, or 1-year treatment with pegylated interferon α-2a, adefovir, or lamivudine had limited impact on disease progression (Fig. 3a for HBeAg-positive CHB and Fig. 3b for HBeAg-negative CHB). In contrast, both lamivudine plus adefovir as rescue medication or adefovir plus lamivudine as rescue medication administered for up to 5 years substantially decrease the rate of disease progression. For example, at the end of the treatment phase the proportion of the HBeAg-positive cohort that had developed complications had been reduced by approximately 70% in those receiving these therapies (Fig. 4a). The corresponding impact on disease progression in the HBeAg-negative cohort is shown in Fig. 4b. A comparison of these figures shows that disease progression occurred at a faster rate in HBeAg-negative patients than in HBeAg-positive patients.
Fig. 3.
Short-duration treatment of (a) HBeAg-positive chronic hepatitis B (CHB); (b) HBeAg-negative CHB – proportion of cohort in compensated cirrhosis (CC), decompensated cirrhosis (DCC) or hepatocellular carcinoma (HCC) states during each cycle.
Fig. 4.
Long-duration treatment of (a) HBeAg-positive chronic hepatitis B (CHB); (b) HBeAg-negative CHB – proportion of cohort in compensated cirrhosis (CC), decompensated cirrhosis (DCC) or hepatocellular carcinoma (HCC) states during each cycle.
Outcomes, costs, cost-effectiveness of treatment alternatives
The discounted incremental costs, benefits and cost-effectiveness ratios (ICERs) for treatment alternatives compared with no treatment and lamivudine 1-year are given in Tables 5a and 6a, respectively, for patients with HBeAg-positive CHB, and Tables 5b and 6b, respectively, for patients with HBeAg-negative CHB. (Similar results were obtained in the absence of discounting).
Table 5.
(a) The discounted incremental costs, benefits and cost-effectiveness ratios (ICERs) for treatment alternatives in comparison with no treatment of HBeAg-positive chronic hepatitis B (CHB); (b) The discounted ICERs for treatment alternatives in comparison with no treatment of HBeAg-negative CHB
| Treatment alternatives | Cost (S$) | Life year gained | QALY | Cost per Life Year gained (S$/LY) | Cost per QALY gained (S$/QALY) |
|---|---|---|---|---|---|
| (a) | |||||
| IFN | 6818.65 | 0.273 | 0.110 | 24 952.35 | 61 940.92 |
| PEG-IFN (1 year) | 19 216.05 | 0.566 | 0.570 | 33 977.06 | 33 718.47 |
| Lamivudine (1 year) | 2247.74 | 0.324 | 0.319 | 6943.59 | 7053.58 |
| Adefovir (1 year) | 2982.83 | 0.275 | 0.298 | 10 830.09 | 9993.50 |
| Lamivudine (5 years) | 8031.34 | 0.644 | 0.631 | 12 476.32 | 12 724.48 |
| Adefovir (5 years) | 11 237.25 | 0.738 | 0.740 | 15 223.13 | 15 176.56 |
| Lamivudine + adefovir as rescue (5 years) | 8455.30 | 0.872 | 0.854 | 9695.47 | 9905.72 |
| Adefovir + lamivudine as rescue (5 years) | 10 845.16 | 0.805 | 0.813 | 13 472.82 | 13 344.85 |
| (b) | |||||
| PEG-IFN (1 year) | 19 785.52 | 0.642 | 0.486 | 30 833.44 | 40 707.14 |
| Lamivudine (1 year) | 1928.59 | 0.625 | 0.568 | 3086.05 | 3393.32 |
| Adefovir (1 year) | 2863.18 | 0.516 | 0.471 | 5546.03 | 6078.79 |
| Lamivudine (5 years) | 10 807.30 | 1.133 | 1.077 | 9535.13 | 10 034.00 |
| Adefovir (5 years) | 13 471.57 | 1.731 | 1.665 | 7784.26 | 8090.25 |
| Lamivudine + adefovir as rescue (5 years) | 10 750.93 | 1.838 | 1.740 | 5848.71 | 6178.02 |
| Adefovir + lamivudine as rescue (5 years) | 13 111.95 | 1.887 | 1.816 | 6948.67 | 7218.64 |
Table 6.
(a) The incremental costs, benefits and cost-effectiveness ratios (ICERs) for treatment alternatives in comparison with 1-year course of lamivudine of HBeAg-positive chronic hepatitis B (CHB); (b) The ICERs for treatment alternatives in comparison with 1-year course of lamivudine of HBeAg-negative CHB
| Treatment alternatives | Cost (S$) | Life year gained | QALY | Cost per Life Year gained (S$/LY) | Cost per QALY gained (S$/QALY) |
|---|---|---|---|---|---|
| (a) | |||||
| IFN | 4570.91 | −0.050 | −0.209 | nv | nv |
| PEG-IFN (1 year) | 16 968.32 | 0.242 | 0.251 | 70 161.69 | 67 540.78 |
| Adefovir (1 year) | 735.09 | −0.048 | −0.020 | nv | nv |
| Lamivudine (5 years) | 5783.60 | 0.320 | 0.313 | 18 073.04 | 18 507.18 |
| Adefovir (5 years) | 8989.51 | 0.414 | 0.422 | 21 689.93 | 21 313.88 |
| Lamivudine + adefovir as rescue (5 years) | 6207.56 | 0.548 | 0.535 | 11 319.95 | 11 604.85 |
| Adefovir + lamivudine as rescue (5 years) | 8597.43 | 0.481 | 0.494 | 17 864.69 | 17 403.02 |
| (b) | |||||
| PEG-IFN (1 year) | 17 856.93 | 0.017 | −0.082 | 1 065 894.63 | nv |
| Adefovir (1 year) | 934.59 | −0.109 | −0.097 | nv | nv |
| Lamivudine (5 years) | 8878.72 | 0.508 | 0.509 | 17 461.19 | 17 453.01 |
| Adefovir (5 years) | 11 542.99 | 1.106 | 1.097 | 10 439.73 | 10 524.11 |
| Lamivudine + Adefovir as rescue (5 years) | 8822.34 | 1.213 | 1.172 | 7271.76 | 7528.61 |
| Adefovir + Lamivudine as rescue (5 years) | 11 183.36 | 1.262 | 1.248 | 8861.37 | 8960.64 |
nv, alternative treatments dominated by 1-year course of lamivudine (i.e. better outcomes achieved at lower costs when lamivudine is used).
A cost-effectiveness threshold of approximately S$ 53 000 was estimated for Singapore based on an international cost-effectiveness threshold of US$ 50 000 per QALY and after adjusting for differences in PPP-based GNI per capita (2001) [13,14].
In comparison with no treatment of HBeAg-positive CHB, short-course α-interferon was not a cost-effective therapy (Table 5a). In contrast, base-case estimates of 1-year treatment with pegylated interferon α-2a were below the international threshold for cost-effectiveness in both HBeAg-positive and HBeAg-negative CHB (Table 5a,b respectively).
Lamivudine administered for 1 year is highly cost-effective in comparison with no treatment of both HBeAg-positive and HBeAg-negative CHB (Table 5a,b). One-year treatment of HBeAg-positive CHB with lamivudine resulted in better outcomes (life years and QALYs) at lower cost than either short-course α-interferon or 1-year treatment with adefovir. Although pegylated interferon α-2a resulted in better outcomes than 1-year treatment with lamivudine, it was not cost-effective in comparison with lamivudine (Table 6a). One-year treatment of HBeAg-negative CHB with lamivudine resulted in better outcomes at lower cost than either adefovir or pegylated interferon α-2a (Table 6b).
In comparison with treatment of CHB for 1 year with lamivudine, longer duration antiviral therapies (lamivudine plus adefovir as rescue medication or adefovir plus lamivudine as rescue medication) administered for up to 5 years were found to be highly cost-effective by international standards in both HBeAg-positive and HBeAg-negative CHB. The base-case cost/QALY for treatment with lamivudine plus adefovir as rescue medication for 5 years was S$ 11 605 in HBeAg-positive CHB (Table 6a) and S$ 7529 in HBeAg-negative CHB (Table 6b). These values are only 22% and 14% of the internationally accepted cost-effectiveness threshold value respectively. These conclusions of cost-effectiveness were found to be robust to uncertainties in model inputs as demonstrated by the sensitivity analyses.
Sensitivity analyses
The following model inputs were found to have the greatest effect on the cost-effectiveness of the longer duration antiviral therapies (lamivudine plus adefovir as rescue medication or adefovir plus lamivudine as rescue medication) administered for up to 5 years in the treatment of HBeAg-positive or HBeAg-negative CHB:
Discounting of costs and benefits: In the base case analyses, costs and benefits were discounted at 5% per annum, which is a conservative approach. The ICER estimates decreased (i.e. became more cost-effective) when there was no discounting or when only costs were discounted.
Rates of disease progression: When the maximum rates of disease progression obtained from Lin et al. (2005) [19] were used, particularly for the rate of progression from CHB to CC, the ICER estimates decreased. Higher ICER estimates were obtained when minimum rates of disease progression were used.
-
Treatment effects: The following elements of treatment effect were investigated individually and in combination by increasing these model inputs by a magnitude of 25% or decreasing them by 20%:
– Annual seroconversion rates (HBeAg-positive CHB) or annual HBV response rates (HBeAg-negative CHB)
– Annual seroreversion rates (HBeAg-positive CHB) or annual rate of loss of HBV response (HBeAg-negative CHB)
– Annual antiviral resistance rates
– Magnitude of treatment reductions on the rates of disease progression in those patients who do not achieve HBeAg-seroconversion (HBeAg-positive CHB) or who do not have a positive HBV treatment response (HBeAg-negative CHB). The biggest effect on the ICER estimates occurred when varying the magnitude of treatment reduction on the rate of disease progression from CHB to CC.
Health state utilities: A 25% increase in health state utilities resulted in a 20% decrease in the ICER estimates, whereas a 20% decrease in health state utilities resulted in a 25% increase in ICER estimates.
Healthcare costs (for the different health states and CHB antiviral drug costs): A 25% increase in healthcare costs resulted in a 25% increase in the ICER estimates, whereas a 20% decrease in healthcare costs resulted in a 20% decrease in ICER estimates.
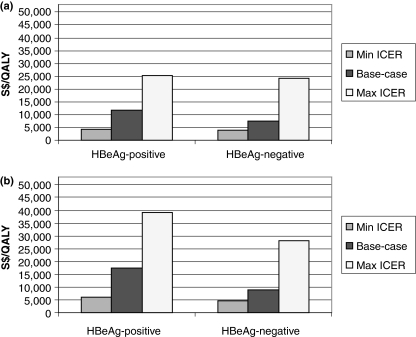
The minimum, base-case and maximum estimated ICERs for lamivudine plus adefovir as rescue medication administered for up to 5 years in the treatment of HBeAg-positive or HBeAg-negative CHB are shown in Fig. 5a. The corresponding estimated ICERs for adefovir plus lamivudine as rescue medication for up to 5 years are shown in Fig. 5b.
Fig. 5.
Summary of sensitivity analyses for (a) lamivudine plus adefovir as rescue (5 years) vs lamivudine (1 year) (b) adefovir plus lamivudine as rescue (5 years) vs lamivudine (1 year).
Discussion
Effectiveness and cost-effectiveness of 5-year antiviral treatment
In comparison with no treatment (Table 5a,b), the most cost-effective short-course treatment for CHB is lamivudine, administered for 1 year. However, short-course and 1-year CHB therapies have limited impact on disease progression (Fig. 3a,b).
In contrast to short-course and 1-year CHB treatment options, lamivudine plus adefovir as rescue medication or adefovir plus lamivudine as rescue medication, administered for up to 5 years, substantially decreased the rate of disease progression in both HBeAg-positive and HBeAg-negative CHB (Fig. 4a,b). Furthermore, by reducing the rates of disease progression, 5-year antiviral treatments decrease non-drug healthcare costs, partially off-setting the antiviral drug acquisition costs. As a result, in comparison with no treatment or treatment with lamivudine for 1-year, both lamivudine plus adefovir as rescue medication or adefovir plus lamivudine as rescue medication administered for up to 5 years are highly cost-effective, with lamivudine plus adefovir as rescue medication having the lowest cost per QALY (Tables 5a,b and 6a,b respectively).
Choice of antiviral treatment regimen
Although lamivudine plus adefovir as rescue medication had a lower cost per QALY than adefovir plus lamivudine as rescue medication in both HBeAg-positive CHB (Tables 5a & 6a) and HBeAg-negative CHB (Tables 5b & 6b), both regimens were highly cost-effective by international standards. Therefore, physicians have a choice as to which antiviral treatment option to use in the long-term management of their CHB patients. Initiating patients with adefovir therapy has the clinical advantage of a lower risk of resistance emerging, especially in the early stages of treatment, whereas lamivudine has a lower acquisition drug cost for those patients of more modest means who need to pay out of pocket for their medication.
Comparisons with other CHB economic evaluations
Crowley et al. (2002) [16] found that antiviral treatment (lamivudine) for 4 years was an economically dominant strategy compared with no treatment or short-duration treatment with α-interferon. However, the analysis by Crowley et al. (2002) [16] was limited in so far as the evaluation only included patients with HBeAg-positive CHB and there was a failure to take into account the emergence of anti-viral resistance during therapy.
Kanwal et al. (2005) [20] used a Markov model incorporating the emergence of antiviral resistance to evaluate the cost-effectiveness of alternative CHB treatment strategies. Their economic evaluation concluded that lamivudine plus adefovir as rescue (salvage) therapy may be highly cost-effective across most healthcare settings. The main differences between the economic modelling performed by Kanwal et al. (2005) [20] and the present evaluation are:
The price differential between adefovir and lamivudine is much greater in the US than in Singapore (i.e. an approximate 250% increase vs a 30% increase in the acquisition cost of adefovir in comparison with lamivudine respectively) or in other Asian countries.
The present study investigated antiviral treatment of CHB for up to 5 years in comparison with life-time treatment. This reduced the extent of assumptions that needed to be made concerning treatment effectiveness and rates of emergence of anti-viral resistance beyond those observed in clinical trials.
These differences may partly explain why Kanwal et al. (2005) [20] concluded that α-interferon might be most cost-effective in healthcare systems with tight budgetary constraints and with a high prevalence of HBeAg-negative disease. However as pointed out by Weitzman and Jacobson (2005) [21], α-interferon treatment is equivalent to a ‘do nothing’ strategy for more than 70% of patients. In contrast, the present evaluation found that short-course α-interferon was not cost-effective in HBeAg-positive CHB. However, when compared with no treatment, 1-year treatment with pegylated interferon α-2a was below the international threshold for cost-effectiveness in both HBeAg-positive and HBeAg-negative CHB (Tables 5a & 6a).
Sullivan et al. (2006) [22] evaluated the cost-effectiveness of 1-year treatment with pegylated interferon α-2a compared with 1-year lamivudine in HBeAg-positive CHB from the perspective of the Taiwan Bureau of Nation Health Insurance. They concluded that pegylated interferon α-2a provided QALY gains at a favourable cost-effectiveness ratio. Sullivan et al. (2006) [22] used a Markov model structure that was similar to that of Crowley et al. (2000) [15], i.e. emergence of anti-viral resistance was not included. When we repeated the economic evaluation using the present model based on Taiwanese drug acquisition and healthcare costs, similar results and conclusions were obtained to those of Sullivan et al. (2006) [22]. Thus, conclusions of cost-effectiveness cannot be extended from one country to another without performing a country-specific economic evaluation. In addition, our analysis found that up to 5 years treatment with lamivudine and adefovir as rescue therapy provided better outcomes at lower cost than pegylated interferon α-2a in Taiwan.
We also conducted threshold analyses to estimate the acquisition cost in Singapore that would be required for pegylated interferon α-2a to achieve a similar cost per QALY (in comparison with no treatment) to that achieve with long duration sequential antiviral therapies. In HBeAg-positive CHB, the acquisition cost of pegylated interferon α-2a would need to be decreased by approximately 55% to be as cost-effective as adefovir plus lamivudine as rescue medication for up to 5 years and by 65% to be as cost-effective as lamivudine plus adefovir as rescue medication. In HBeAg-negative CHB, the acquisition cost of peg-IFN would need to be decreased by approximately 80% to be as cost-effective as the long-duration sequential antiviral therapies.
Study limitations
The economic evaluation was limited to CHB patients, with elevated ALT levels at least twice upper limit of normal and who, on initiation of treatment, did not have cirrhosis or HCC. If patients with lower baseline ALT levels or advanced liver disease were included, it might be expected that the benefits of long-duration nucleoside analogue therapies over both short-term therapies including interferons, would be further enhanced given that high pre-treatment levels of ALT is a predictive factor for end-of-treatment response and interferons are contra-indicated in patients with DCC [23].
There was limited published data on the annual loss of response following treatment with pegylated interferon α-2a. More recently, longer duration post-treatment data have been published as abstracts. Lau et al. (2006) [24] appeared to provide a 12-month seroreversion rate in HBeAg-positive patients of 9% (n = 6/69) for pegylated interferon α-2a. This compares with our derived estimate of 4.4%. As Lau et al. (2006) [24] referred to ‘relapse/no response’, there is reason to suspect that an estimate of 9% would be an over-estimate. Moreover, a seroreversion rate of 4.4% would favour pegylated interferon α-2a in comparison with antiviral treatment alternatives. Marcellin et al. (2006) [25] provided 6- to 24-month post-treatment data in HBeAg-negative patients. However, the HBV response data were based on a HBV–DNA cut-off of 100 000 copies/mL level. This is much higher than the threshold for not-detectable HBV-DNA used for the comparative treatments (300–400 copies/mL) and hence these data from Marcellin et al. (2006) [25] could not be used in the model.
Another limitation of the present economic evaluation is that patient adherence during long-term antiviral treatment in the real-world setting may be worse than occurred in the adefovir and lamivudine clinical trials. A lower level of adherence may be expected to decrease the rate of HBeAg seroconversion or HBV treatment response, and decrease the magnitude of treatment effect on disease progression. Nonetheless, it would require the choice of pessimistic estimates across several variables before there would be any real uncertainty as to the cost-effectiveness of 5-year antiviral treatment with adefovir or lamivudine as rescue medication in comparison with no treatment or 1-year treatment with lamivudine, in both HBeAg-positive and HBeAg-negative CHB.
This economic evaluation pertains specifically to Singapore. However, given the highly cost-effective conclusions in relation to 5-year antiviral therapies in both HBeAg-positive and HBeAg-negative CHB, these conclusions might be applicable to other countries that have similar ratios of annual antiviral acquisition costs to the healthcare costs of managing the complications of disease progression. However, country-specific economic evaluations would be required to confirm this.
This economic evaluation only included direct healthcare costs. However, if indirect costs (such as productivity losses for time off work due to CHB-related sickness) had been taken into account, it would be expected that the cost-effectiveness of long-duration antiviral therapies from a societal perspective would be further enhanced.
Lastly, newer anti-viral treatments, such as entecavir, sequential interferon/antiviral based-strategies and combination lamivudine plus adefovir as initial therapy, were not investigated in this economic evaluation.
Conclusions
In Singapore, antiviral treatment of CHB with lamivudine or adefovir for up to 5 years using the alternative antiviral agent as rescue medication (upon emergence of anti-viral drug resistance) is predicted to substantially decrease the rate of disease progression at the end of the treatment period, in both HBeAg-positive and HBeAg-negative CHB. By reducing the rates of disease progression, 5-year antiviral treatments decrease non-drug healthcare costs, partially off-setting the antiviral drug acquisition costs.
In Singapore, antiviral treatment of CHB with lamivudine or adefovir for up to 5 years using the alternative antiviral agent as rescue medication was found to be highly cost-effective, in comparison with no treatment or 1-year treatment with lamivudine, in both HBeAg-positive and HBeAg-negative CHB, with lamivudine and adefovir as rescue medication being the most cost-effective strategy.
Sensitivity analyses indicate that these economic conclusions are robust to uncertainties in model inputs.
Combination therapy was only considered for rescue of patients who had developed resistance to either lamivudine or adefovir monotherapy. Further studies should include combination lamivudine plus adefovir as initial therapy. Moreover, newer antiviral treatments such as entecavir and other sequential treatment strategies (e.g. using interferons and antivirals) should be evaluated.
Glossary
Abbreviations:
- ALT
aminotransferase
- CC
compensated cirrhosis
- CHB
chronic hepatitis B
- DCC
decompensated cirrhosis
- GNI
Gross National Income
- HBeAg
hepatitis B e antigen
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HR QoL
health-related quality of life
- ICERs
incremental cost-effectiveness ratios
- PEG-IFN
pegylated interferon
- PPP
purchasing power parity
- QALY
quality adjusted life year
Appendix
Appendix 1.
Assumptions for model inputs
| Model input | Treatment | Assumption |
|---|---|---|
| HBeAg-seroconversion rates for patients with ALT levels ≥2 × ULN | Lamivudine | Annual seroconversion rate for years 2–5 is the same as for year 1 in the absence of resistance |
| Adefovir | Annual seroconversion rate for years 2–5 remains constant in the absence of resistance | |
| HBeAg-seroconversion lack of durability (% who relapse back to CHB state) | Lamivudine | % Relapse rate per year is average of the annual rates occurring over years 1–3 after stopping treatment (from Dienstag et al. 2003 [26]). The rate of relapse does not change with time |
| IFN | From Crowley et al. 2002 [16], relapse rates are assumed to be the same for IFN and lamivudine. The rate of relapse does not change with time | |
| PEG-IFN | Derived from Dienstag et al. 2003 [26] and Lau et al. 2005 [17] | |
| Adefovir | From Chang et al. 2004 [27], baseline ALT levels unknown. Therefore, assuming that ALT levels ≥2 × ULN. The rate of relapse does not change with time | |
| HBV response rates for patients with ALT levels >2 × ULN | Lamivudine | Annual response rate for years 2–5 in the absence of resistance is the same as for year 1 |
| Adefovir | Annual response rate for adefovir in the absence of resistance for years 2–5 is the same as for year 1 | |
| Annual lack of durability of HBV response (% who relapse back to CHB state) | All treatments | The annual rate of relapse does not change with length of time post-treatment |
| Emergence of resistance during treatment | Lamivudine | Resistance rates for years 1–5 is the average of the annual rates occurring over years 1–4. |
| IFN/PEG-IFN | No resistance occurs during treatment | |
| Adefovir | Resistance rate for years 2–5 is the average of the annual rates occurring over years 2–4. There is no resistance in year 1 | |
| Annual rate of progression from CHB to CC for those who do not seroconvert or have HBV response | In absence of treatment | Rate used is applicable to Singaporeans |
| % Decrease in rate of progression from CHB to CC in the absence of resistance | Lamivudine | For years 2–5 it is assumed that control is maintained in those who do not develop resistance |
| IFN/PEG-IFN | It is assumed that the direct effect of IFN/PEG-IFN on disease progression during treatment is the same as for lamivudine | |
| Adefovir | Assumes that control is as good with adefovir as with lamivudine in those who do not develop resistance | |
| % Decrease in rate of progression from CHB to CC in presence of resistance | All treatments | Assumption of no effect |
| Annual rate of progression from CHB to HCC | In absence of treatment | Rate used is applicable to Singaporeans |
| % Decrease in rate of progression from CHB to HCC in presence of resistance | All treatments | Assumption of no effect |
| Annual rate of progression from CC to DCC | In absence of treatment | Rate used is applicable to Singaporeans |
| % Decrease in rate of progression from CC to DCC in absence of resistance | Lamivudine | Increase in Child-Pugh score is indicative of progression to DCC. |
| IFN/PEG-IFN | It is assumed that the effect of IFN/PEG-IFN during treatment is the same as for lamivudine | |
| Adefovir | Assumes that control is as good with Adefovir as with lamivudine in those who do not develop resistance | |
| % Decrease in rate of progression from CC to DCC in presence of resistance | All treatments | It is assumed that the effect of IFN/PEG-IFN during treatment is the same as for lamivudine. |
| Annual rate of progression from CC to HCC | In the absence of treatment | Rate used is applicable to Singaporeans |
| % Decrease in rate of progression from CC to HCC in absence of resistance | Lamivudine | For years 2–5, it is assumed that control is maintained in those who do not develop resistance |
| IFN/PEG-IFN | It is assumed that the effect of IFN/PEG-IFN during treatment is the same as for lamivudine | |
| Adefovir | It is assumed that control is as good with adefovir as with lamivudine in those who do not develop resistance | |
| % Decrease in rate of progression from CC to HCC in presence of resistance | All treatments | Assumption of no effect |
| Annual rate of progression from DCC to HCC | In the absence of treatment | Rate used is applicable to Singaporeans |
| Annual rate of progression from seroconversion or HBV response to death | Life table values(Singapore 2001) | From age-specific life tables |
| Annual rate of progression from CHB to death | In the absence of treatment | Rate used is applicable to Singaporeans |
| Annual rate of progression from CC to death | In the absence of treatment | Rate used is applicable to Singaporeans |
| Annual rate of progression from DCC to death | In the absence of treatment | Rate used is applicable to Singaporeans |
| Annual rate of progression from HCC to death | In the absence of treatment | Rate used is applicable to Singaporeans |
| Annual acquisition costs (wholesale) of drugs | All treatments | IFN costs are average for Roferon-A and Intron-A and 6–12 months treatment durations. PEG-IFN costs are the average of those for Pegasys and Peg-Intron for a duration of 12 months |
| Annual cost following HBeAg-seroconversion (S$) | In the absence of treatment | Not available from Li et al. 2004 [6]. Assumed that the annual costs are 50% of the cost of CHB (from Crowley et al. 2002) [16] |
| Utility for CHB | All treatments | Lamivudine and adefovir are well tolerated in CHB patients and have at least the same utility as untreated patients. PEG-IFN is better tolerated than IFN but less well tolerated than lamivudine or adefovir, resulting in a utility mid-way between that for IFN and lamivudine/adefovir |
| Utility for resistant CHB | All treatments | No effect of resistance per se on the health state utility For other assumptions refer to those for Utility for CHB |
| Utility for resistant CC | Irrespective of treatment | No effect of resistance per se on the health state utility |
| Utility for resistant DCC | Irrespective of treatment | No effect of resistance per se on the health state utility |
| Utility for resistant HCC | Irrespective of treatment | No effect of resistance per se on the health state utility |
References
- 1.Hepatitis B. World Health Organisation (WHO) publications; WHO/CDS/C5R/LYO/2002. 2:Hepatitis B ( http://www.who.int/emc) [Google Scholar]
- 2.McMahon BJ. The natural history of chronic hepatitis B virus infection. Semin Liver Dis. 2004;24(Suppl. 1):17–21. doi: 10.1055/s-2004-828674. [DOI] [PubMed] [Google Scholar]
- 3.Chen C-J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum HBV DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 4.Iloeje U, et al. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678–686. doi: 10.1053/j.gastro.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 5.Liaw Y-F, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. New Engl J Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 6.Li SC, et al. A cost comparison of management of chronic hepatitis B and its associated complications in Hong Kong and Singapore. J Clin Gastroenterol. 2004;38:S136–S143. doi: 10.1097/00004836-200411003-00004. [DOI] [PubMed] [Google Scholar]
- 7.Liaw Y-F, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2005 update. Liver Int. 2005;25:472–489. doi: 10.1111/j.1478-3231.2005.01134.x. [DOI] [PubMed] [Google Scholar]
- 8.Marcellin P. Treatment of chronic hepatitis B. J Viral Hepat. 2005;12:333–345. doi: 10.1111/j.1365-2893.2005.00599.x. [DOI] [PubMed] [Google Scholar]
- 9.Locarnini S, et al. Management of antiviral resistance in patients with chronic hepatitis B. Antivir Ther. 2004;9:679–693. [PubMed] [Google Scholar]
- 10.Lai CL, et al. Prevalence and clinical correlates of YMDD variants during lamivudine therapy for patients with chronic hepatitis B. Clin Infect Dis. 2003;36:687–696. doi: 10.1086/368083. [DOI] [PubMed] [Google Scholar]
- 11.Marcellin P, et al. Long term efficacy and safety of adefovir dipivoxil (ADV) 10 mg in HBeAg+ chronic hepatitis B (CHB) patients: increasing serologic, virologic and biochemical response over time. Hepatology. 2004;40(Suppl. 1):655A. [Google Scholar]
- 12.Kobelt G. Cost utility analysis. In: Health economics - an introduction to economic evaluation. London. Office of Health Economics. 1996:21–25. [Google Scholar]
- 13.Towse A. What is NICE's threshold? An external view. In: Devlin N, editor. Cost-effectiveness Thresholds: Economic and Ethical Issues. London: King's Fund/Office for Health Economics; 2002. pp. 25–30. chapter 2. [Google Scholar]
- 14.Ngee WS. Purchasing Power Parity (PPP): The International Comparison Program (ICP) Statistics Singapore Newsletter; 2004. 3–6 ( http://www.singstat.gov.sg/) [Google Scholar]
- 15.Crowley SJ. Cost-effectiveness analysis of lamivudine for the treatment of chronic hepatitis B. Pharmacoeconomics. 2000;17:409–427. doi: 10.2165/00019053-200017050-00001. [DOI] [PubMed] [Google Scholar]
- 16.Crowley S, et al. Introduction of lamivudine for the treatment of chronic hepatitis B: expected clinical and economic outcomes based on 4-year clinical trial data. J Gastroenterol Hepatol. 2002;17:153–164. doi: 10.1046/j.1440-1746.2002.02673.x. [DOI] [PubMed] [Google Scholar]
- 17.Lau GKK, et al. Peginterferon alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352:2682–2695. doi: 10.1056/NEJMoa043470. [DOI] [PubMed] [Google Scholar]
- 18.Marcellin P, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2004;351:1206–1217. doi: 10.1056/NEJMoa040431. [DOI] [PubMed] [Google Scholar]
- 19.Lin X, et al. Chronic hepatitis B virus infection in the Asia-Pacific region and Africa: review of disease progression. J Gastroenterol Hepatol. 2005;20:833–843. doi: 10.1111/j.1440-1746.2005.03813.x. [DOI] [PubMed] [Google Scholar]
- 20.Kanwal F, et al. Treatment alternatives for chronic hepatitis B virus infection: a cost-effectiveness analysis. Annals Intern Med. 2005;142:821–831. doi: 10.7326/0003-4819-142-10-200505170-00007. [DOI] [PubMed] [Google Scholar]
- 21.Weitzman G. Cost-effectiveness in hepatitis B. Annals Intern Med. 2005;142:757–758. doi: 10.7326/0003-4819-143-10-200511150-00021. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan SD, et al. Cost-effectiveness of peginterferon alfa-2a compared to lamivudine treatment in patients with hepatitis B e antigen positive chronic hepatitis B in Taiwan. J Gastroenterol Hepatol. 2006 doi: 10.1111/j.1440-1746.2006.04539.x. Available Online. [DOI] [PubMed] [Google Scholar]
- 23.de Francis R, et al. EASL International Consensus Conference on Hepatitis B. 13–14 September, 2002 Geneva, Switzerland. Consensus statement (long version) J Hepatol. 2003;39(Suppl. 1):S3–25. [PubMed] [Google Scholar]
- 24.Lau G, et al. Durability of response and occurrence of late response to peginterferon alpha-2a (40 kd) [one year post-treatment in patients with HBeAg-positive chronic hepatitis] J Hepatol. 2006;44:S23. [Google Scholar]
- 25.Marcellin P, et al. The majority of patients with HBeAg-negative chronic hepatitis B treated with peginterferon alpha-2a (40 kd) sustain responses 2 years post-treatment. J Hepatol. 2006;44:S275. [Google Scholar]
- 26.Dienstag JL, et al. Durability of serologic response after lamivudine treatment of chronic hepatitis B. Hepatology. 2003;37:748–755. doi: 10.1053/jhep.2003.50117. [DOI] [PubMed] [Google Scholar]
- 27.Chang TT, et al. Durability of HBeAg seroconversion after adefovir dipivoxil treatment for chronic hepatitis B (CHB) J Hepatol. 2004;40(Suppl. 1):126. [Google Scholar]
- 28.Singapore Life Tables. WHO Statistical Information System (WHOSIS), Life Tables for WHO member states: WHO; 2001. ( http://www.who.int/whosis/en/) [Google Scholar]
- 29.Perrillo RP, et al. Predictors of HBeAg loss after lamivudine treatment for chronic hepatitis B. Hepatology. 2002;36:186–194. doi: 10.1053/jhep.2002.34294. [DOI] [PubMed] [Google Scholar]
- 30.Marcellin P, et al. Baseline ALT predicts histologic and serologic response in patients with HBeAg+ chronic hepatitis B treated with adefovir dipivoxil (ADV) J Hepatol. 2002;36(Suppl. 1):122–123. [Google Scholar]
- 31.Locarnini S, et al. Incidence of predictors of emergence of adefovir resistant HBV during four years of adefovir dipivoxil (ADV) therapy for patients with chronic hepatitis B (CHB) Hepatology. 2005;42(Suppl. 2):17. [Google Scholar]
- 32.Hadziyannis SJ, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med. 2003;348:800–807. doi: 10.1056/NEJMoa021812. [DOI] [PubMed] [Google Scholar]
- 33.Hadziyannis SJ, et al. Long-term treatment with adefovir dipivoxil for HBeAg-negative chronic hepatitis B. N Engl J Med. 2005;352:2673–2681. doi: 10.1056/NEJMoa042957. [DOI] [PubMed] [Google Scholar]