Abstract
Using embryos transgenic for the TOP-GFP reporter, we show that the two zebrafish β-catenins have different roles in the organizer and germ-ring regions of the embryo. β-catenin-activated transcription in the prospective organizer region specifically requires β-catenin-2, whereas the ventrolateral domain of activated transcription is abolished only when both β-catenins are inhibited. chordin expression during zebrafish gastrulation has been previously shown in both axial and paraxial domains, but is excluded from ventrolateral domains. We show that this gene is expressed in paraxial territories adjacent to the domain of ventrolateral β-catenin-activated transcription, with only slight overlap, consistent with the now well-known inhibitory effects of Wnt8 on dorsal gene expression. Eliminating both Wnt8/β-catenin signaling and organizer activity by inhibition of expression of the two β-catenins results in massive ectopic circumferential expression of chordin and later, by formation of a distinctive embryonic phenotype (‘ciuffo’) that expresses trunk and anterior neural markers with correct relative anteroposterior patterning. We show that chordin expression is required for this neural gene expression. The Nodal gene squint has been shown to be necessary for optimal expression of chordin and is sufficient in some contexts for its expression. However, chordin is not normally expressed in the ventrolateral germ-ring despite robust expression of squint in this domain. We show the ectopic circumferential expression of chordin and other dorsal genes to be completely dependent on Nodal and FGF signaling, and to be independent of a functional organizer. We propose that whereas the axial domain of chordin expression is formed by cells that are derived from the organizer, the paraxial domain is the result of axial-derived anti-Wnt signals, which relieve the repression that otherwise is set by the Wnt8/β-catenin/vox,vent pathway on latent germ-ring Nodal/FGF-activated expression.
Keywords: β-catenin, Wnt8, Vox, Vent, Nodal signaling, FGF signaling, dorsoventral patterning, ichabod (ich)
1. Introduction
Dorsoventral patterning of the zebrafish embryo, as in other vertebrates, is a consequence of signaling by canonical Wnt, BMP, Nodal, and FGF pathways (reviewed in Harland and Gerhart, 1997; Schier and Talbot, 1998, 2005; De Robertis and Kuroda, 2004). Both mesodermal and ectodermal dorsoventral tissue identities are set at least in part by the level of BMP signaling (Wilson et al., 1997; Dosch et al., 1997; Nguyen et al., 1998; Eimon and Harland, 1999; Barth et al., 1999; Ramel et al., 2005), which itself is under the control of the secretion of anti-BMP factors produced in the dorsal organizer region (reviewed in Harland and Gerhart, 1997; Yamamoto and Oelgeschlager, 2004). These BMP antagonists include Chordin (Sasai et al., 1994, 1995; Piccolo et al., 1996), Noggin (Smith and Harland, 1992; Zimmerman et al., 1996; Groppe et al., 2002) and Follistatin (Hemmati-Brivanou et al., 1994; Fainsod et al., 1997).
β-catenin-mediated signaling is essential in the development of the vertebrate dorsal embryonic signaling center. A functional β-catenin gene is required for formation of the Spemann organizer in amphibians (Heasman et al., 1994; Wylie et al., 1996; Heasman, 2000) and for normal axis formation in the mouse (Haegel et al., 1995; Huelsken et al., 2000). In the zebrafish, severe ventralization of embryos bred from females homozygous for the maternal effect ichabod (ich) mutation is accompanied by a failure of nuclear localization of β-catenin in dorsal cells and the dorsal YSL (Kelly et al., 2000). These effects are due to an impairment of maternal expression of a second β-catenin gene, β-catenin-2, which maps in proximity to the ich mutation and is required for organizer and axis formation (Bellipanni et al., 2006).
In both amphibians and teleosts, chordin (chd) expression in the organizer region is clearly dependent on β-catenin. In Xenopus, dorsal expression of chd prior to the onset of gastrulation requires an active β-catenin pathway, and ectopic β-catenin expression is sufficient to induce expression of chd and other BMP antagonists (Wessely et al., 2001). In the zebrafish, chd is first expressed in the mid-blastula on the future dorsal side of the embryo and later in the embryonic shield in both axial and paraxial domains (Miller-Bertoglio et al., 1997; Schulte-Merker et al., 1997; Shimizu et al., 2000). chd expression is absent or greatly reduced in ich embryos at all stages, but can be induced by β-catenin produced from injected RNA (Kelly et al., 2000; Bellipanni et al., 2006; Maegawa et al., 2006). Inhibition of β-catenin function by expression of a dominant negative form of Tcf3 (dnTcf3) (Pelegri and Maischein, 1998) or by expression of axin (Shimizu et al., 2000) results in elimination of chd expression at blastula stages (but not at shield stage, see Discussion).
Wnt signaling pathways are not only important in establishment of the organizer, but also have a modifying or opposing role during gastrulation of teleost and amphibian embryos. Wnt8 is expressed in the ventrolateral germ-ring of zebrafish embryos (Kelly et al., 1995) as bicistronic transcripts (Lekven et al., 2001) and is required for formation of ventrolateral and posterior mesoderm, spinal cord and posterior brain (Lekven et al., 2001; Erter et al., 2001; Momoi et al., 2003; Ramel and Lekven, 2004). Ventrolateral Wnt signaling during gastrulation, as well as the earlier dorsal β-catenin-mediated gene activation, can be visualized in zebrafish embryos carrying a transgene in which GFP is expressed from a β-catenin-responsive promoter (Dorsky et al., 2002). In Xenopus, wnt-8 is expressed in a wide vegetal region including the ventrolateral marginal zone (Christian et al., 1991; Smith and Harland, 1991; Christian and Moon, 1993) and has a similar ventralizing and posteriorizing role (Christian and Moon, 1993; Hoppler et al., 1996; Fredieu et al., 1997). Wnt8 represses organizer function in the zebrafish embryo by inducing the expression of the homeobox genes vox/vega1 and vent/vega2 and this induction requires β-catenin (Ramel and Lekven, 2004). The zygotic expression of a third member of this homeobox family, ved (Shimizu et al., 2002; Gilardelli et al., 2004), is also partially under wnt8 control (Ramel et al., 2005). These homeobox proteins act as redundant transcriptional repressors to limit the expression of genes such as goosecoid (gsc), bozozok (boz) and chordin (chd) to the organizer region (Kawahara et al., 2000a, 2000b; Melby et al., 2000; Imai et al., 2001; Ramel and Lekven, 2004; Ramel et al., 2005; Shimizu et al., 2005). Similar ventral homeobox genes (Xvents) also function in Xenopus to repress organizer gene expression (including gsc and chd) (Onichtchouk et al., 1996; Hoppler and Moon, 1998; Melby et al., 1999; Trindale et al., 1999). Loss of function of zebrafish wnt8 or of both vox/vega1 and vent/vega2 results in expansion of axial mesoderm with similar expansion of chd and other dorsal markers to the lateral germ-ring (Lekven et al., 2001; Erter et al., 2001; Imai et al., 2001; Ramel and Lekven, 2004; Ramel et al., 2005). These results are consistent with the finding that in Xenopus, Vox can directly repress chd transcription (Melby et al., 1999). Transcription of vox and vent is also under control of BMP signaling, but mainly starting at 70–75% epiboly (Kawahara et al., 2000a; Melby et al., 2000; Imai et al., 2001; Ramel and Lekven, 2004). Thus, in contrast to the dependence of chd expression on β-catenin in the organizer and dorsal regions of the embryo during blastula stages, chd expression during early and mid-gastrula is under negative control of Wnt signaling and β-catenin (through Vox and Vent) in the ventrolateral germ-ring of the zebrafish embryo.
β-catenin induction of chd in the zebrafish is dependent on the homeodomain protein Bozozok and the Nodal-related factor Squint. squint (sqt) (Feldman et al., 1998; Erter et al., 1998; Rebagliati et al., 1998) and bozozok/dharma/nieuwkoid (boz) (Yamanaka et al., 1998; Koos and Ho, 1998; Fekany et al., 1999) act in parallel to establish the organizer (Sirotkin et al., 2000; Shimizu et al., 2000; reviewed in Schier and Talbot, 2005). These two genes are induced by β-catenin (Kelly et al., 2000; Shimizu et al., 2000; Maegawa et al., 2006) and embryos lacking both genes fail to express chd, although single mutant embryos still can express chd transcript (Sirotkin et al., 2000; Shimizu et al., 2000; Maegawa et al., 2006). As in amphibians, zebrafish embryos lacking mesendoderm, due to loss of function of Nodal genes (in this case, sqt and cyclops) or absence of One-eyed pinhead, can still express chd and form neurectoderm (Feldman et al., 1998; Gritsman et al., 1999; Shimizu et al., 2000; Maegawa et al., 2006). Expression of chd has been shown to be under the control of FGF signaling in both Xenopus (Mitchell and Sheets, 2001) and zebrafish (Londin et al., 2005; Maegawa et al., 2006). Moreover, dorsalization of zebrafish embryos by FGF3 does not occur in embryos (chordino mutants) lacking chd function (Koshida et al, 2002), and we have recently shown that FGF signaling is required for induction of chordin by both Boz and Sqt (Maegawa et al., 2006). There is thus a firm basis for a pathway of chd induction by β-catenin mediated by both Nodal pathway and FGF pathway signaling.
Insight into the multiple roles of β-catenin in the patterning of zebrafish embryos has come from work with the maternal effect mutant ich. Embryos bred from homozygous ich females are highly ventralized and fail to form a functional organizer (Kelly et al., 2000). The zebrafish genome contains two β-catenin genes and formation of the dorsal organizing center was found to be dependent on maternal expression of β-catenin-2 but not β-catenin-1 (Bellipanni et al., 2006). The deficiency in β-catenin-2 function is due to a genome rearrangement in close proximity to β-catenin-2 (S. Maegawa, M. Nimmakayalu, B. Emanuel, E.S. Weinberg, unpublished results). Wild-type embryos treated with antisense morpholino oligonucleotides (MOs) directed against β-catenin-2 transcript (βcat2MO), but not against β-catenin-1 transcript (βcat1MO), show ventralized phenotypes similar to that of the ich mutant, and βcat2MO treatment of ich embryos results in a shift towards the most ventralized phenotypes (Bellipanni et al., 2006). When ich embryos were treated with βcat1MO(or with both βcat1MO and βcat2MO), or when wild-type embryos were treated with both MOs, a new phenotype was observed (termed ‘ciuffo’), which is characterized by a protrusion of tissue extending from the vegetal end of the yolk, robust expression of neurectodermal markers in apparently correct anteroposterior order, and formation of neurons (Bellipanni et al., 2006). In ich embryos, which lack a functional organizer and have low amounts of maternal β-catenin-2 transcript, β-catenin-1 expression alone is able to repress the expression of all neural markers tested. However, in wild-type embryos, inhibition of β-catenin-1 does not lead to an expansion of neural tissue or patterning defects. Thus, only when expression of both β-catenin genes is inhibited do we find a de-repression of neural markers (Bellipanni et al., 2006). That chd was expressed ectopically around the germ-ring of such embryos starting at 50% epiboly (Bellipanni et al., 2006) is consistent with the formation of neurectoderm in these treated embryos, but the network of control of ventrolateral chd expression was not clear.
We show here that in contrast to the specific requirement for β-catenin-2 in organizer formation, the two zebrafish β-catenins act redundantly in response to Wnt8 to induce expression of vox and vent and to repress and limit chd expression to the dorsal and paraxial regions of the embryo. We demonstrate that ventrolateral β-catenin-activated transcription of a reporter gene expressed from a promoter with multiple Tcf3 sites (TOP-GFP) is abolished only when both β-catenins are inhibited, whereas inhibition of only β-catenin-2 results in failure to activate transcription in the dorsal organizing center domain. We show that chd expression is normally excluded from the ventrolateral domain of Wnt/β-catenin signaling activity. Elimination of this signaling results in a massive circumferential ectopic expression of chd and development of embryos with the ‘ciuffo’ phenotype. Consistent with previous work in which wnt8 or vox and vent expression has been inhibited directly (Erter et al., 2001; Imai et al., 2001; Lekven et al., 2001; Ramel and Lekven, 2004; Ramel et al., 2005) or indirectly (Reim and Brand, 2006), we showed that in ich embryos lacking maternal β-catenin-2, inhibition of β-catenin-1 expression leads to ventrolateral germ-ring expression of chd (Bellipanni et al., 2006). In addition we now show that at mid-gastrula, inhibition of both β-catenins, wnt8, or vox and vent results in a broad circumferential chd expression domain that encompasses approximately half of the embryo. The normal pathway of repression of chd in the ventrolateral germ-ring is thus dependent on Wnt8 (Lekven et al., 2001; Erter et al., 2001) induction of Vox and Vent (Imai et al., 2001; Ramel et al., 2004), shown here to be mediated by both β-catenins. We also present evidence that the ectopic germ-ring chd expression is completely dependent on germ-ring-derived Nodal and FGF signaling. Thus, without a functional Wnt8 signaling pathway, the same factors that normally induce chd on the dorsal side of the embryo are able to function ventrolaterally to induce the gene.
2. Results
2.1. Dorsal TOP-GFP expression is dependent only on β-catenin-2 whereas β-catenin-1 or β-catenin-2 is sufficient for later germ-ring TOP-GFP expression
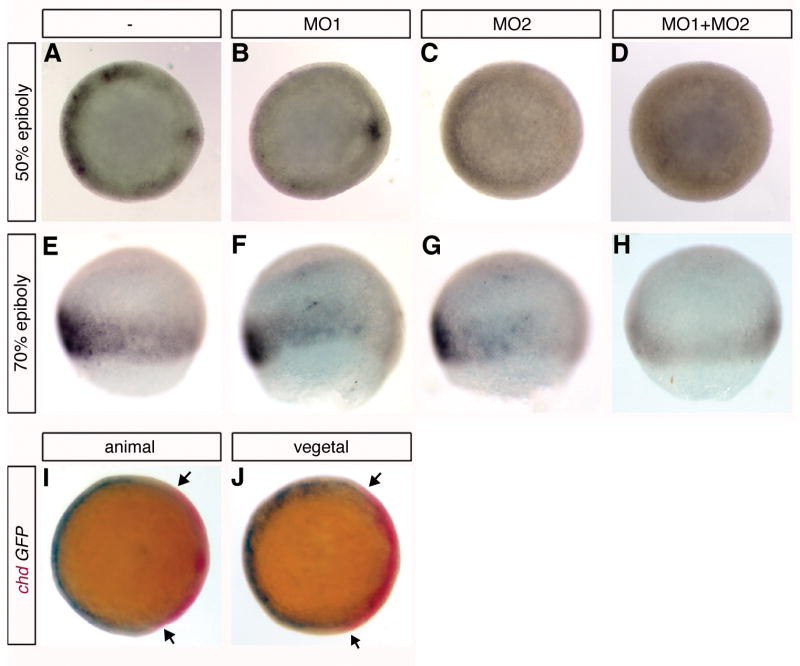
To test our hypothesis that germ-ring Wnt8 signaling can be mediated by either of the two β-catenins, we made use of the Tg(TOP:GFP)w25 transgenic line of zebrafish in which β-catenin-dependent transcriptional activity can be monitored by expression of a GFP reporter driven from a promoter containing multiple Tcf/Lef elements (Dorsky et al., 2002). As already shown (Dorsky et al., 2002), β-catenin-mediated gene activation can be observed in both the incipient embryonic shield and in the ventrolateral germ-ring at 50% epiboly (Fig. 1A). At 70% epiboly, germ-ring gene activation increases and the dorsal domain disappears (Fig. 1E). When βcat1MO is injected into these embryos, there is some decrease in the ventrolateral gene response at 50% epiboly, but no decrease in dorsal activity (Fig. 1B). Injection of βcat2MO, in contrast, completely abolishes dorsal activity and also decreases the ventrolateral activity at this developmental time (Fig. 1C). At 70% epiboly, when only the ventrolateral germ-ring activity can be detected in untreated embryos (Fig. 1E), both βcat1MO and βcat2MO decrease the signaling response in dorsolateral regions (Fig. 1F,G), but when both MOs are injected, the β-catenin-mediated signaling activity completely disappears (Fig. 1H). We conclude that β-catenin-mediated transcription in the organizer is completely dependent on expression of β-catenin-2, consistent with our previous finding that maternal β-catenin-2 expression is required for formation of the organizer (Bellipanni et al., 2006). We further conclude that the ventrolateral germ-ring signaling is dependent on both β-catenins, confirming our previous finding that repression of chd in this domain, coincident with expression of wnt8 (Kelly et al., 1995; Erter et al, 2001; Lekven et al., 2001), was relieved only when expression of both β-catenins is inhibited (Bellipanni et al., 2006). In contrast to the apparently equal involvement of both β-catenins in ventrolateral Wnt signaling at mid-gastrula, the germ-ring expression at 50% epiboly appears to be more dependent on β-catenin-2 than β-catenin-1.
Fig. 1.
The ventrolateral domain of Wnt/β-catenin signaling is dependent on both β-catenin-1 and β-catenin-2, whereas organizer β-catenin-mediated gene activation is dependent only on β-catenin-2. (AH) TOP-GFP embryos were injected with βcat1MO (MO1) (B,F), MO2 (C,G), or both βcat1MO and βcat2MO (MO2) (D,H) and compared to uninjected embryos (A,B) after in situ hybridization with probe for GFP at 50% epiboly (A–D) or 70% epiboly (E–H). Embryos are shown in animal view (A–D) or lateral view (E–H). (I,J) chd expression is excluded from the ventrolateral Wnt/β-catenin signaling domain. TOP-GFP embryos at 70% epiboly were assayed by two color in situ hybridization for both GFP (blue) and chd (red) expression. A single embryo is presented in both animal pole (I) and vegetal pole (J) views to show the completmentarity of chd and Wnt signaling domains at this stage. The arrows in (I, J) point to a slight region of overlap of the chordin expression and Wnt signaling domains. All embryos are shown with dorsal to the right; (E–H) animal pole is to the top.
2.2. Exclusion of chordin expression from the mid-gastrula Wnt signaling domain
To examine the relationship of chd expression to the ventrolateral domain of Wnt8 signaling, we performed two-color double in situ hybridizations to assay both chordin and GFP transcripts in TOP-GFP embryos. At 70% epiboly, chd is expressed in a butterfly-like pattern with an axial domain and winged paraxial domains, and is not expressed in the ventrolateral germ-ring (Miller-Bertoglio et al., 1997). In TOP-GFP embryos assayed at this stage, chd expression is clearly present in dorsal and paraxial regions which do not exhibit β-catenin-activated transcription (Fig 1I,J). Marginal ventrolateral Wnt8/β-catenin signaling decreases towards the dorsal side of the embryo and fades out in the region where paraxial chd expression domain can be detected. At 70% epiboly, the most lateral extent of paraxial chordin expression only slightly overlaps the boundary of marginal β-catenin-activated transcription (arrows in Fig. 1I,J). These patterns are consistent with an inhibitory threshold of Wnt8 signaling on dorsal gene expression.
2.3. Inhibition of Wnt-β-catenin signaling causes ectopic expression of dorsal markers in the germ-ring
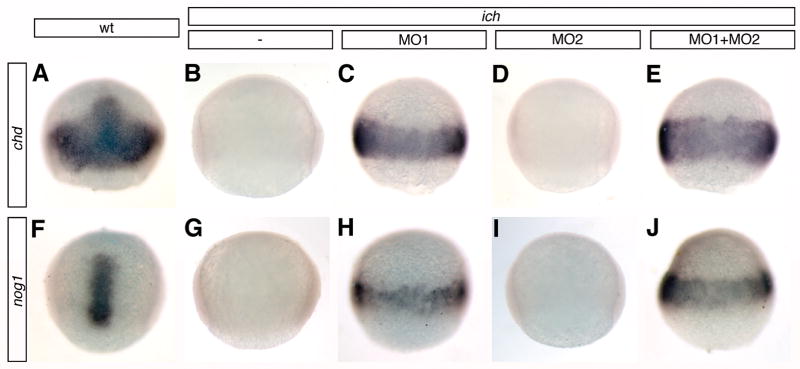
We previously reported circumferential ectopic germ-ring expression of gsc and chd at 50% epiboly in ich embryos treated with βcat1MO (Bellipanni et al., 2006). These embryos, deficient in expression of both β-catenin-1 and β-catenin-2, exhibit the ‘ciuffo’ neurectodermal-containing protrusions at 24 hpf. As ectopic chd expression is first noticeable in the germ-ring of these embryos only at 50% epiboly (1.3 hr later than when expression of the gene is first detected in wild-type embryos, Miller-Bertoglio et al., 1997), it was of interest to look somewhat later in gastrulation to see if this expression is sustained. Although expression is not observed in untreated or βcat2MO-injected ich embryos at 70% epiboly (Fig. 2B,D), a massive amount of ectopic chd transcript is found all around the germ-ring at 70% epiboly in βcat1MO-treated ich embryos (Fig. 2C). A similar circumferential expression of chd was observed in wild-type embryos treated with βcat1MO and βcat2MO (data not shown). We conclude that either one of the two β-catenins is sufficient to prevent the ectopic expression of chd, but when expression of both β-catenins is inhibited, this repression is relieved. The pattern of expression is quite different from the winged-type pattern (dorsal and flanking paraxial domains) observed in wild-type embryos at this stage (Fig. 2A; Miller-Bertoglio et al., 1997). We often observed a somewhat wider band of expression in ich embryos treated with both MOs (Fig. 2E), suggesting that even the low amounts of endogenous maternal β-catenin-2 in ich embryos (Bellipanni et al., 2006) may act to repress chd expression to some extent. We also examined the expression of noggin1 (nog1) in embryos lacking one or both β-catenins. Like chd, nog1 expression is first observed at 4 hpf in the presumptive organizer area in wild-type embryos and is then strongly expressed in axial mesendoderm (Fürthauer et al., 1999; Fig. 2F). A more diffuse transverse stripe of nog1 expression extending into paraxial mesoderm can be detected at the end of gastrulation (Fürthaeur et al., 1999; not shown in Fig. 2F, which is at an earlier stage). The gene is not expressed in untreated or βcat2MO-injected ich embryos (shown at 70% epiboly in Fig. 2G,I), but is first detected in βcat1MO-injected ich embryos as a faint ring of ectopic expression in the germ-ring at 50% epiboly (5.3 hpf) (not shown), and like chd, is massively ectopically expressed at 70% epiboly all around the germ-ring (Fig. 2H). These data extend our findings that the two β-catenins mediate the repression of dorsal-specific genes in the germ-ring domain during early gastrula stages, (Bellipanni et al., 2006), but in addition, we demonstrate that relief of this repression results in dorsal gene expression in a extremely wide marginal band coincident with the zone of ventrolateral Wnt signaling at 70% epiboly (Fig. 1E). This band of expression is much wider and more intense than in other studies in which lateral ectopic expression of chd has been observed (Pelegri and Maischein, 1998, Shimizu et al., 2000, 2002; Imai et al., 2001; Ramel and Lekven, 2004; Ramel et al., 2005; Bellipanni et al., 2006), and the strong expression extends completely around the embryo. In these prior studies, the massive mid-gastrula expression was not reported, as the latest embryonic stage examined for chd expression was the shield stage. We also examined expression of bmp4 in ich embryos treated with both β-catenin MOs and found that although inhibition of β-catenins resulted in a decrease in bmp4 expression, there was still considerable bmp4 transcript throughout the embryo (compare Fig. 6M,S with 6P,V). The remaining bmp4 expression in the marginal zone represents an unusual case of uncoupling of the feedback control that regulates the reciprocal expression of chd and BMP genes.
Fig. 2.
Elimination of β-catenin signaling induces the ectopic expression of dorsal genes around the germ-ring. At 75% epiboly, wild-type embryos have characteristic patterns of chd (A) and nog1 (F) expression, whereas ich embryos do not express these genes (B,G). Injection of βcat1MO (MO1) into ich embryos induces expression of chd (H) and nog1 (H) around the germ-ring, but βcat2MO (MO2) has no effect (D,I). Co-injection of the two MOs into ich embryos causes somewhat increased expression of chd (E) and nog1 (J) around the germ-ring. Concentrations of MOs injected were 3 mM for the single MO1 or MO2 injections and 3mM each for the double MO injection. Wild-type embryos are shown in dorsal view (A,F) and all other embryos are shown in lateral views. Expression (or lack of) was identical for all embryos for each treatment (>16 in each case) except for (I) in which 1/19 embryos showed very weak expression.
Fig. 6.
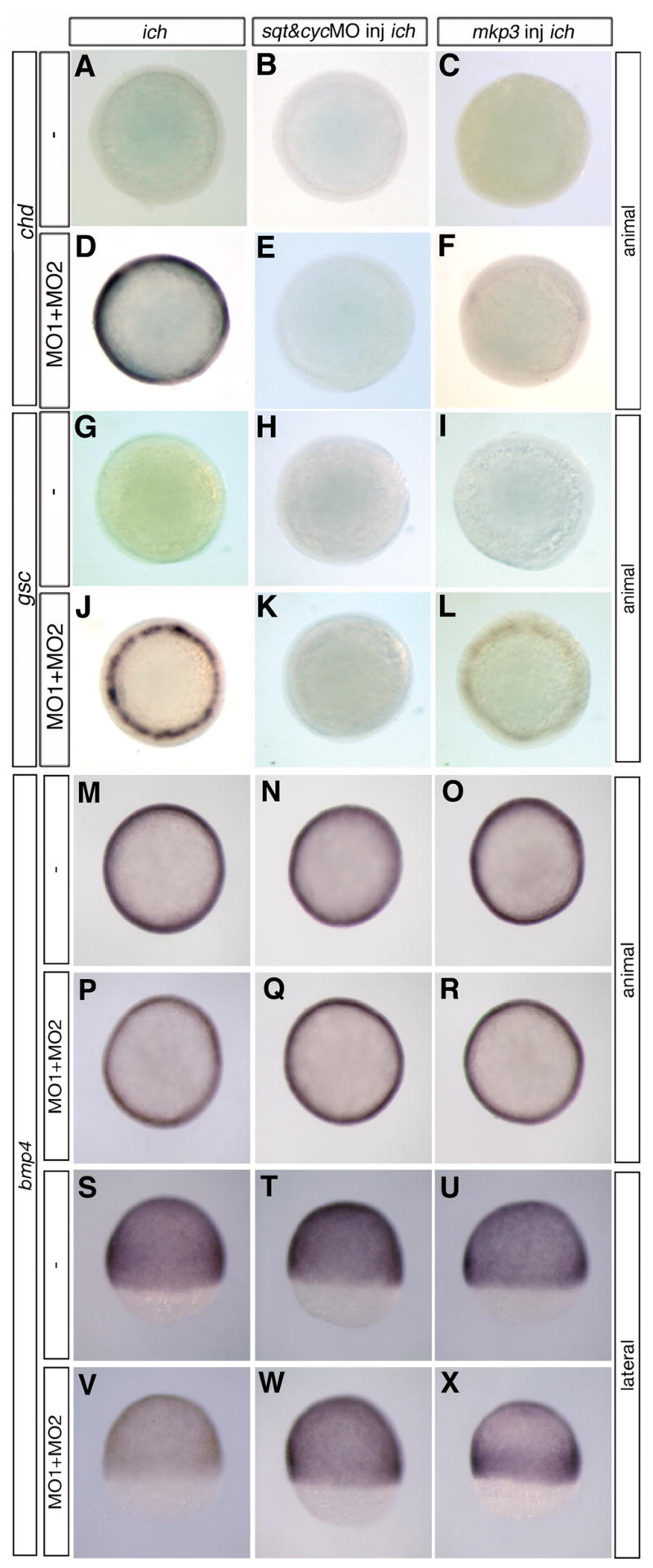
Germ-ring expression of chordin and goosecoid in embryos with impaired Wnt8/β-catenin signaling is dependent on Nodal and FGF signaling pathways. Circumferential germ-line expression of chd (D) and gsc (J) in ich embryos depleted of β-catenins by co-injection of βcat1MO and βcat2MO (MO1+MO2) was eliminated in embryos co-injected with additional MOs against sqt and cyc (E, K) or with mkp3 RNA to inhibit FGF signaling (F,L). Uninjected control ich embryos and ich embryos injected solely with sqtMO+cycMO or with mkp3 RNA did not express chd (A–C) or gsc (G–I). The widespread ubiquitous expression of bmp4 in ich embryos (M,S) is somewhat reduced when the embryos are co-injected with βcat1MO and βcat2MO (P,V). Injection of ich embryos with sqtMO+cycMO (N,T) or with mkp3 RNA (O,U) does not reduce bmp4 expression. Injection of the four MOs, sqtMO+cycMO+βcat1MO+βcat2MO (Q,W), or mkp3 RNA + the two βcat MOs (R,X), also did not reduce bmp4 expression, except in the latter case, the expression was less uniform with greater expression towards the margin. Results for eve1 expression were essentially identical to that of bmp4 (data not shown). Although only one embryo is shown per panel, results were completely uniform for all embryos in each panel (n>25 in each case). Embryos are all at 70% epiboly and are shown in animal views (A–R) and for the embryos of (M–R), also shown in lateral view (S–X).
2.4. Chordin is required for the ‘ciuffo’ phenotype and for expression of neural markers when both β-catenins are inhibited
To test whether the ectopic expression of chd is essential for establishment of the neurectoderm in ‘ciuffo’ embryos, we inhibited expression of both chd and the two β-catenins in ich embryos. Injection of an MO against chd (chdMO) into ich embryos resulted in a flatter tail protrusion (compare Fig. 3A,B) and, as in untreated mutant embryos (Fig. 3E,I,M), no expression of the neural markers emx1 (Fig. 3E) and krox20 (Fig 3I). (That this MO yielded a flatter tail region may indicate a low level of tail chd expression in ich embryos that has not been detected by other means.) When ich embryos were injected with βcat1MO and βcat2MO to eliminate expression of both β-catenins, the embryos developed massive ‘ciuffo’ protrusions at 24 hpf (Fig. 3C and Bellipanni et al., 2006), in which patterned expression of emx1 (Fig. 1G) and krox 20 (Fig. 1K) could be observed. emx1 expression was found close to the yolk (arrow, Fig. 3G), indicating the position of tissue with telencephalon identity (Morita et al., 1995), and krox20 expression was found further toward the tip of the protrusion, often in two stripes or rings (arrowheads, Fig. 3K), corresponding to tissue of rhombomere 3 and 5 identity (Oxtoby and Jowett, 1993). These results show that the embryo can express neural markers with correct relative antero-posterior pattern, with posterior oriented toward the tip of the ‘ciuffo’ protrusion, in embryos inhibited in expression of both β-catenins.
Fig. 3.
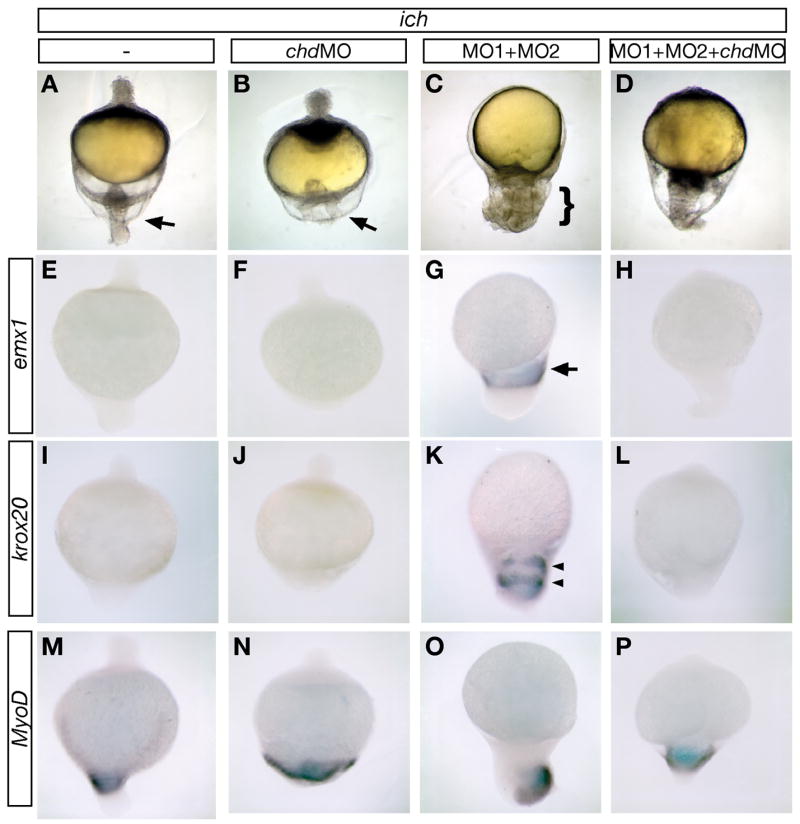
chordin expression is required for expression of neural markers when both β-catenins are inhibited. 24 hpf untreated ich embryos (A,E,I,M), and ich embryos injected with chdMO alone (B,F,J,N), βcat1MO and βcat2MO ([MO1+MO2] C,G,K,O), or all three MOs (D,H,L,P) were photographed alive (A–D) or after in situ hybridization with probe for emx1 (E–H) krox20 (I–L), or myoD (M–P). ich embryos, which are highly ventralized if untreated (A), do not express emx1 (E), or krox20 (I), but do express myoD in the tail region (M). When depleted of β-catenin-1 and β-catenin-2 expression by MO treatment, embryos develop the "ciuffo" phenotype (C, bracket indicates the protrusion that has very different morphology from the tail region of ich embryos, indicated by arrow in A) and express emx1 (G, arrow points to single band of expression), and krox20 (K, arrowheads point to two bands of expression), in addition to myoD (O). ich embryos injected with chdMO alone remain highly ventralized, with a broader but less protrusive tail (B, arrow) than in untreated ich embryos, and show no induction of emx1 (F) or krox20 (J). When ich embryos are injected with chdMO as well as the two β-catenin MOs, the posterior protrusion is no longer ‘ciuffo-like’, but rather is tail-like, and the anterior necrotic protrusion seen in ich embryos (A, arrowhead) and embryos treated with chdMO alone (B, arrowhead) fails to form (D). The embryos treated with the three MOs are inhibited in both emx1 (H) and krox20 (L) expression but do express myoD in the tail region (P), indicating that the failure of emx1 and krox20 expression in the triple MO-injected embryos was not due to non-specific toxic effects of the MOs. Over 24 embryos were examined for each marker and each condition, with results identical to embryo shown in the panel (except for panel N in which 1 of 24 embryos did not express myoD and panel K in which 2 of 28 embryos did not express krox20). Concentrations of MOs injected were 3 mM for each of the β-catenin MOs and 0.6 mM chdMO.
The role of Chordin in expression of these markers was tested by co-injection of chdMO along with βcat1MO and βcat2MO. This treatment resulted in suppression of the ‘ciuffo’ phenotype, with all embryos developing a broad tail-like appendage (much larger than that formed by the uninjected ich embryos) and failure to form the necrotic animal pole protrusion (found in both untreated and chdMO-injected ich embyos) (Fig. 3D). The expression of neural markers in these embryos was suppressed (emx1, Fig. 3H; krox20, Fig. 3L), with virtually all embryos examined showing no detectable expression (27/27 embryos for emx1, 26/28 embryos for krox20).
We also examined expression of myoD in these four classes of embryos (Fig. 3M–P) and found that this gene, which is normally expressed in the tail regions of ich embryos (Fig. 3M) is not inhibited by chdMO, βcat1MO and βcat2MO, or a combination of all three MOs (Fig. 3N–P). The strong effect of the triple MO injection on neural marker expression without concomitant inhibition of myoD expression indicates that the loss of neural marker expression is a specific response to the chdMO, and not merely due to toxicity or other non-specific effects of the administration of multiple MOs. We conclude, therefore, that chd expression is required for formation of hindbrain and anterior neurectoderm in embryos lacking expression of the two β-catenins.
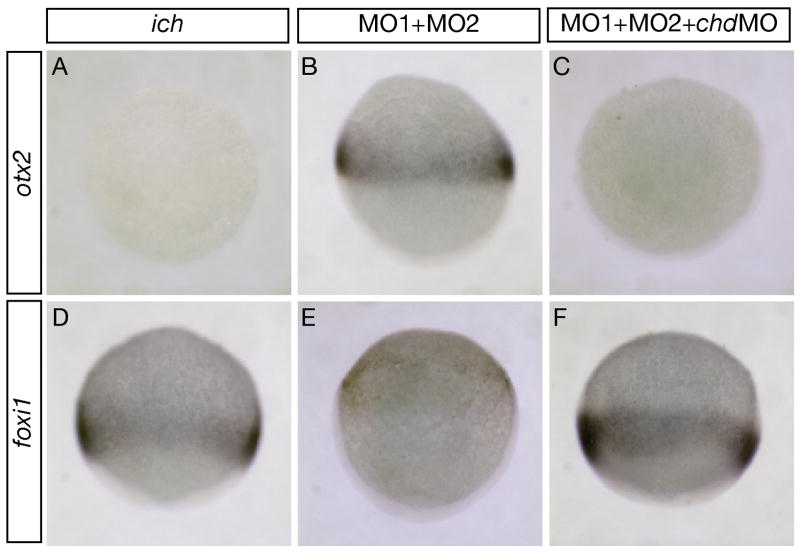
To determine whether the effects of inhibition of chd resulted in ectopic expression of neural markers in even earlier ‘ciuffo’ embryos, we assayed expression of otx2 (Fig. 4A–C) in 10 hpf embryos, comparing uninjected ich embryos, βcat1MO and βcat2MO ‘ciuffo’ embryos, and embryos co-injected with chdMO along with βcat1MO and βcat2MO. As expected, otx2 was not expressed in ich embryos (Fig. 4A). Injection of the two βcat MOs into ich embryos clearly resulted in induction of otx2 in an equatorial band extending completely around the embryo (Fig. 4B), and the additional injection of chd MO completely suppressed this induction (Fig. 4C). We also assayed for expression of the epidermal marker foxi1 at this stage in the three types of embryos (Fig. 4D–F). foxi1 is expressed ubiquitously in ich embryos, with the most intense expression found toward the vegetal pole (Fig. 4D). Injection of the two βcat MOs into ich embryos resulted in a marked suppression of expression of foxi1 (Fig. 4E), except in the animal pole region which most probably gives rise to the epidermal tissue covering the yolk cell in 24 hpf ‘ciuffo’ embryos. Intense foxi1 expression was restored in ich embryos injected with all three MOs (Fig. 4F). These experiments suggest that the lack of neural gene expression in 24 hpf ‘ciuffo’ embryos treated with chdMO (Fig. 3H,L) is not due to the loss of neurectodermal cells that might have been present in these embryos at earlier developmental times. Analysis of 10 hpf embryos clearly shows ‘ciuffo’ embryos can robustly express an early neural marker, and that expression of this marker is completely abolished on inhibition of chd. The lack of chd expression appears to respecify ectoderm from neurectodermal to epidermal identity.
Fig. 4.
chordin expression is required for otx2 expression and suppresses foxi1 expression in 10 hpf embryos inhibited in expression of both β-catenins. Untreated ich embryos (A,D) and ich embryos injected with βcat1MO and βcat2MO ([MO1+MO2] (B,E), or additionally with chdMO (C,F) were assayed for expression of otx2 (A–C) or foxi1 (D–F). Inhibition of expression of the two β-catenins results in induction of the anterior neural marker otx2 at the expense of expression of the epidermal marker foxi1. The inhibition of Chd in such embryos prevents otx2 expression and restores the foxi1 expression. Concentrations of MOs injected were as in Fig. 3 and embryos are shown in lateral views.
2.5. Wnt8 signaling, mediated by vox/vent and β-catenin-1/-2, prevents expression of chordin in the germ ring
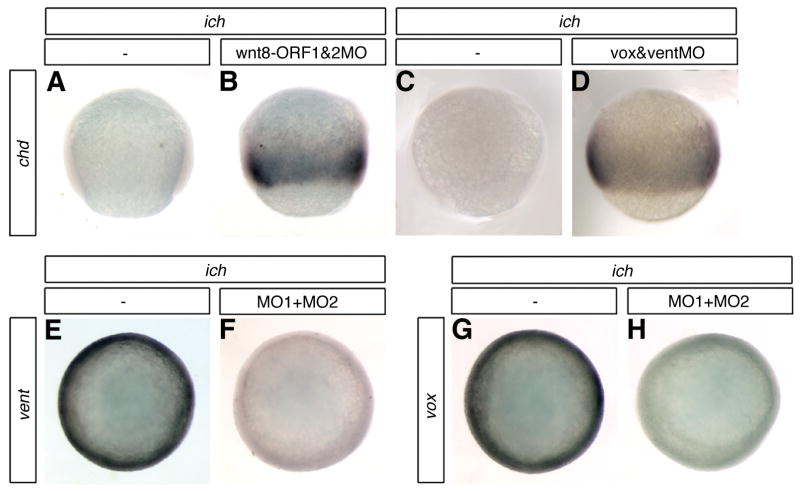
If the Wnt8/Vox/Vent pathway acts to prevent germ-ring chd expression, we would predict that loss of function of wnt8 or of vox and vent in ich embryos would have the same effect on chd expression as the loss of expression of the two β-catenins. Co-injection into ich embryos of MOs that target the two wnt8 transcripts (Fig. 5B) or co-injection of MOs targeting vox and vent transcripts (Fig. 5D) resulted in the same intense broad circumferential band of chd expression at 70% epiboly as observed in ich embryos injected with βcat1MO or βcat1MO + βcat2MO (Fig. 2C,E). chd expression was not observed in embryos injected with just one or the other wnt8 MO or with voxMO or ventMO alone (data not shown), indicating that either wnt8 ORF product or either Vox or Vent alone is sufficient to repress the ectopic chd expression in ich embros, as has been shown in wild-type embryos (Lekven et al., 2001; Imai et al., 2001; Ramel and Lekven, 2004). To test whether β-catenin depletion had an effect on vox and/or vent transcript levels, we examined the expression of these two genes in ich embryos injected with βcat1MO +βcat2MO. Each of these genes is expressed ubiquitously in untreated ich embryos (Figs. 5E,G), as would be expected in embryos lacking boz expression (Kawahara et al., 2000a, 2000b, Imai et al., 2001). In ich embryos injected with the two β-catenin MOs, both vox and vent were found to be down-regulated at 70% epiboly compared to their expression in uninjected ich control embryos (compare Fig. 5E to 5F and Fig. 5G to 5H). βcat1MO or βcat2MO treatment of wild-type embryos did not reduce vox and vent transcript levels (data not shown), indicating that either of the two β-catenins is sufficient to mediate wnt8 activation of vox and vent. Taken together, these results show that even in the absence of the organizer, wnt8 represses the expression of chd by activation of vox and vent expression and that either of the two β-catenins can transduce the wnt8 signal. These conclusions are consistent with the finding that only the combined inhibition of both β-catenin transcripts can inhibit endogenous Wnt signaling in wild-type embryos (Fig. 1H).
Fig. 5.
Germ-ring expression of chordin is repressed by Wnt8 through the transcriptional repressors Vox and Vent. ich embryos co-injected with MOs targeted against both ORFs of wnt8 (B) or with MOs against both vox and vent (D) show ectopic expression of chd around the germ-ring. Uninjected ich embryos do not express chd (A,C). Suppression of β-catenin signaling in the germ-ring of ich embryos by injection of βcat1MO + βcat2MO (MO1+MO2) causes a decrease of vox and vent expression (compare E with F, and G with H). Embryos are shown in lateral (A–D) or animal (E–H) views at 70% epiboly. Concentrations of MOs injected were 0.6 mM of each of the two Wnt8MOs, 0.7 mM of each of voxMO and ventMO, and 3 mM of each of the two β-catenin MOs.
Both the combination of vox and vent MOs or of the two Wnt8 MOs, when injected into ich embryos, also gave rise to many embryos with a ‘ciuffo’-like phenotype (data not shown). These embryos all expressed krox20, whereas none of the uninjected control ich embryos expressed this marker (data not shown). These results indicate that wnt8, the two β-catenin genes, and vox and vent all act in a pathway of repression of germ-ring ectopic chd, which when expressed result in neurectoderm formation in the absence of the organizer.
2.6. Nodal and FGF signaling is required for ectopic germ-ring chordin and goosecoid expression
We next investigated the factors that act to induce chd expression in the absence of Wnt8 signaling. The ventrolateral germ-ring normally expresses several genes (sqt, fgf3, fgf8) that are expressed somewhat earlier in the presumptive organizer. chd expression in the organizer is partially dependent on a pathway in which FGFs are induced by sqt, and in turn FGFs then signal cells to activate chd (Maegawa et al., 2006; see Discussion). Both sqt and FGF genes are also expressed in the germ-ring of ich embryos, indicating that this domain of expression is independent of the organizer. We therefore considered the possibility that the very same Nodal and FGF pathway that activates chd in the organizer could potentially function in the germ-ring to yield ectopic chd transcripts. In wild-type embryos, we hypothesize that this latent activity is prevented by signaling by Wnt8, acting through the two β-catenins.
To test this hypothesis, we blocked either Nodal or FGF signaling in βcat1MO + βcat2MO treated ich embryos and examined chd expression at 70% epiboly (Fig. 6A–F). sqtMO or cycMO, alone, each reduced the level of ectopic germ-ring chd expression produced when the two β-catenins are inhibited (data not shown), and the combination of sqtMO and cycMO completely eliminated this expression (compare Fig. 6D with 6E). This result demonstrates that ectopic germ-ring chd expression that occurs when gastrula wnt8 signaling is eliminated is totally dependent on Nodal signaling. To test for the role of FGF signaling, we injected mkp3 RNA, which is effective in blocking the transduction of FGF signals in zebrafish embryos (Tsang et al., 2004). MAP kinase phosphatase 3 (MKP3) has been shown to inhibit FGF/MAPK signaling activity by de-phosphorylation of MAPK proteins (reviewed in Tseng et al., 2004). Expression of mkp3 completely eliminated the ectopic germ-ring chd expression produced in ich embryos injected with the two β-catenin MOs (compare Fig. 6D with 6F). Similar results were obtained for goosecoid (gsc) expression (Fig. 6G–L). To test whether the administration of multiple MOs causes non-specific toxic effects that would lead to failure of chd or gsc expression, we also examined the effects of these treatments on bmp4 (Fig. 6M–X) and eve1 (data not shown) expression. Both of these markers were robustly expressed under exactly the same conditions that resulted in failure to express chd and gsc. Thus, both Nodal and FGF signaling are required for the activation of chd and gsc expression that occurs on inhibition of Wnt8-β-catenin signaling. As we have already demonstrated in the previous section that wnt8 and vox and vent can repress germ-ring chd expression in ich embryos (that still express β-catenin-1), it is likely that Wnt8 signaling counteracts latent Sqt/FGF activation of chd.
3. Discussion
3.1 β-catenin-1 and β-catenin-2 are each sufficient to transduce Wnt signaling and prevent chordin expression in the germ-ring
Two distinct areas of β-catenin-mediated gene activation, a dorsal domain in the prospective organizer and a ventrolateral germ-ring domain correlated with Wnt8 signaling, can be visualized in TOP-GFP transgenic zebrafish embryos (Dorsky et al., 2002; Fig. 1A). These two domains have opposite consequences for dorsoventral patterning of the zebrafish embryo: the organizer promotes dorsal fates, and the ventrolateral Wnt8 signaling ventralizes mesoderm and posteriorizes neurectoderm. Using MOs specific for each β-catenin, we show that ventrolateral germ-ring TOP-GFP activity at 70% epiboly is decreased by either βcat1MO or βcat2MO, but is eliminated only when expression of both β-catenins is inhibited (Fig. 1F-H) (at 50% epiboly, it is more dependent on β-catenin-2; Fig. 1A–C). In contrast, the early dorsal domain of TOP-GFP activity is completely dependent on β-catenin-2 (Fig. 1A–C), as expected from our results showing that maternal β-catenin-2 expression is required for formation of the organizer (Bellipanni et al., 2006). This difference in regulation cannot be due to lack of expression of β-catenin-1 in the dorsal region, as transcripts of both genes are detected in the dorsal and ventrolateral germ-ring embryonic domains (Bellipanni et al., 2006). This finding suggests that there may be functional differences between the two β-catenins, differences in stability or sub-cellular localization of the two proteins in the dorsal region, or a block to translation of β-catenin-1 in this region. In wild-type embryos, chd expression and TOP-GFP expression only overlap slightly (Fig. 1I,J). Class 1–3 ich embryos, deficient in maternal β-catenin-2 expression, do not express chd at all (Kelly et al., 2000; Bellipanni et al., 2006; Fig. 2B). However, when these embryos are also depleted of β-catenin-1 activity by βcat1MO injection, massive chordin expression is induced circumferentially in a wide band extending from the germ-ring (Fig. 2C). nog1 and gsc are also induced in this region in βcat1MO-injected ich embryos (Fig. 2H; Bellipanni et al., 2006). Injection of βcat1MO or βcat2MO alone do not cause induction of these genes in wild-type embryos, but the presence of the two MOs together also induces circumferential expression of chd in wild-type embryos (data not shown). Thus, expression of either of the two β-catenins is sufficient to repress chd expression in the germ-ring. Only when expression of both β-catenins is prevented (either by MO treatment and, for β-catenin-2, as a result of the ich mutation), is chd expressed in this region.
3.2. In the absence of organizer, de-repression of chordin is required for patterned expression of neural markers
When expression of both β-catenins is inhibited, embryos form a distinctive phenotype (‘ciuffo’), in which epiboly movements propel tissue away from the vegetal pole in a protrusion that expresses trunk and anterior neural markers. These neural genes are expressed in correct anteroposterior pattern, with posterior markers expressed towards the tip of the protrusion and the most anterior markers expressed close to the yolk (Fig. 3G,K; Bellipanni et al., 2006). The ‘ciuffo’ phenotype and expression of emx1 and krox20 neural markers was completely suppressed in 24 hpf embryos, and expression of otx2 was suppressed in 10 hpf embryos, when chd MO was co-injected into ich embryos along with MOs for both β-catenins (compare Fig. 3G,K with 3H,L; and Fig. 4B with 4C). Moreover, induction of otx2 in ‘ciuffo’ embryos and its repression in ‘ciuffo’ embryos inhibited in chd expression was accompanied by the reciprocal repression and induction of the epidermal marker foxi1. chd expression is thus required in ‘ciuffo’ embryos for neural specification of ectoderm, which is reversed to epidermal identify in the absence of Chd. Inhibition of chd expression in the embryos injected with MOs or both β-catenins, however, does not fully restore the ich phenotype (compare Fig. 3A,D), indicating that not all the effects of loss of both β-catenins are mediated by chd.
The massive expression of chd in βcat1MO +βcat2MO-treated ich embryos would be expected to cause a profound inhibition of BMP signaling due to direct binding of Chd to ligand (Piccolo et al., 1996). The neural induction default model, based on experiments with amphibian embryos, posits that neural induction occurs in ectoderm in the absence of BMP (Wilson and Hemmati-Brivanlou, 1995, 1997; reviewed in Harland, 2000). Our results suggest that inhibition of BMP signaling is sufficient for neuralization in zebrafish as well, whereas in chick, Chordin and Noggin cannot induce ectopic neural markers (reviewed in Streit and Stern, 1999). We find that neuralization can also be induced in ich embryos by injection of a MO for bmp2b, circumventing the need for chd induction and that such neurectoderm also exhibits correct relative anteroposterior marker expression (M. Varga, S. Maegawa, E.S. Weinberg, unpublished observations). These results are similar to those of Reversade et al. (2005), who showed that large amounts of neurectoderm with a degree of normal AP pattern could be formed in Xenopus embryos lacking organizer, if expression of BMP genes was inhibited.
βcat1MO +βcat2MO-treated ich embryos (‘ciuffo’) do not resemble swirl/bmp2b mutant embryos, which neuralize the entire ectoderm in its normal position, not in a protrusion at the vegetal pole as in ‘ciuffo’ embryos (Mullins et al., 1996). swirl/bmp2b embryos have expanded organizer activity, and although dorsalized, still retain an axis and dorsoventral pattern, whereas ‘ciuffo’ embryos are completely radicalized and have a distinctive vegetal protrusion. There are at least two possible reasons for this difference. First, as BMP signaling is involved in convergence movements (von der Hardt et al., 2007), the circumferential expression of chd in gastrulating ‘ciuffo’ embryos would reorient the BMP activity gradient from vegetal to animal (instead of ventral to dorsal), thus increasing the migration of cells towards the vegetal pole and subsequently past the pole into a protrusion, which effectively would replace the dorsal axis as the destination of converging cells. These ‘ciuffo’ embryos exhibit a significant level of BMP transcript throughout the embryo (shown for bmp4 expression at 70% epiboly in Fig. 6V). Such BMP expression would be absent in swirl embryos. Second, there may be functions of one or the other β-catenins required for convergence movements, thus eliminating restraints on epiboly in ‘ciuffo’ embryos.
3.3. Repression of chordin by Wnt8 signaling
How Vox and Vent act to repress chd is not yet known, but this repression is clearly initiated by Wnt8/β-catenin-1 and -2 induction of vox and vent. vent/vox/ved and wnt8 expression is low or absent in the dorsal marginal area that expresses chd at 40% epiboly and shield stages (Kaawahara et al., 2000a, 2000b; Ramel and Lekven, 2004; Shimizu et al., 2005). Here, consistent with the studies of Ramel and Lekven (2004) done at shield stage, we show that in embryos lacking organizer and expression of both β-catenins (ich embryos injected with βcat1MO or wild-type embryos injected with βcat1MO + βcat2MO), wnt8 (ich embryos injected with wnt8-ORF1&2MOs), vox and vent (ich embryos injected with vox and vent MOs), the same broad intense ectopic circumferential expression of chd is observed bordering the mid-gastrula germ-ring (Figs. 2C,E, 4B,D, 7E,E′). Ectopic chd expression in ich embryos treated with βcat1MO (or both βcat1MO and βcat2MO) is much more intense than previously reported in wild-type embryos inhibited in wnt8 or vox and vent expression or in mutants for these genes (Imai et al., 2001; Ramel and Lekven, 2004; Ramel et al., 2005), but these studies used earlier embryonic stages than the 70% epiboly embryos that we investigated.
Fig. 7.
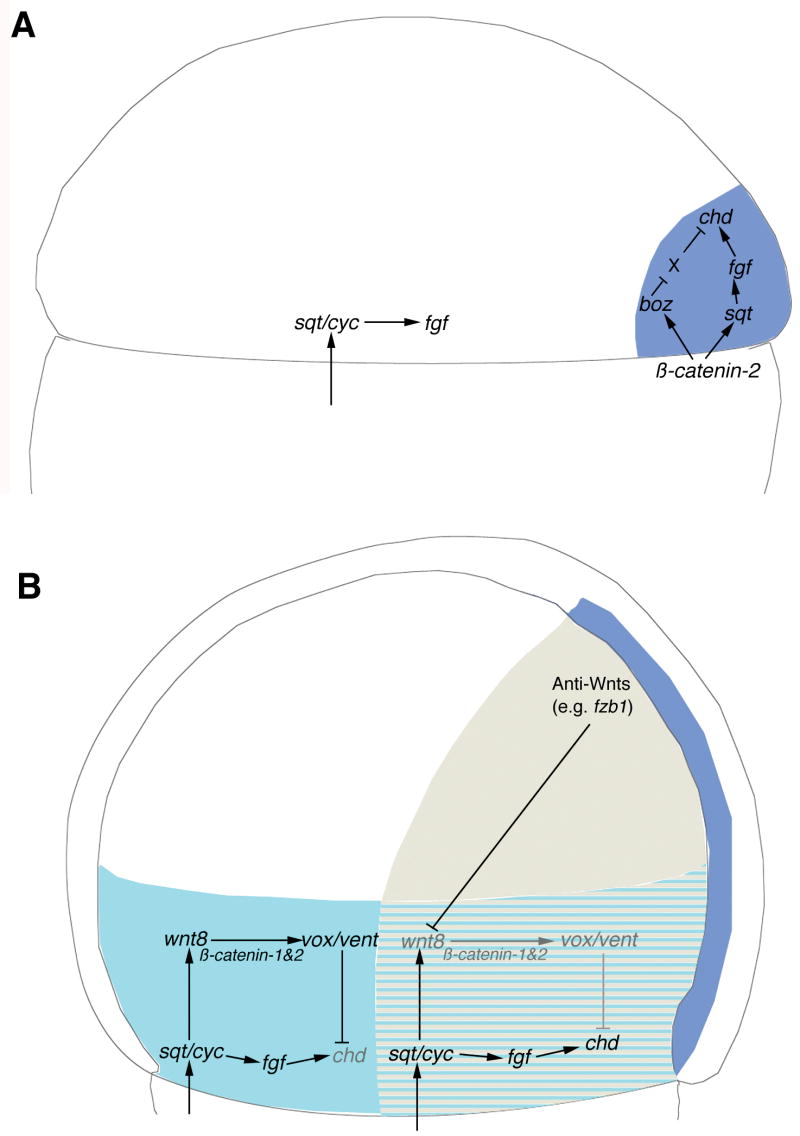
Two-step model of chordin gene regulation in the zebrafish gastrula. (A) During early- and mid-blastula stages, β-catenin-2 enters the nuclei of the dorsal YSL and dorsal blastomeres triggering the transcriptional activation of boz and sqt, which in turn induce axial expression of chd (expressed in the region shown in dark blue) in a process dependent on FGF signaling. boz is shown activating chd by inhibition of a repressor, X, which could be one or more of the Vox/Vent/Ved class of homeobox repressor proteins. Approximately 30 minutes after the first dorsal appearance of sqt and FGF transcripts, Nodal signals from the YSL induce sqt expression in the lateral and ventral germ-ring, and germ-ring Sqt induces expression of FGF genes in this region. (B) By mid-gastrula stage, axial mesoderm (shown in dark blue) continues to express chd and is also the source of anti-Wnt secreted proteins such as Fzb1. These anti-Wnt and anti-BMP proteins will diffuse laterally and form a region (shown in beige) in which Wnt and BMP signaling is inhibited In cells around the germ-ring, wnt8 is induced by Nodal signaling. A broad band of potential germ-ring derived chd expression (light blue) is established by sqt/cyc acting through Fgfs. In the paraxial regions where Wnt8 is inhibited by anti-Wnt proteins (light blue/grey stipes), chd is expressed, whereas in the more lateral and ventral regions, Wnt8, acting through Vox and Vent, inhibits the expression of chd. Indicated in grey typeface and arrows are those parts of the pathway that normally do not operate in that particular region due to the normal inhibitory network.
Relief of inhibition of germ-ring chd and gsc during gastrulation is not only observed when both β-catenins are inhibited (Bellipanni et al., 2006; this work), but also when embryos express a dominant negative (dn) Tcf3 (Pelegri and Maischein, 1998; Lewis et al., 2004) or of axin (Shimizu et al., 2000), each of which would be expected to inhibit the function of both β-catenins. These studies are also consistent in showing that inhibition of β-catenin (β-catenin-2 in our work) early in development abolishes organizer-dependent expression of chd. The expression of dnTcf3 (Pelegri and Maischein, 1998) and axin (Shimuzu et al., 2000) led to different effects depending on the time of development. At sphere stage, expression of these proteins resulted in the elimination of dorsal markers, including chd, whereas at shield stage, expression of dorsal markers was expanded. The work we present here clarifies the role of the β-catenins at these two developmental times. At sphere stage, activation of dorsal genes is completely dependent on β-catenin-2, whereas at shield stage, the repression of dorsal genes can be mediated by either of two β-catenins in the ventrolateral germ-ring. A decrease in β-catenin function or levels by a mechanism that does not discriminate between the two β-catenins, thus has opposite outcomes at the two developmental stages.
Maintenance of vox and vent expression during late blastula in both non-marginal and marginal zones is also dependent on maternal pou2 (spg) function and MZspg embryos show ectopic ventrolateral expansion of chd, nog1, and gsc (Reim and Brand, 2006). This pathway acts in parallel to Wnt8 during late blastula stages but would most likely not have been perturbed in the embryos we treated with β–catenin MOs. vox and vent expression becomes dependent on BMP signaling, predominantly starting at 70–75% epiboly (Kawahara et al., 2000a; Melby et al., 2000; Imai et al., 2001), but decreases in expression of these genes is more severe in wnt8;swr(bmp2b) mutants than in wnt8 mutants alone (Ramel and Lekven, 2004), indicating that even at shield stage, vox and vent expression depend on at least three pathways controlled respectively by wnt8, bmp2b, and pou2. The effects on dorsal gene expression that we observe at 70% epiboly as a consequence of β-catenin MO treatment of ich embryos are most likely entirely due to elimination of Wnt8 signaling.
3.4. Germ-ring potential for chordin expression
During zebrafish organizer formation both FGF signaling (Maegawa et al., 2006) and either Nodals or Bozozok are required for the dorsal induction of chd (Sirotkin et al., 2000; Shimizu et al., 2000; Maegawa et al., 2006). Dependence of FGF expression on Nodal signaling has previously been shown for mesodermal precursors in the zebrafish embryo (Mathieu et al., 2004; Bennett et al., 2007) and we have demonstrated that this is also the case for prospective organizer tissue (Maegawa et al., 2006). Several studies have shown that FGFs can induce chd (Mitchell and Sheets, 2001; Koshida et al., 2002; Londin et al., 2005), and we have found a requirement for FGF signaling in β-catenin and Sqt induction of chd (Maegawa et al., 2006). Since both sqt and FGF genes are expressed in the germ-ring of wild-type and ich embryos, we tested whether the same pathway that activates chd in the organizer could also induce chd transcripts in the germ-ring. Indeed, blocking either Nodal or FGF signaling in βcat1MO + βcat2MO treated ich embryos (Fig. 6A–F) prevented the germ-ring ectopic chd expression that normally results from inhibition of both β-catenins. A similar requirement for Nodal and FGF signaling was also demonstrated for the induction of germ-ring gsc expression in βcat1MO + βcat2MO injected ich embryos (Fig. 6G–L). In wild-type embryos, this latent activity can be prevented by signaling by Wnt8, acting through vox and vent and the two β-catenins. Our evidence suggests that Wnt8 signaling in the germ-ring is required to inhibit the induction of chordin by a pathway dependent on germ-ring expression of both Nodal and FGF ligands.
3.5. Control of axial and paraxial domains of chordin expression
We propose a model to explain the restriction of chd expression to axial and paraxial domains, and the control of expression within each of these domains. During early stages of development (Fig. 7A), dorsal canonical Wnt/β-catenin signaling (driven by β-catenin-2) induces the expression of early dorsal marker genes (e.g., boz, sqt) (Fekany et al., 1999; Shimizu et al., 2000). chd is induced dorsally by Sqt and Boz (Sirotkin et al., 2000; Shimizu et al., 2000) and this activation is dependent on FGF signaling (Maegawa et al., 2006). Slightly later than the time of first dorsal expression of sqt, signals emanating from the YSL induce the expression of Nodal genes in germ-ring blastomeres (Chen and Kimelman, 2000) (Fig. 7B). The germ-ring Nodals will in turn induce the expression of FGF genes (Gritsman et al., 1999; Mathieu et al., 2004) and wnt8 (Erter et al., 2001), which activates expression of the transcription of vox and vent (Ramel and Lekven, 2004) (Fig. 7B). As both Vox and Vent are known repressors of chd (Kawahara et al., 2000a, 2000b), it would be expected that they have this effect in the whole germ ring, except for the most dorsal/axial region, where wnt8 is not expressed (Lekven et al., 2001). However, chd is expressed in extensive paraxial domains at this stage (e.g, Fig. 2A, 7A). We propose that this later paraxial expression (Fig. 7B, striped region) is dependent on germ-ring sqt and FGF expression, and not directly on organizer-derived sqt and FGFs. We posit that in these paraxial regions, the potential inhibition of chd expression by Wnt8 and Vox/Vent is inhibited by secreted anti-Wnt proteins derived from axial tissues (e.g., fzb1, Fig. 7B). This model is substantiated in wild-type embryos by: 1) increases in the chd paraxial domain obtained by increasing the amount of the Wnt antagonist Fzb1 by injecting fzb1 RNA (frzb -- Zebrafish Information Network) RNA (Shinya et al., 2001) (data not shown) and reducing the transduction of Wnt8 signaling by injection of MOs targeted against vox and vent or by using vox;vent mutant embryos (data not shown; Imai et al., 2001, Ramel and Lekven, 2004, and Ramel et al., 2005); 2) elimination of all paraxial and all but a small amount of axial chd expression when Nodal activity was eliminated by injection of antivin RNA (data not shown); and 3) by decrease in axial chd expression by injection of boz-pNA or in boz mutant embryos (data not shown; Shimizu et al., 2000). These experiments are all consistent with the two-step model of chd expression, which defines a non-expressing ventrolateral zone, a germ-ring Nodal-dependent/axial anti-Wnt factor-dependent paraxial zone, and an axial zone dependent on organizer genes. chd is thus expressed in an initial step in the organizer and axial regions dependent on the β-catenin-2 → Sqt [and Boz] → FGF pathway (Fig. 7A), and a later paraxial step in which is also dependent on germ-ring Sqt and FGFs (Fig. 7B). Chd expression from both of these domains is available to diffuse and bind to BMP ligands, creating a dorsoventral gradient of BMP activity thought to be a major determinant in dorsoventral patterning of the embryo (reviewed in Schier and Talbot, 2005).
3.6. Comparative aspects of chordin expression
The relevance of the model of control of chd expression presented above to control of this gene in other model animals is still not clear. Unfortunately, expression pattern data from other teleost species (Medaka, Fugu and Tetraodon) is not yet available for the genes involved. However a number of findings in Xenopus are useful to consider. Xenopus chd is expressed strongly in the dorsal lip during gastrulation (Stages 10–11). At Stage 11.5 chd expression, though still dorsal, becomes a bit more diffuse, and continues to be expressed in axial mesoderm (but not paraxial mesoderm) even during neurulation (Sasai et al., 1994). Repressive effects of ventral Xvent1 and Xvent2 factors on chd and gsc expression have been demonstrated (Gawantka et al., 1995; Onichtchouk et al., 1998; Melby et al., 1999) and some of these ventral factors are upregulated by Xwnt8 (Hoppler and Moon, 1998; Friedle and Knöchel, 2002). Despite these similarities, however, inhibition of ®-catenin by MOs in Xenopus does not result in a circumferential upregulation of chd (Reversade et al., 2005). The most plausible explanation for this may be that there are a high number of functionally redundant ventralizing homeobox factors in Xenopus. The Xvent1 and Xvent2 subfamilies have several members: Xvent1 (Gawantka et al., 1995), PV.1 (Ault et al., 1996), Xvent1B (Rastegar et al., 1999), Xvent2 (Onichtchouk et al., 1996), Xbr-1b (Papalopulu and Kintner, 1996), Xom (Ladher et al., 1996), Vox (Melby et al., 1999), Xvent2B (Rasteger et al., 1999). These factors appear to be regulated by different mechanisms. While all can be activated by BMP signaling, this regulation can be direct (Xvent2B) or indirect, through other intermediate molecules such as GATA-2 (Xvent1B) (Friedle and Knöchel, 2002). Thus far, only three members of the Xvent subfamilies have been shown to be under Xwnt8 control (xvent1, vox [Hoppler and Moon, 1998] and Xvent1B [Friedle and Knöchel, 2002]). It is possible that some of the other ventralizing homeodomain factors are not regulated by Wnt signaling. Therefore, even in a β-catenin-MO treated Xenopus embryo, one or more of these factors would still be able to repress the expression of dorsal genes in ventrolateral regions.
In the chick, chd is first found in the pre-primitive streak embryo in the posterior epiblast just anterior to Koller’s sickle and in a few middle layer cells, both of which contribute to the organizer, and is then expressed in the anterior tip of the primitive streak, in Hensen’ node, and subsequently in the head process and notochord (Streit et al., 1998). Chick chd can be induced by a combination of Vg1, Wnt1, and the secreted protein Tsukushi (Ohta et al., 2004), but the network of control of chd expression has not yet been elucidated. As in the zebrafish, both Nodal and FGF signaling are implicated as essential for induction of chd in the chick embryo (Bertocchini et al., 2002, 2004). Wnt-3a, Wnt5a, and Wnt-8c are all expressed in the primitive streak and node region (Hume and Dodd, 1993; Hollyday et al., 1995; Baranski et al., 2000; Rodriguez-Esteban et al., 2001). Loss-of-function experiments have not been reported that would explain which of the Wnts are involved in induction of dorsal genes, including chd, and which if any might be involved in repression of these genes in posterior (i.e., ventrolateral) regions of the streak. The roles of Chd in the chick are somewhat different than observed in frogs and fish. Although Chd can induce an ectopic primitive streak in the chick, it is not sufficient for generation of somites and intermediate mesoderm or for neural induction (Streit et al., 1998; Streit et al.,1999a,b). In the mouse embryo, chd is first expressed in the anterior primitive streak and then in the node and in axial mesoderm, but not in the anterior visceral endoderm (Bachiller et al., 2000). In mouse embryos, Wnt3 is expressed in the primitive streak and around the node, (Liu et al., 1999) and Wnt3−/− embryos lack these tissues and do not express a variety of dorsal markers (Liu et al., 1999). Wnt3a is also expressed in the primitive streak and dorsal posterior node (Nakaya et al., 2005), but null mice do form a primitive streak and node but have an ectopic neural tube instead of posterior somites, suggesting that this gene is required for the cell fate decision between paraxial mesoderm and neurectoderm (Takeda et al., 1994; Yoshikowa et al., 1997). Expression of chd has not been reported in these mutants. Phenotypes of loss-of-function have not been reported for other Wnt genes expressed in the primitive streak during gastrulation, which include Wnt8 (Bouillet et al., 1995) and Wnt2b (Kemp et al., 2005). In mouse embryos, as in the chick, the relationship of expression of each Wnt species to the expression and repression of chd is still unkown.
Taken together, these data suggest that particular aspects of the mechanism of chd regulation (e.g. expression in the paraxial mesoderm) appear to be rather specific to zebrafish or perhaps teleosts. Other vertebrates appear to use different mechanisms to fine-tune the expression of chd to modulate BMP-signaling during gastrulation. In the absence of experimental data from other fish species, it is not possible to know whether the paraxial mesodermal expression of chd expression is a general characteristic of teleosts. The comparison of chd regulation between different fish species, as well as between fish and frog, could provide important insights into the evolution of BMP regulation in different phylogenetic lineages.
4. Experimental procedures
4.1. Zebrafish strains and husbandry
Wild-type embryos were derived from AB parents. ichabodp1 (ich) embryos were obtained by breeding homozygous ich females with brass males. Only those ich females that reproducibly yielded severely ventralized (Class 1 and 1a; see Kelly et al., 2000; Maegawa et al., 2006) embryos were used in this work. Adult zebrafish were maintained under standard conditions (Westerfield, 1993). To examine endogenous β-catenin activity, we monitored GFP expression in Tg(TOP:GFP)w25 embryos.
4.2. DNA clones, RNA preparation, hybridization, and RNA injection
The following clones were used to prepare antisense probes for hybridization and sense RNAs for embryo injection: goosecoid in pBS-SK (Stachel et al., 1993), chd in pCS2+ (Miller-Bertoglio, 1997), noggin1 in pCS2+ (Furthauer et al., 1999), vox (vega1) and vent (vega2) in pBS (Kawahara et al., 2000a, 2000b), mkp3 in pCS2+ (Tsang et al., 2004), krox20 (Oxtoby and Jowett, 1993), emx1 (Morita et al., 1995), foxi1 in pCS2+ (Solomon et al., 2003), otx2 in pCS2+ (Li et al., 1994), and gfp in pCS2+ (Dorsky et al., 2002). Antisense RNA probes were synthesized and single probe in situ hybridization was carried out as previously described (Bellipanni et al., 2006). For two color in situ hybridization, we combined a modification of the protocol of Teroaka et al. (2004) with out standard method as follows. After staining with Purple AP substrate (Roche) was completed, samples were washed three times in PBST and treated with 0.1 M Glycine-HCl (pH 2.2), 0.1% Tween-20 for 10 minutes at room temperature to incativate the phosphatase. After several PBST washes, embryos were incubated in 1:1000 dilution of alkaline-phosphatase conjugated anti-fluorescein Fab fragments (Roche) overnight at 4C. Subsequently, samples were washed six times with PBST (for 15 minutes each) and three times with 0.1M Tris-HCl (pH 8.2)/0.1% Tween-20 (5 minutes each). Finally embryos were stained with Fast-Red (Roche) following the manufacturer’s protocol.
Sense RNAs to be injected into embryos were synthesized and capped using an mMessage mMachine Kit (Ambion), following the manufacturer’s protocol. These RNAs were stored in dH2O at −80° C and injection solutions were prepared by diluting a 2x stock of RNA in dH2O with an equal volume of Dulbecco’s modified phosphate-buffered saline containing 5% phenol red (Sigma). Approximately 1 nl of RNA solution was injected through the intact chorion into the yolk at the base of blastomeres of 1–4 cell embryos, using an agar mold to hold the embryos. The concentration of mkp3 mRNAs injected was 0.4 μg/μl.
4.3. Morpholino antisense oligonucleotide injections
Morpholino antisense oligonucleotides (MOs) were obtained from Gene Tools (Philomath, OR). β-catenin-1-MO1 (βcat1MO) (Bellipanni et al., 2006), β-catenin-2-MO1 (βcat2MO) (Bellipanni et al., 2006), bmp2bMO (Imai and Talbot, 2001), chdMO (Nasevicius and Ekker, 2000), wnt8-ORF1MO and wnt8-ORF2MO (Lekven et al., 2001), sqtMO (Feldman and Stemple, 2001), and cycMO (Karlen and Rebagliati, 2001) were manufactured by Gene Tools (Philomath, OR) and the sequences of each are as published. The amounts of these antisense reagents that were injected per embryo were as follows: βcat1MO and βcat2MO (3 mM each, when injected alone or together), bmp2bMO (0.4 mM), sqtMO and cycMO (0.6 mM each, when injected alone or together), chdMO (0.6 mM), wnt8MOs (0.6 mM each, injected together), ventMO and voxMO (0.7 mM each, when injected alone or together).
Acknowledgments
This work was supported by National Institute of Health grant RO1-HD39272 to E.S.W. We are grateful to Richard Dorsky for arranging for us to obtain the Tg(TOP:GFP)w25 line of fish and to Tetsurohito Kudoh for the foxi1 plasmid. We thank Mary Mullins, and Daniel Kessler for helpful discussions and Joshua Bradner, Jianyan He, Richard Gill Jr., and Carol Hu for fish care and general laboratory assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ault KT, et al. A novel homeobox gene PV.1 mediates induction of ventral mesoderm in Xenopus embryos. Proc Natl Acad Sci USA. 1996;93:6415–6420. doi: 10.1073/pnas.93.13.6415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachiller D, et al. The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature. 2000;403:658–661. doi: 10.1038/35001072. [DOI] [PubMed] [Google Scholar]
- Baranski M, et al. The dynamic expression pattern of frzb-1 suggests multiple roles in chick development. Dev Biol. 2000;217:25–41. doi: 10.1006/dbio.1999.9516. [DOI] [PubMed] [Google Scholar]
- Barth KA, et al. Bmp activity establishes a gradient of positional information throughout the entire neural plate. Development. 1999;126:4977–4987. doi: 10.1242/dev.126.22.4977. [DOI] [PubMed] [Google Scholar]
- Bellipanni G, et al. Essential and opposing roles of zebrafish ®-catenins in formation of dorsal axial structures and development of neurectoderm. Development. 2006;133:1299–1309. doi: 10.1242/dev.02295. [DOI] [PubMed] [Google Scholar]
- Bennett JT, et al. Nodal signaling activates differentiation genes during zebrafish gastrulation. Dev Biol. 2007;304:525–540. doi: 10.1016/j.ydbio.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betochhini F, Stern CD. The hypoblast of the chick embryo positions the primitive streak by antagonizing nodal signaling. Dev Cell. 2002;3:735–744. doi: 10.1016/s1534-5807(02)00318-0. [DOI] [PubMed] [Google Scholar]
- Bertocchini F, et al. Determination of embryonic polarity in a regulative system: evidence for endogenous inhibitors acting sequentially during primitive streak formation in the chick embryo. Development. 2004;131:3381–3390. doi: 10.1242/dev.01178. [DOI] [PubMed] [Google Scholar]
- Bouillet P, et al. A new mouse member of the Wnt gene family, mWnt-8, is expressed during early embryogenesis and is ectopically induced by retinoic acid. Mech Dev. 1996;58:141–152. doi: 10.1016/s0925-4773(96)00569-2. [DOI] [PubMed] [Google Scholar]
- Chen S, Kimelman D. The role of the yolk syncytial layer in germ layer patterning in zebrafish. Development. 2000;127:4681–4689. doi: 10.1242/dev.127.21.4681. [DOI] [PubMed] [Google Scholar]
- Christian JL, et al. Xwnt-8, a Xenopus Wnt-1/int-1-related gene responsive to mesoderm-inducing growth factors, may play a role in ventral mesodermal patterning during embryogenesis. Development. 1991;111:1045–1055. doi: 10.1242/dev.111.4.1045. [DOI] [PubMed] [Google Scholar]
- Christian JL, Moon RT. Interactions between Xwnt-8 and Spemann organizer signaling pathways generate dorsoventral pattern in the embryonic mesoderm of Xenopus. Genes Dev. 1993;7:13–28. doi: 10.1101/gad.7.1.13. [DOI] [PubMed] [Google Scholar]
- De Robertis EM, Kuroda H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol. 2004;20:285–308. doi: 10.1146/annurev.cellbio.20.011403.154124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsky RI, et al. A transgenic Lef1/®-catenin-dependent reporter is expressed in spatially restricted domains throughout zebrafish development. Dev Biol. 2002;241:229–237. doi: 10.1006/dbio.2001.0515. [DOI] [PubMed] [Google Scholar]
- Dosch R, et al. Bmp-4 acts as a morphogen in dorsoventral mesoderm patterning in Xenopus. Development. 1997;124:2325–2334. doi: 10.1242/dev.124.12.2325. [DOI] [PubMed] [Google Scholar]
- Eimon PM, Harland RM. In Xenopus embryos, BMP heterodimers are not required for mesoderm induction, but BMP activity is necessary for dorsal/ventral patterning. Dev Biol. 1999;216:29–40. doi: 10.1006/dbio.1999.9496. [DOI] [PubMed] [Google Scholar]
- Erter CE, et al. zebrafish nodal-related 2 encodes an early mesendodermal inducer signaling from the extraembryonic yolk syncytial layer. Dev Biol. 1998;204:361–72. doi: 10.1006/dbio.1998.9097. [DOI] [PubMed] [Google Scholar]
- Erter CE, et al. Wnt8 is required in lateral mesendodermal precursors for neural posteriorization in vivo. Development. 2001;128:3571–3583. doi: 10.1242/dev.128.18.3571. [DOI] [PubMed] [Google Scholar]
- Fainsod A, et al. The dorsalizing and neural inducing gene follistatin is an antagonist of BMP-4. Mech Dev. 1997;63:39–50. doi: 10.1016/s0925-4773(97)00673-4. [DOI] [PubMed] [Google Scholar]
- Fekany K, et al. The zebrafish bozozok locus encodes Dharma, a homeodomain protein essential for induction of gastrula organizer and dorsoanterior embryonic structures. Development. 1999;126:1427–1438. doi: 10.1242/dev.126.7.1427. [DOI] [PubMed] [Google Scholar]
- Feldman B, et al. Zebrafish organizer development and germ-layer formation require nodal-related signals. Nature. 1998;395:181–185. doi: 10.1038/26013. [DOI] [PubMed] [Google Scholar]
- Feldman B, Stemple DL. Morpholino phenocopies of sqt, oep, and ntl mutations. Genesis. 2001;30:175–177. doi: 10.1002/gene.1058. [DOI] [PubMed] [Google Scholar]
- Fredieu JR, et al. Xwnt-8 and lithium can act upon either dorsal mesodermal or neurectodermal cells to cause a loss of forebrain in Xenopus embryos. Dev Biol. 1997;186:100–114. doi: 10.1006/dbio.1997.8566. [DOI] [PubMed] [Google Scholar]
- Friedle H, Knöchel W. Cooperative interaction of Xvent-2 and GATA-2 in the activation of the ventral homeobox gene Xvent-1B. J Biol Chem. 2002;277:23872–23881. doi: 10.1074/jbc.M201831200. [DOI] [PubMed] [Google Scholar]
- Fürthauer M, et al. Three different noggin genes antagonize the activity of bone morphogenetic proteins in the zebrafish embryo. Dev Biol. 1999;214:181–196. doi: 10.1006/dbio.1999.9401. [DOI] [PubMed] [Google Scholar]
- Gilardelli CN, et al. Functional and hierarchical interactions among zebrafish vox/vent homeobox genes. Dev Dynamics. 2004;230:494–508. doi: 10.1002/dvdy.20073. [DOI] [PubMed] [Google Scholar]
- Gritsman K, et al. The EGF-CFC protein one-eyed pinhead is essential for nodal signaling. Cell. 1999;97:121–32. doi: 10.1016/s0092-8674(00)80720-5. [DOI] [PubMed] [Google Scholar]
- Gawantka V, et al. Antagonizing the Spemann organizer: role of the homeobox gene Xvent-1. EMBO J. 1995;14(24):6268–6279. doi: 10.1002/j.1460-2075.1995.tb00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppe J, et al. Structural basis of BMP signalling inhibition by the cystine knot protein Noggin. Nature. 2002;420:636–642. doi: 10.1038/nature01245. [DOI] [PubMed] [Google Scholar]
- Haegel H, et al. Lack of ®-catenin affects mouse development at gastrulation. Development. 1995;121:3529–3537. doi: 10.1242/dev.121.11.3529. [DOI] [PubMed] [Google Scholar]
- Harland R. Neural induction. Curr Opin Genet Dev. 2000;19:357–362. doi: 10.1016/s0959-437x(00)00096-4. [DOI] [PubMed] [Google Scholar]
- Harland R, Gerhart J. Formation and function of Spemann’s organizer. Annu Rev Cell Dev Biol. 1997;13:611–667. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- Heasman J, et al. Overexpression of cadherins and underexpression of ®-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell. 1994;79:791–803. doi: 10.1016/0092-8674(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Heasman J, et al. ®-catenin signaling activity dissected in the early Xenopus embryo: a novel antisense approach. Dev Biol. 2000;222:124–134. doi: 10.1006/dbio.2000.9720. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, et al. Follistatin, an antagonist of activin, is expressed in the Spemann organizer and displays direct neuralizing activity. Cell. 1994;77:283–295. doi: 10.1016/0092-8674(94)90320-4. [DOI] [PubMed] [Google Scholar]
- Hollyday M, et al. Wnt expression patterns in chick embryo nervous system. Mech Dev. 1995;52:9–25. doi: 10.1016/0925-4773(95)00385-e. [DOI] [PubMed] [Google Scholar]
- Hoppler S, Moon RT. BMP-2/-4 and Wnt-8 cooperatively pattern the Xenopus mesoderm. Mech Dev. 1998;71:119–129. doi: 10.1016/s0925-4773(98)00004-5. [DOI] [PubMed] [Google Scholar]
- Hoppler S, et al. Expression of a dominant-negative Wnt blocks induction of MyoD in Xenopus embryos. Genes Dev. 1996;10:2805–2817. doi: 10.1101/gad.10.21.2805. [DOI] [PubMed] [Google Scholar]
- Huelsken J, et al. Requirement for ®-catenin in anterior-posterior axis formation in mice. J Cell Biol. 2000;148:567–78. doi: 10.1083/jcb.148.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume CR, Dodd J. Cwnt-8c: a novel Wnt gene with a potential role in primitive streak formation and hindbrain organization. Development. 1993;119:1147–1160. doi: 10.1242/dev.119.4.1147. [DOI] [PubMed] [Google Scholar]
- Imai Y, et al. The homeobox genes vox and vent are redundant repressors of dorsal fates in zebrafish. Development. 2001;128:2407–2420. doi: 10.1242/dev.128.12.2407. [DOI] [PubMed] [Google Scholar]
- Imai Y, Talbot WS. Morpholino phenocopies of the bmp2b/swirl and bmp7/snailhouse mutations. Genesis. 2001;30:160–163. doi: 10.1002/gene.1055. [DOI] [PubMed] [Google Scholar]
- Karlen S, Rebagliati M. A morpholino phenocopy of the cyclops mutation. Genesis. 2001;30:126–128. doi: 10.1002/gene.1046. [DOI] [PubMed] [Google Scholar]
- Kawahara A, et al. Antagonistic role of vega1 and bozozok/dharma homeobox genes in organizer formation. Proc Natl Acad Sci USA. 2000a;97:12121–12126. doi: 10.1073/pnas.97.22.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara A, et al. Functional interaction of vega2 and goosecoid homeobox genes in zebrafish. Genesis. 2000b;28:58–67. doi: 10.1002/1526-968x(200010)28:2<58::aid-gene30>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Kelly C, et al. Maternally controlled ®-catenin-mediated signaling is required for organizer formation in the zebrafish. Development. 2000;127:3899–3911. doi: 10.1242/dev.127.18.3899. [DOI] [PubMed] [Google Scholar]
- Kelly GM, et al. Zebrafish wnt8 and wnt8b share a common activity but are involved in distinct developmental pathways. Development. 1995;121:1787–1799. doi: 10.1242/dev.121.6.1787. [DOI] [PubMed] [Google Scholar]
- Kemp C, et al. Expression of all Wnt genes and their secreted antagonists during mouse blastocyst and postimplantation development. Dev Dynam. 2005;223:1064–1075. doi: 10.1002/dvdy.20408. [DOI] [PubMed] [Google Scholar]
- Koos DS, Ho RK. The nieuwkoid gene characterizes and mediates a Nieuwkoop-center-like activity in the zebrafish. Curr Biol. 1998;8:1199–1206. doi: 10.1016/s0960-9822(07)00509-x. [DOI] [PubMed] [Google Scholar]
- Koshida S, et al. Inhibition of BMP activity by the FGF signal promotes posterior neural development in zebrafish. Dev Biol. 2002;244:9–20. doi: 10.1006/dbio.2002.0581. [DOI] [PubMed] [Google Scholar]
- Lekven AC, et al. Zebrafish wnt8 encodes two Wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev Cell. 2001;1:103–114. doi: 10.1016/s1534-5807(01)00007-7. [DOI] [PubMed] [Google Scholar]
- Ladher R, et al. Xom: a Xenopus homeobox gene that mediates the early effects of BMP-4. Development. 1996;122:2385–2394. doi: 10.1242/dev.122.8.2385. [DOI] [PubMed] [Google Scholar]
- Lewis JL, et al. Reiterated Wnt signaling during zebrafish neural crest development. Development. 2004;131:1299–1308. doi: 10.1242/dev.01007. [DOI] [PubMed] [Google Scholar]
- Li Y, et al. Expression of two zebrafish orthodenticle-related genes in the embryonic brain. Mech Dev. 1994;48:229–244. doi: 10.1016/0925-4773(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Liu P, et al. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- Londin ER, et al. Chordin, FGF signaling, and mesodermal factors cooperate in zebrafish neural induction. Dev Biol. 2005;279:1–19. doi: 10.1016/j.ydbio.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Maegawa S, et al. FGF signaling is required for ®-catenin mediated induction of the zebrafish organizer. Development. 2006;133:3265–3276. doi: 10.1242/dev.02483. [DOI] [PubMed] [Google Scholar]
- Mathieu J, et al. Nodal and Fgf pathways interact through a positive regulatory loop and synergize to maintain mesodermal cell populations. Development. 2004;131:629–641. doi: 10.1242/dev.00964. [DOI] [PubMed] [Google Scholar]
- Melby AE, et al. Regulation of dorsal gene expression in Xenopus by the ventralizing homeodomain gene Vox. Dev Biol. 1999;211:293–305. doi: 10.1006/dbio.1999.9296. [DOI] [PubMed] [Google Scholar]
- Melby AE, et al. Patterning the early zebrafish by the opposing actions of bozozok and vox/vent. Dev Biol. 2000;192:537–550. doi: 10.1006/dbio.2000.9780. [DOI] [PubMed] [Google Scholar]
- Miller-Bertoglio VE, et al. Differential regulation of chordin expression domains in mutant zebrafish. Dev Biol. 1997;192:537–550. doi: 10.1006/dbio.1997.8788. [DOI] [PubMed] [Google Scholar]
- Mitchell TS, Sheets MD. The FGFR pathway is required for the trunk-inducing functions of Spemann’s organizer. Dev Biol. 2001;237:295–305. doi: 10.1006/dbio.2001.0385. [DOI] [PubMed] [Google Scholar]
- Morita T, et al. Differential expression of 2 zebrafish emx homeoprotein messenger-RNAs in the developing brain. Neurosci Letters. 1995;198:131–135. doi: 10.1016/0304-3940(95)11988-9. [DOI] [PubMed] [Google Scholar]
- Momoi A, et al. Analysis of Wnt8 for neural posteriorizing factor by identifying Frizzled 8c and Frizzled 9 as functional receptors for Wnt8. Mech Dev. 2003;120:477–489. doi: 10.1016/s0925-4773(03)00003-0. [DOI] [PubMed] [Google Scholar]
- Mullins MC, et al. Genes establishing dorsoventral pattern formation in the zebrafish embryo: The ventral specifying genes. Development. 1996;123:81–93. doi: 10.1242/dev.123.1.81. Sp. Iss. SI. [DOI] [PubMed] [Google Scholar]
- Nakaya M, et al. Wnt3a links let-right determination with segmentation and anteroposterior axis elongation. Development. 2005;132:5425–5436. doi: 10.1242/dev.02149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Nguyen VH, et al. Ventral and lateral regions of the zebrafish gastrula, including the neural crest progenitors, are established by a bmp2b/swirl pathway of genes. Dev Biol. 1998;199:93–110. doi: 10.1006/dbio.1998.8927. [DOI] [PubMed] [Google Scholar]
- Ohta K, et al. Tsukushi functions as an organizer inducer by inhibition of BMP activity in cooperation with chordin. Dev Cell. 2004;7:347–358. doi: 10.1016/j.devcel.2004.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onichtchouk D, et al. The Xvent-2 homeobox gene is part of the BMP-4 signaling pathway controlling dorsoventral patterning of Xenopus mesoderm. Development. 1996;122:3045–3053. doi: 10.1242/dev.122.10.3045. [DOI] [PubMed] [Google Scholar]
- Onichtchouk D, et al. Requirement for Xvent-1 and Xvent-2 gene function in dorsoventral patterning of Xenopus mesoderm. Development. 1998;125:1447–1456. doi: 10.1242/dev.125.8.1447. [DOI] [PubMed] [Google Scholar]
- Oxtoby E, Jowett T. Cloning of the zebrafish drox-20 gene (krx-20) and its expression during hindbrain development. Nucleic Acids Res. 1993;21:1087–1095. doi: 10.1093/nar/21.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papalopulu N, Kintner C. A Xenopus gene, Xbr-1, defines a novel class of homeobox genes and is expressed in the dorsal ciliary margin of the eye. Dev Biol. 1996;174:104–114. doi: 10.1006/dbio.1996.0055. [DOI] [PubMed] [Google Scholar]
- Pelegri F, Maischein HM. Function of zebrafish ®-catenin and TCF-3 in dorsoventral patterning. Mech Dev. 1998;77:63–74. doi: 10.1016/s0925-4773(98)00132-4. [DOI] [PubMed] [Google Scholar]
- Piccolo S, et al. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel MC, Lekven AC. Repression of the vertebrate organizer by Wnt8 is mediated by Vent and Vox. Development. 2004;131:3991–4000. doi: 10.1242/dev.01277. [DOI] [PubMed] [Google Scholar]
- Ramel MC, et al. WNT8 and BMP2B co-regulate non-axial mesoderm patterning during zebrafish gastrulation. Dev Biol. 2005;287:237–248. doi: 10.1016/j.ydbio.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Rastegar S, et al. Transcriptional regulation of Xvent homeobox genes. Mech Dev. 1999;81:139–49. doi: 10.1016/s0925-4773(98)00239-1. [DOI] [PubMed] [Google Scholar]
- Rebagliati MR, et al. cyclops encodes a nodal-related factor involved in midline signaling. Proc Natl Acad Sci USA. 1998;95:9932–9937. doi: 10.1073/pnas.95.17.9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reim G, Brand M. Maternal control of vertebrate dorsoventral axis formation and epiboly by the POU domain protein Spg/Pou2/Oct4. Development. 2006;133:2757–2770. doi: 10.1242/dev.02391. [DOI] [PubMed] [Google Scholar]
- Reversade B, et al. Depletion of Bmp2, Bmp4, Bmp7 and Spemann organizer signals induces massive brain formation in Xenopus embryos. Development. 2005;132:3381–3392. doi: 10.1242/dev.01901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Esteban C, et al. Wnt signaling and PKA control Nodal expression and left-right determination in the chick embryo. Developmetn. 2001;128:3189–3195. doi: 10.1242/dev.128.16.3189. [DOI] [PubMed] [Google Scholar]
- Sasai Y, et al. Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994;79:779–790. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y, et al. Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature. 1995;376:333–336. doi: 10.1038/376333a0. [DOI] [PubMed] [Google Scholar]
- Schier AF, Talbot WS. The zebrafish organizer. Curr Opin Genet Dev. 1998;8:464–471. doi: 10.1016/s0959-437x(98)80119-6. [DOI] [PubMed] [Google Scholar]
- Schier AF, Talbot WS. Molecular genetics of axis formation in zebrafish. Annu Rev Genet. 2005;39:561–613. doi: 10.1146/annurev.genet.37.110801.143752. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, et al. The zebrafish organizer requires chordino. Nature. 1997;387:862–863. doi: 10.1038/43092. [DOI] [PubMed] [Google Scholar]
- Shimizu T, et al. Cooperative roles of Bozozok/Dharma and Nodal-related proteins in the formation of the dorsal organizer in zebrafish. Mech Dev. 2000;91:293–303. doi: 10.1016/s0925-4773(99)00319-6. [DOI] [PubMed] [Google Scholar]
- Shimizu T, et al. A novel repressor-type homeobox gene, ved, is involved in dharma/bozozok-mediated dorsal organizer formation in zebrafish. Mech Dev. 2002;118:125–138. doi: 10.1016/s0925-4773(02)00243-5. [DOI] [PubMed] [Google Scholar]
- Shimizu T, et al. Interaction of Wnt and caudal-related genes in zebrafish posterior body formation. Dev Biol. 2005;279:125–141. doi: 10.1016/j.ydbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Shinya M, et al. Zebrafish Dkk1, induced by the pre-MBT Wnt signaling, is secreted from the prechordal plate and patterns the anterior neural plate. Mech Dev. 2000;98:3–17. doi: 10.1016/s0925-4773(00)00433-0. [DOI] [PubMed] [Google Scholar]
- Sirotkin HI, et al. bozozok and squint act in parallel to specify dorsal mesoderm and anterior neuroectoderm in zebrafish. Development. 2000;127:2583–2592. doi: 10.1242/dev.127.12.2583. [DOI] [PubMed] [Google Scholar]
- Smith WC, Harland RM. Injected Xwnt-8 RNA acts early in Xenopus embryos to promote formation of a vegetal dorsalizing center. Cell. 1991;67:753–765. doi: 10.1016/0092-8674(91)90070-f. [DOI] [PubMed] [Google Scholar]
- Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- Solomon KS, et al. Zebrafish foxi1 mediates otic placode formation and jaw development. Development. 2003;130:929–940. doi: 10.1242/dev.00308. [DOI] [PubMed] [Google Scholar]
- Stachel SE, et al. Lithium perturbation and goosecoid expression identify a dorsal specification pathway in the pregastrula zebrafish. Development. 1993;117:1261–1274. doi: 10.1242/dev.117.4.1261. [DOI] [PubMed] [Google Scholar]
- Streit A, Stern CD. Mesoderm patterning and somite formation during node regression: differential effects of chordin and noggin. Mech Dev. 1999a;85:85–96. doi: 10.1016/s0925-4773(99)00085-4. [DOI] [PubMed] [Google Scholar]
- Streit A, Stern CD. Neural induction a bird’s eye view. Trends Genet. 1999b;15:20–24. doi: 10.1016/s0168-9525(98)01620-5. [DOI] [PubMed] [Google Scholar]
- Streit A, et al. Chordin regulates primitive streak development and the stability of induced neural cells, but is not sufficient for neural induction in the chick embryo. Development. 1998;125:507–519. doi: 10.1242/dev.125.3.507. [DOI] [PubMed] [Google Scholar]
- Takada S, et al. Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes Dev. 1994;8:174–189. doi: 10.1101/gad.8.2.174. [DOI] [PubMed] [Google Scholar]
- Trindale M, et al. DNA-binding specificity and embryological function of Xom (Xvent-2) Dev Biol. 1999;216:442–456. doi: 10.1006/dbio.1999.9507. [DOI] [PubMed] [Google Scholar]
- Tsang M, et al. A role for MKP3 in axial patterning of the zebrafish embryo. Development. 2004;131:2769–2779. doi: 10.1242/dev.01157. [DOI] [PubMed] [Google Scholar]
- von der Hardt S, et al. The Bmp gradient of the zebrafish gastrula guides migrating lateral cells by regulating cell-cell adhesion. Curr Biol. 2007;17:475–487. doi: 10.1016/j.cub.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Brachydanio rerio) Eugene, OR: Univ. of Oregon Press; 1993. [Google Scholar]
- Wilson PA, Hemmati-Brivanlou A. Induction of epidermis and inhibition of neural facte by Bmp-4. Nature. 1995;376:331–333. doi: 10.1038/376331a0. [DOI] [PubMed] [Google Scholar]
- Wilson PA, Hemmati-Brivanlou A. Vertebrate neural induction: inducers, inhibitors and a new synthesis. Neuron. 1997;18:699–710. doi: 10.1016/s0896-6273(00)80311-6. [DOI] [PubMed] [Google Scholar]
- Wilson PA, et al. Concentration-dependent patterning of the Xenopus ectoderm by BMP4 and its signal transducer Smad1. Development. 1997;124:3177–3184. doi: 10.1242/dev.124.16.3177. [DOI] [PubMed] [Google Scholar]
- Wylie C, et al. Maternal ®-catenin establishes a ‘dorsal signal’ in early Xenopus embryos. Development. 1996;122:2987–2996. doi: 10.1242/dev.122.10.2987. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Oelgeschlager M. Regulation of bone morphogenetic proteins in early embryonic development. Naturwissenschaften. 2004;91:519–534. doi: 10.1007/s00114-004-0575-z. [DOI] [PubMed] [Google Scholar]
- Yamanaka Y, et al. A novel homeobox gene, dharma, can induce the organizer in a non-cell-autonomous manner. Genes Dev. 1998;12:2345–2353. doi: 10.1101/gad.12.15.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa Y, et al. Evidence that absence of Wnt-3a signaling promotes neuralization insead of paraxial mesoderm development in the mouse. Dev Biol. 1997;183:234–242. doi: 10.1006/dbio.1997.8502. [DOI] [PubMed] [Google Scholar]
- Zimmerman LB, et al. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]