Abstract
Glycosyl phosphatidylinositol (GPI)–linked receptors and receptor protein tyrosine phosphatases (RPTPs), both play key roles in nervous system development, although the molecular mechanisms are largely unknown. Despite lacking a transmembrane domain, GPI receptors can recruit intracellular src family tyrosine kinases to receptor complexes. Few ligands for the extracellular regions of RPTPs are known, relegating most to the status of orphan receptors. We demonstrate that PTPα, an RPTP that dephosphorylates and activates src family kinases, forms a novel membrane-spanning complex with the neuronal GPI-anchored receptor contactin. PTPα and contactin associate in a lateral (cis) complex mediated through the extracellular region of PTPα. This complex is stable to isolation from brain lysates or transfected cells through immunoprecipitation and to antibody-induced coclustering of PTPα and contactin within cells. This is the first demonstration of a receptor PTP in a cis configuration with another cell surface receptor, suggesting an additional mode for regulation of a PTP. The transmembrane and catalytic nature of PTPα indicate that it likely forms the transducing element of the complex, and we postulate that the role of contactin is to assemble a phosphorylation-competent system at the cell surface, conferring a dynamic signal transduction capability to the recognition element.
Keywords: PTPα, tyrosine phosphatase, glycosyl phosphatidylinositol, neural signal transduction
The neural cell adhesion molecule contactin (F3/F11) contains Ig-like domains and FN-III repeats, and is attached to the cell surface through a glycosyl phosphatidylinositol (GPI) anchor ( Ranscht and Dours 1988; Brümmendorf et al. 1989; Gennarini et al. 1989; Zisch et al. 1992). This mode of membrane attachment precludes direct transfer of signals into the cell, but allows high mobility in the plane of the membrane, and as such ideally suits contactin for responding to macromolecular stimuli presented to the cell ( Vaughan 1996). The modulation of signal transduction would critically depend on cis association with transmembrane components. Lateral cis interactions occur with the Ig superfamily transmembrane receptors Ng-CAM and Nr-CAM ( Brümmendorf et al. 1993; Morales et al. 1993; Sakurai et al. 1997) and with Caspr ( Peles et al. 1997). Intriguingly, contactin can be isolated in a complex with the intracellular src family tyrosine kinase fyn ( Zisch et al. 1995). The mechanism of this association is unknown, but likely requires an intermediate membrane-spanning component.
As many signal transduction pathways are initiated through activation of intrinsic or associated receptor tyrosine kinase activity, the association of fyn with contactin may be central to contactin signaling. Several protein tyrosine phosphatases (PTPs) can activate src family kinases in various systems, suggesting a PTP to be a candidate component of a contactin signaling complex.
The transmembrane nature of the receptor-like PTPs (RPTPs) indicates that they transduce extracellular signals, although few such signals/ligands are known. The identification of extracellular ligands for RPTPs could provide critical insights into the specific functions and regulation of these enzymes. PTPα is one such RPTP for which a ligand is sought. The short glycosylated extracellular region of PTPα, unlike many other RPTPs, lacks known adhesion motifs. It is linked via the transmembrane segment to the intracellular region that contains two homologous catalytic domains, both of which have intrinsic phosphatase activity ( Wang and Pallen 1991). Several properties of PTPα indicate that it may, among other things, be involved in neuronal signaling. PTPα is particularly abundant in the brain and implicated in mediating neuronal differentiation, cell proliferation, and transformation, by its ability to activate the tyrosine kinase src ( Zheng et al. 1992; den Hertog et al. 1993). PTPα also associates with, dephosphorylates, and activates brain fyn ( Bhandari et al. 1998), a src family kinase that plays a role in axonal growth, myelination, spatial learning and memory ( Lowell and Soriano 1996). Mice lacking PTPα have reduced activities of brain src and fyn, demonstrating that PTPα is a positive physiological regulator of these kinases ( Ponniah et al. 1999; Su et al. 1999).
The shared and complementary features of PTPα and contactin suggested that they may form a signaling complex. Both proteins are expressed in some of the same neuronal cell types such as migrating cerebellar granule cell neurons ( Faivre-Sarrailh et al. 1992; Fang et al. 1996). At a molecular level, the physical association of PTPα and fyn raises the possibility that PTPα provides a transmembrane link between contactin and fyn. Furthermore, the ability of PTPα to activate fyn indicates that PTPα could thereby transduce a signal originating from the extracellular engagement of contactin. We demonstrate that PTPα and contactin associate and define certain requirements for interaction. These findings indicate that contactin is a novel extracellular partner of PTPα and that this complex may regulate aspects of neuronal development.
Materials and Methods
Expression Plasmids
Numbering of the human PTPα amino acid sequence is according to Krueger et al. 1990. The pXJ41 vectors expressing PTPα, VSVG-tagged PTPα, fyn, and CD45 have been described ( Zheng et al. 1992; Bhandari et al. 1998). To construct pXJ41-contactin-neo, two contactin cDNA fragments comprising nucleotides 38–1103 and 177–3961 were obtained from pSP72-contactin/F11 ( Zisch et al. 1995) and subcloned in two steps into pXJ41-neo to reconstitute the contactin coding sequence. To construct pXJ41-myr-PTPα-neo, the PCR-amplified region of human src cDNA encoding the NH2-terminal myristylated sequence MGSNKSKPKDASQ was cloned into pGEM-T (Promega Corp.), and the reverse orientation was selected with the multiple cloning NotI site at the 5′ end of the myristylation signal. A NotI-XhoI fragment was excised and cloned into pXJ41-neo, creating pXJ41-myr-neo. A PCR fragment of PTPα encoding the entire intracellular region (amino acids 148–774), and flanked with engineered SalI sites, was inserted in-frame into XhoI-cut pXJ41-myr-neo. To construct pXJ41-PTPα/CD45-neo encoding the chimeric RPTP, a region of the VSVG–PTPα cDNA encoding the signal peptide and amino acids 1–121 of PTPα was amplified by PCR using forward and reverse primers with engineered NotI and BglII sites, respectively. This NotI-BglII fragment was inserted into a pXJ41-neo intermediate vector. A CD45 cDNA fragment encoding 35 extracellular juxtamembrane amino acids, the transmembrane, and the entire intracellular regions was released from pXJ41-CD45-Hy with BglII and inserted into the intermediate vector.
Cell Culture and Transient Transfections
COS-1 cells were maintained and transiently transfected as described ( Bhandari et al. 1998). The empty expression plasmid pXJ41-neo was used to normalize the amount of DNA in each transfection. After 24 h of coculture, the cells were harvested and processed as described below. For tunicamycin treatment, 20 μg/ml tunicamycin (Boehringer Mannheim) in DME/10% FCS was added to the cells 6 h after transfection, and the cells were harvested after a further 42 h of culture.
COS-7 cells cultured on 1 ml polylysine or alcian blue–coated glass 12-mm-diam coverslips were processed for immunocytochemistry 24–48 h after transfection. Images were taken using a Zeiss Axiovert 100M equipped with a Hamamatsu C5810 3 color CCD cooled camera. Images were processed directly with Adobe Photoshop.
Antibody-mediated Copatching of Contactin and PTPα in Transfected Cells
Copatching of contactin or VSVG-PTPα was performed on COS-7 cells 24–48 h after transfection as described ( Zisch et al. 1995). Cells were incubated on ice with 10 μg anticontactin (4D1) or anti–VSVG antibodies. Bound antibodies were clustered by incubation with a 1:100 dilution of FITC- or RITC-labeled goat anti–mouse antibodies (Tago), followed by fixation with 4% paraformaldehyde and permeabilization (when appropriate) with 0.2% Triton X-100. Affinity-purified rabbit antibodies to contactin or PTPα (see below) were used complementary to the mAbs, together with goat anti–rabbit antibodies labeled with FITC or RITC (Tago).
Western Blots and Immunoprecipitation
Six embryonic chick brains (15-d-old) were mechanically suspended in 30 ml of ice-cold PBS buffer, filtered through a nylon mesh, homogenized by 10 strokes in a glass Dounce homogenizer, and washed three times in PBS. Chick brain cells or transfected COS cells were lysed in 10 mM Tris-Cl, pH 8, 150 mM NaCl, 1 mM EDTA, 1% Brij-96, 20 μg/ml aprotinin, and 2 mM PMSF, and the lysates were clarified by centrifugation. Antibodies toward VSVG (Sigma Chemical Co.), contactin (4D1) ( Zisch et al. 1995), CD45 or fyn (Transduction Laboratories), PTPα (antiserum 2205, raised against PTPα-D1), and NCAM (Chemicon International, Inc.) were used for immunoprecipitation and/or immunoblotting.
Phosphatase Assays
The phosphatase activity of 5 μl immunoprecipitate toward 2 μM phosphotyrosyl-RR-src peptide was measured at 30°C for 15 min as described ( Lim et al. 1997).
Results
PTPα and Contactin Are Associated in Chick Brain
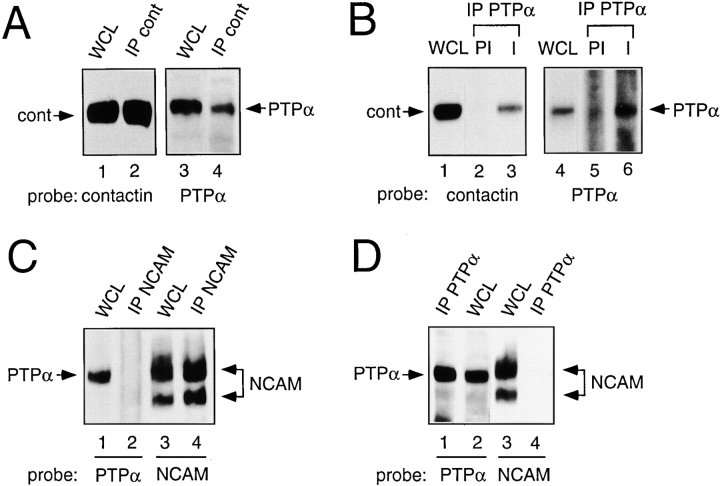
Contactin and PTPα immunoprecipitates from embryonic chick brain lysates were probed for the presence of contactin and PTPα. PTPα was present in anticontactin immunoprecipitates ( Fig. 1 A), and contactin was present in PTPα immunoprecipitates, but was not precipitated by preimmune serum ( Fig. 1 B). Thus, PTPα and contactin exist in a complex in brain lysates. To check the specificity of the association of PTPα and contactin, we examined the interaction of PTPα with NCAM, another fyn-associated ( Beggs et al. 1997) cell adhesion molecule highly expressed in the brain. The 120- and 140-kD NCAM isoforms were detected in anti-NCAM immunoprecipitates from mouse brains, but PTPα was not present ( Fig. 1 C). Likewise, NCAM could not be detected in anti-PTPα immunoprecipitates prepared from these lysates ( Fig. 1 D) using the same anti-PTPα antiserum as employed with the chick brain lysates (raised to the species conserved intracellular D1 region of PTPα).
Figure 1.
PTPα associates with contactin in chick brain. Embryonic chick (A and B) or adult mouse (C and D) brain lysates (WCL) and immunoprecipitates made with anticontactin antibody (A), anti–NCAM antibody (C), or affinity-purified anti–PTPα antibody or preimmune (PI) serum (B and D), were probed for the presence of contactin, NCAM, or PTPα as indicated.
Association of Ectopically Expressed PTPα and Contactin
To investigate the molecular basis of the association between PTPα and contactin, we used a transient expression system where the interaction of different forms could be manipulated. PTPα and contactin coimmunoprecipitated with one another from COS cells coexpressing PTPα (tagged in its extracellular region with an epitope of VSVG to facilitate immunoprecipitation [ Bhandari et al. 1998]) and contactin ( Fig. 2 A). In other experiments, the immunocomplexes were assayed for PTP activity ( Fig. 2 B). Anti-VSVG immunoprecipitates from cells expressing PTPα alone or with contactin contained comparable levels of phosphatase activity, whereas virtually no activity was detectable in anti-VSVG immunoprecipitates from cells expressing contactin alone. Anticontactin immunoprecipitates from coexpressing cells contained about a fivefold higher phosphatase activity than those from cells expressing contactin or PTPα alone. These results indicate that the contactin–PTPα complexes are functionally active. The levels of PTPα protein and phosphatase activity were much lower in anticontactin immunoprecipitates than in anti-VSVG immunoprecipitates from the coexpressing cells, likely because only a portion of the expressed PTPα associates with contactin.
Figure 2.
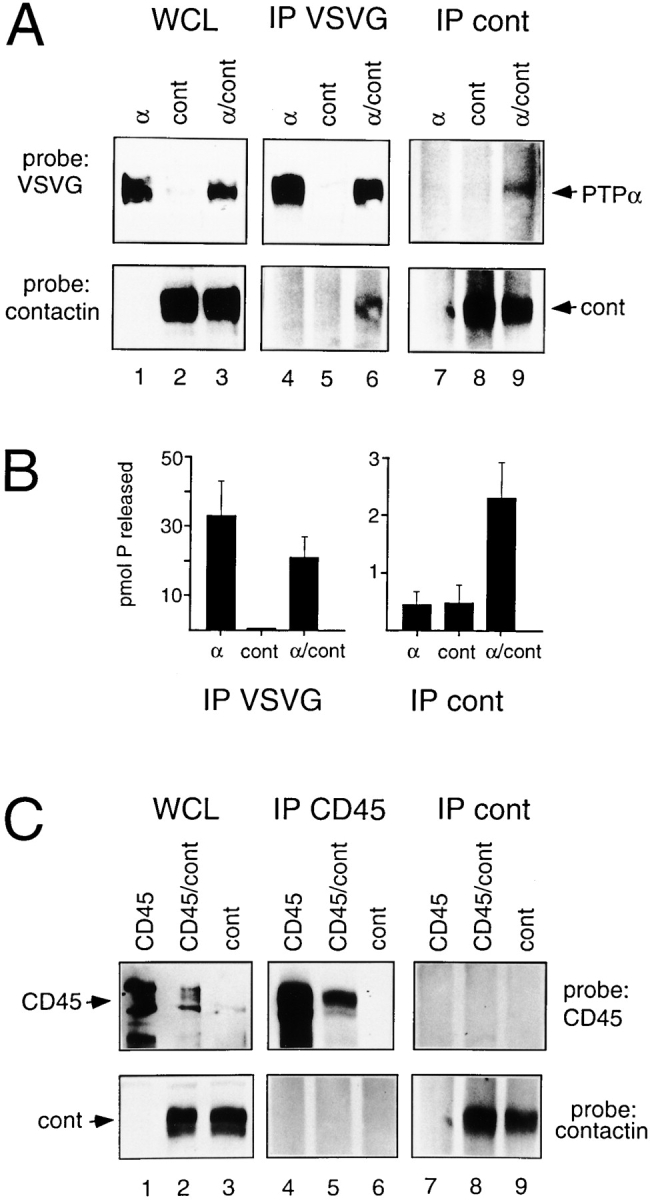
Association of ectopically expressed PTPα and contactin. COS-1 cells were transfected with VSVG-PTPα (α), contactin (cont), or VSVG-PTPα and contactin (α/cont) cDNAs. (A) Whole cell lysates (WCL), and anti-VSVG or anticontactin immunoprecipitates (IP) were probed with anti-VSVG or anticontactin antibodies. (B) Anti-VSVG and anticontactin immunoprecipitates were assayed for phosphatase activity as described in Materials and Methods. The bars represent the values of the mean phosphatase activity ± SEM measured in three independent experiments. (C) COS-1 cells were transfected with CD45, contactin (cont), or CD45 and contactin (CD45/cont) cDNAs. Whole cell lysates (WCL), and anti-CD45 or anticontactin immunoprecipitates (IP) were probed with anti-CD45 or anticontactin antibodies.
Similar experiments were carried out with contactin and CD45, a receptor-like PTP with structural similarity to PTPα. No coimmunoprecipitation of contactin and CD45 from coexpressing cells was detected ( Fig. 2 C), indicating that the interaction of PTPα and contactin is specific and not merely due to heterologous expression.
Colocalization and Coclustering of Contactin and PTPα
In situ localization of contactin and PTPα in cotransfected COS cells revealed a similar distribution for both proteins within the plane of the plasma membrane ( Fig. 3A and Fig. B). In contrast, control transfections of contactin together with the RPTP CD45, gave a completely different pattern of distribution to that of contactin (data not shown), indicating that they do not associate in the same cellular complexes.
Figure 3.
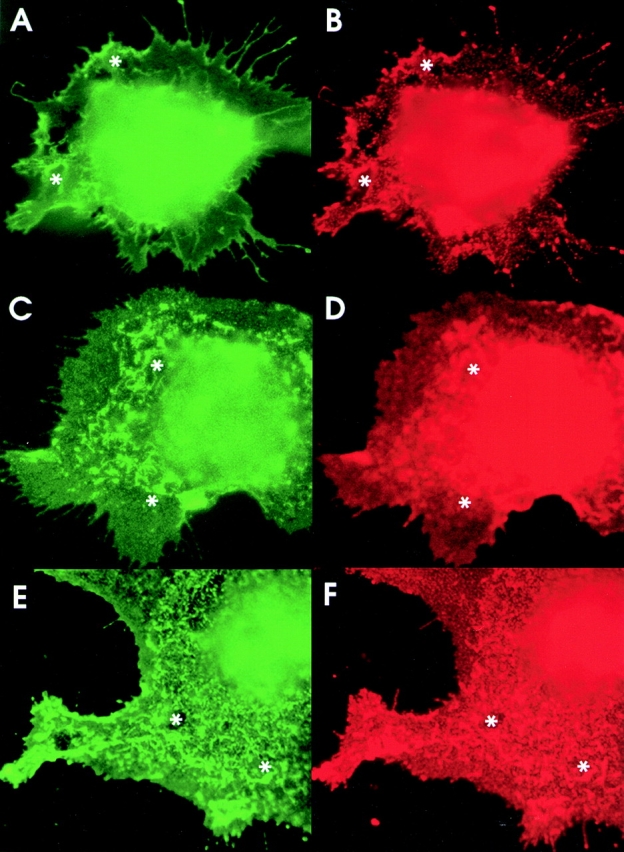
Ectopically expressed PTPα and contactin colocalize. (A and B) COS-7 cells cotransfected with contactin and VSVG-tagged PTPα cDNAs were fixed and incubated with a mouse anti–VSVG antibody and with a rabbit anticontactin antibody, with detection by FITC-labeled goat-anti–rabbit (A) and RITC-labeled goat anti–mouse antibodies (B). (C–F) In COS-7 cells cotransfected with contactin and PTPα, contactin was induced to form cell surface clusters by incubation with mouse 4D1 antibody (C and D) and PTPα was induced to form cell surface clusters by incubation with mouse anti-VSVG antibody (E and F), in each case followed by FITC-labeled goat anti–mouse antibodies. In the PTPα-induced clustering, contactin was detected with rabbit anticontactin, and in the 4D1-induced clustering, PTPα was detected with rabbit anti-PTPα, followed in both cases by RITC-labeled goat anti–rabbit antibodies. C and F show the distribution of contactin and D and E that of PTPα, with focus levels close to the plane of cell-substratum attachment and with corresponding structures in each panel marked (asterisk).
We examined whether an enforced redistribution of either contactin or PTPα, induced by incubating the live cells with antibodies to the extracellular domains of these molecules, would lead to coclustering of the respective partner. Clustering of PTPα ( Fig. 3 E) caused contactin to redistribute to closely match the PTPα pattern ( Fig. 3 F). The close similarity of these two patterns supports the efficacy of clustering via the free-standing VSVG tag on the NH2-terminal of PTPα, leading to little or no interference with the interactions between PTPα and contactin. Coclusters of contactin and PTPα could also be induced by 4D1 mAb specific for contactin ( Fig. 3C and Fig. D). Clusters of various sizes were induced in individual cells, with the PTPα localization largely matching the contactin pattern.
The Extracellular Region of PTPα Is Required for Association with Contactin
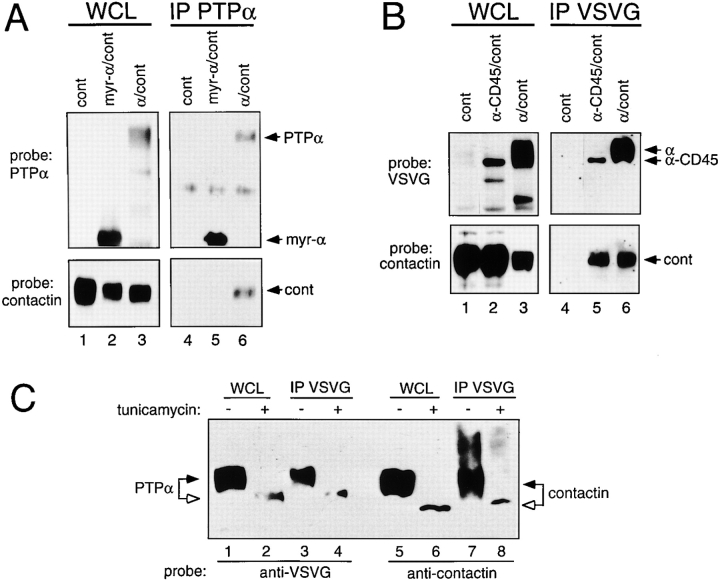
To identify the region of PTPα involved in the interaction with contactin, we generated a membrane-associated intracellular form of PTPα by replacing its extracellular and transmembrane regions with the myristylation signal of src (myr-PTPα). Expressed myr-PTPα was associated with the membrane fraction (data not shown), however, contactin only associated with wild-type PTPα and not with myr-PTPα ( Fig. 4 A). Thus, contactin does not interact with the PTPα lacking the extracellular and transmembrane regions.
Figure 4.
The extracellular region of PTPα is required for association with contactin. (A) Lysates (WCL) and anti-PTPα immunoprecipitates (IP) from COS-1 cells transiently expressing contactin (cont), myr-PTPα/contactin (myr-α/cont), or PTPα/contactin (α/cont) were probed for PTPα and contactin. (B) Whole cell lysates (WCL) and anti-VSVG immunoprecipitates (IP) of COS cells transiently expressing contactin (cont), VSVG-PTPα-CD45/contactin (α-CD45/cont), or VSVG-PTPα/contactin (α/cont) were probed with anti-VSVG or anticontactin antibodies. (C) COS-1 cells were cotransfected with VSVG-PTPα and contactin cDNAs and treated with 20 μg/ml tunicamycin (lanes 2, 4, 6, and 8) or left untreated (lanes 1, 3, 5, and 7). Whole cell lysates (WCL) and anti-VSVG immunoprecipitates (IP) were probed with anti-VSVG or anticontactin antibodies.
Since CD45 does not associate with contactin, we created a PTPα/CD45 hybrid molecule where most of the extracellular region of CD45 was replaced with that of PTPα. PTPα or the PTPα-CD45 hybrid was coexpressed with contactin. Anti-VSVG–immunoprecipitated PTPα-CD45 hybrid and PTPα were complexed with contactin ( Fig. 4 B), demonstrating that contactin associates with the extracellular region of PTPα. Furthermore, the transmembrane region of PTPα is not specifically involved in the association with contactin since it can be replaced with that of CD45.
N-linked Glycosylation Is Not Required for the Association of PTPα and Contactin
The mature PTPα protein contains both N- and O-linked oligosaccharides ( Daum et al. 1994). Chick contactin has nine potential sites for N-linked glycosylation ( Ranscht and Dours 1988; Brümmendorf et al. 1989). When COS cells coexpressing PTPα and contactin were cultured with tunicamycin, an inhibitor of N-linked glycosylation, faster migrating, less diffuse forms of PTPα and contactin were detected on SDS-PAGE ( Fig. 4 C), which is consistent with a loss of N-linked oligosaccharides. Nevertheless, contactin was present in anti-VSVG immunoprecipitates from cells treated with or without tunicamycin ( Fig. 4 C, lanes 7 and 8), indicating that the association of contactin and PTPα occurs independently of N-linked glycosylation of either protein.
PTPα and Contactin Associate In Cis but Not In Trans
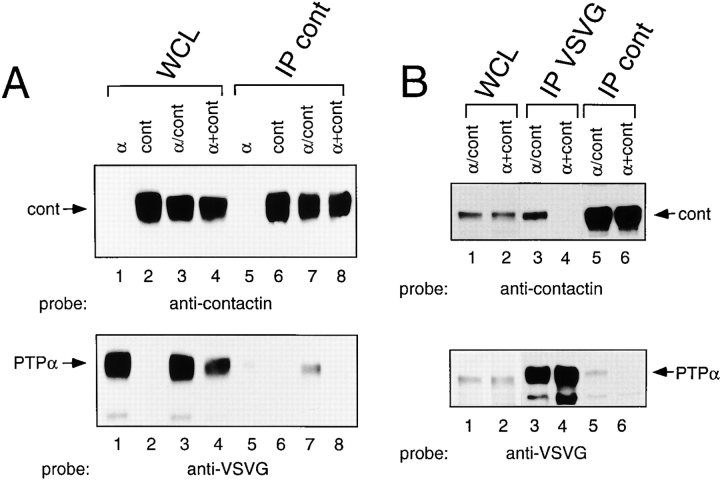
Two experiments were carried out to address the question of whether PTPα and contactin associate in a cis or trans conformation. First, anticontactin precipitates were prepared from lysates of COS cells expressing either PTPα or contactin, or from cells coexpressing both PTPα and contactin ( Fig. 5 A, lanes 1–3), as well as from another sample made by mixing lysates from the cells expressing either contactin or PTPα ( Fig. 5 A, lane 4). Anticontactin immunoprecipitates prepared from coexpressing cells contained PTPα, but those from mixed cell lysates did not ( Fig. 5 A, bottom, lanes 7 and 8). The lack of detectable association of PTPα and contactin in the mixed lysates suggests that interaction cannot take place in a trans conformation. Still, this may require a particular presentation of these cell surface molecules in growing cells that cannot form in solubilized cell lysates. Therefore, contactin-expressing cells were trypsinized 24 h after transfection and replated in dishes containing PTPα-expressing cells (these were not trypsinized for replating because this resulted in a large decrease in PTPα expression). After 24 h of coculture, the cells were lysed and immunoprecipitates were prepared. As a positive control for contactin–PTPα association, PTPα- and contactin-cotransfected cells were cultured for 48 h, harvested, and processed the same way. PTPα and contactin coimmunoprecipitated from cotransfected cells ( Fig. 5 B, top, lanes 3 and 5), but not from cocultured cells ( Fig. 5 B, top, lanes 4 and 6). Thus, even when cells expressing contactin are cultured together with other cells expressing PTPα, no association in trans of these two receptor proteins occurs.
Figure 5.
PTPα and contactin do not associate in a trans configuration. (A) COS cells were transfected with VSVG-PTPα (α), contactin (cont), or VSVG-PTPα and contactin cDNAs (α/cont). Whole cell lysates (WCL) (lanes 1–3), a mixed lysate (α+cont) (lane 4) made of equal parts of the VSVG-PTPα–expressing cell lysate, the contactin-expressing cell lysate, and corresponding anticontactin immunoprecipitates (IP) (lanes 5–8) were probed with anticontactin (top) and anti-VSVG (bottom) antibodies. (B) Cells coexpressing VSVG-PTPα and contactin (α/cont) were cultured for 48 h and harvested. Contactin-expressing cells were trypsinized 24 h after transfection, replated onto VSVG-PTPα–expressing cells (α+cont), and harvested after coculture for 24 h. Whole cell lysates (WCL) (lanes 1 and 2), anti-VSVG (lanes 3 and 4) and anticontactin (lanes 5 and 6) immunoprecipitates (IP) were probed with anticontactin (top) or anti-VSVG (bottom) antibodies.
Discussion
We demonstrate that a receptor protein tyrosine phosphatase forms a membrane-spanning complex with a neuronal GPI-anchored receptor. PTPα and contactin associate with one another in a lateral (cis) forming complex mediated through the extracellular region of PTPα. This complex is sufficiently stable to permit its isolation from brain lysates or transfected cells through immunoprecipitation of either component, and to permit coclustering of PTPα with contactin upon antibody-induced clustering of contactin within cells and vice versa. Our findings that contactin–PTPα complexes form in cotransfected cells, but not upon coculture or in mixed lysates of PTPα-expressing cells and contactin-expressing cells, provides compelling evidence that PTPα and contactin can only associate within the same cell; thus, forming a receptor complex rather than a ligand–receptor pair.
This is the first identification of an extracellular partner and potential regulator of PTPα. As contactin lacks any intracellular region and is tethered to the external face of the plasma membrane through a GPI linkage, PTPα could, thus, link an extracellular contactin-mediated signal to an intracellular response. Fyn is complexed with contactin and is transiently activated upon aggregation of contactin ( Zisch et al. 1995). Likewise, fyn is associated with PTPα and is dephosphorylated and activated by PTPα ( Bhandari et al. 1998; Harder et al. 1998; Ponniah et al. 1999; Su et al. 1999). The association of PTPα and contactin suggests that PTPα might act as an intermediary molecule in a tripartite complex of contactin, PTPα, and fyn, in accord with its proposed role as a transducer. Zisch et al. 1995 reported that antibody-mediated cross-linking of contactin results in the activation of associated fyn, raising the possibility that cross-linking of contactin–PTPα and contactin–fyn complexes brings PTPα into proximity with fyn, allowing the subsequent dephosphorylation and activation of fyn. In view of the constitutive activity of PTPα (and other RPTPs), such a contactin-regulated access of PTPα to its substrate provides an attractive mechanism for mediating RPTP activity and the action of GPI-anchored receptors as modulators in larger signaling complexes.
Contactin interacts with multiple ligands and the identification of PTPα as a novel contactin-associated protein extends the number and the nature of possible contactin-containing receptor complexes. Contactin is found in regions of active neuronal migration or outgrowth in the developing brain and in areas of synaptic development and activity ( Faivre-Sarrailh et al. 1992; D'Alessandri et al. 1995; Zisch et al. 1995). The role of this mobile GPI-anchored receptor may well be to deliver the components of a phosphorylation-competent machinery to receptor complexes mediating neuronal motility or synaptic activity. Evidence for such a role has been obtained for the closely related GPI receptor axonin-1/TAG-1, where neurite fasciculation mediated by complexes of axonin-1 and NgCAM is accompanied by a rapid downregulation of fyn phosphorylation ( Kunz et al. 1996).
An intriguing possibility arising from this study is that contactin acts as an adapter to bring together two RPTPs, namely RPTPζ/β and PTPα. The NH2-terminal carbonic anhydrase-like region of the transmembrane and secreted extracellular forms of glial cell RPTPζ/β associates in trans with contactin, and in doing so promotes neurite growth ( Peles et al. 1995). If PTPα functions as a signaling component of a RPTPζ/β–bound contactin–PTPα complex, this would represent a novel mode of RPTP interaction and regulation.
The proposed signaling through a contactin–PTPα complex represents a new paradigm of receptor-mediated tyrosine kinase activation. Receptors with intrinsic tyrosine kinase activity or directly associated with active nonreceptor tyrosine kinases have been well documented. Contactin, lacking the intracellular region required for either of these mechanisms, may utilize an associated RPTP, PTPα, to effect intracellular activation of tyrosine kinases. This is reminiscent of the recent finding that the GPI-anchored cell surface receptors GDNFR-α and NTNR-α form functional coreceptor complexes with the transmembrane tyrosine kinase Ret ( Buj-Bello et al. 1997; Klein et al. 1997) and underlines the concept that GPI-anchored receptor signaling is achieved by modulation of protein tyrosine phosphorylation. Our study unites previous progress in two areas of research: the interaction of extracellular ligands with the neural cell adhesion molecule contactin, and the intracellular signaling events mediated by PTPα. The components of a novel signal transduction pathway, thus, have been identified and can now be tested for function and physiological relevance to aspects of neuronal development.
Acknowledgments
We thank G. Koretzky for the CD45 cDNA, P. Bello for the pp60c-src cDNA, V. Bhandari, J.E. Walker, and K.L. Lim for construction of pXJ41-PTPα/CD45-neo, pXJ41-contactin-neo, and pXJ41-myr-PTPα-neo, and C. Cagnoli for help with preparation of anti–PTPα antibodies.
This work was supported by the Krebsforschung Schweiz and the Swiss National Science Foundation (to L. Vaughan) and the National Science and Technology Board of Singapore (to C.J. Pallen).
Footnotes
L. Zeng, L. D'Alessandri, and M.B. Kalousek contributed equally to this work.
M.B. Kalousek's present address is Laves-Arzneimittel GmbH, CH-6247 Schötz, Switzerland.
Abbreviations used in this paper: FN-III, fibronectin type III; GPI, glycosyl phosphatidylinositol; PTP, protein tyrosine phosphatase; RPTP, receptor protein tyrosine phosphatase; VSVG, vaccinia stomatitis virus glycoprotein.
References
- Beggs H.E., Baragona S.C., Hemperly J.J., Maness P.F. NCAM140 interacts with the focal adhesion kinase p125fak and the SRC related tyrosine kinase p59fyn . J. Biol. Chem. 1997;272:8310–8319 . doi: 10.1074/jbc.272.13.8310. [DOI] [PubMed] [Google Scholar]
- Bhandari V., Lim K.L., Pallen C.J. Physical and functional interactions between receptor-like protein-tyrosine phosphatase α and p59fyn . J. Biol. Chem. 1998;273:8691–8698 . doi: 10.1074/jbc.273.15.8691. [DOI] [PubMed] [Google Scholar]
- Brümmendorf T., Wolff J.M., Frank R., Rathjen F.G. Neural cell recognition molecule F11homology with fibronectin type III and immunoglobulin type C domains. Neuron. 1989;2:1351–1361 . doi: 10.1016/0896-6273(89)90073-1. [DOI] [PubMed] [Google Scholar]
- Brümmendorf T., Hubert M., Treubert U., Leuschner R., Tàrnok A., Rathjen F.G. The axonal recognition molecule F11 is a multifunctional proteinspecific domains mediate interactions with Ng-CAM and restrictin. Neuron. 1993;10:711–727 . doi: 10.1016/0896-6273(93)90172-n. [DOI] [PubMed] [Google Scholar]
- Buj-Bello A., Adu J., Pinon L.G.P., Horton A., Thompson J., Rosenthal A., Chinchetru M., Buchman V.L., Davies A.M. Neurturin responsiveness requires a GPI-linked receptor and the Ret receptor tyrosine kinase. Nature. 1997;387:721–724 . doi: 10.1038/42729. [DOI] [PubMed] [Google Scholar]
- D'Alessandri L., Ranscht B., Winterhalter K.H., Vaughan L. Contactin/F11 and tenascin-C co-expression in the chick retina correlates with formation of the synaptic plexiform layers. Curr. Eye Res. 1995;14:911–926 . doi: 10.3109/02713689508995131. [DOI] [PubMed] [Google Scholar]
- Daum G., Regenass S., Sap J., Sclessinger J., Fischer E.H. Multiple forms of the human tyrosine phosphatase RPTPα. J. Biol. Chem. 1994;269:10524–10528 . [PubMed] [Google Scholar]
- den Hertog J., Pals C.E., Peppelenbosch M.P., Tertoolen L.G., de Laat S.W., Kruijer W. Receptor protein tyrosine phosphatase α activates pp60c-src and is involved in neuronal differentiation. EMBO (Eur. Mol. Biol. Organ.) J. 1993;12:3789–3798 . doi: 10.1002/j.1460-2075.1993.tb06057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre-Sarrailh C., Gennarini G., Goridis C., Rougon G. F3/F11 cell surface molecule expression in the developing mouse cerebellum is polarized at synaptic sites and within granule cells. J. Neurosci. 1992;12:257–267 . doi: 10.1523/JNEUROSCI.12-01-00257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang K.S., Martins-Green M., Williams L.T., Hanafusa H. Characterization of chicken protein tyrosine phosphatase alpha and its expression in the central nervous system. Mol. Brain Res. 1996;37:1–14 . doi: 10.1016/0169-328x(95)00240-s. [DOI] [PubMed] [Google Scholar]
- Gennarini G., Cibelli G., Rougon G., Mattei M.-G., Goridis C. The mouse neuronal cell surface protein F3a phosphatidylinositol-anchored member of the immunoglobulin superfamily related to chicken contactin. J. Cell Biol. 1989;109:775–788 . doi: 10.1083/jcb.109.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder K.W., Moller N.P.H., Peacock J.W., Jirik F.R. Protein-tyrosine phosphatase α regulates src family kinases and alters cell-substratum adhesion. J. Biol. Chem. 1998;273:31890–31900 . doi: 10.1074/jbc.273.48.31890. [DOI] [PubMed] [Google Scholar]
- Klein R.D., Sherman D., Ho W.H., Stone D., Bennett G.L., Moffat B., Vandlen R., Simmons L., Gu Q., Hongo J.-A. A GPI-linked protein that interacts with Ret to form a candidate neurturin receptor. Nature. 1997;387:717–721 . doi: 10.1038/42722. [DOI] [PubMed] [Google Scholar]
- Krueger N.X., Streuli M., Saito H. Structural diversity and evolution of human receptor-like protein tyrosine phosphatases. EMBO (Eur. Mol. Biol. Organ.) J. 1990;9:3241–3252 . doi: 10.1002/j.1460-2075.1990.tb07523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz S., Ziegler U., Kunz B., Sonderegger P. Intracellular signaling is changed after clustering of the neural cell adhesion molecules axonin 1 and Ngcam during neurite fasciculation. J. Cell Biol. 1996;135:253–267 . doi: 10.1083/jcb.135.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim K.L., Lai D.S.Y., Kalousek M.B., Wang Y., Pallen C.J. Kinetic analysis of two closely related receptor-like protein-tyrosine-phosphatases, PTPα and PTP∈. Eur. J. Biochem. 1997;245:693–700 . doi: 10.1111/j.1432-1033.1997.00693.x. [DOI] [PubMed] [Google Scholar]
- Lowell C.A., Soriano P. Knockouts of Src-family kinasesstiff bones, wimpy T cells, and bad memories. Genes Dev. 1996;10:1845–1857 . doi: 10.1101/gad.10.15.1845. [DOI] [PubMed] [Google Scholar]
- Morales G., Hubert M., Brümmendorf T., Treubert U., Tàrnok A., Schwarz U., Rathjen F.G. Induction of axonal growth by heterophilic interactions between the cell surface recognition proteins F11 and Nr-CAM/Bravo. Neuron. 1993;11:1113–1122 . doi: 10.1016/0896-6273(93)90224-f. [DOI] [PubMed] [Google Scholar]
- Peles E., Nativ M., Campbell P.L., Sakurai T., Martinez R., Lev S., Clary D.O., Schilling J., Barnea G., Plowman G.D., Grumet M., Schlessinger J. The carbonic anhydrase domain of receptor tyrosine phosphatase β is a functional ligand for the axonal cell recognition molecule contactin. Cell. 1995;82:251–260 . doi: 10.1016/0092-8674(95)90312-7. [DOI] [PubMed] [Google Scholar]
- Peles E., Nativ M., Lustig M., Grumet M., Schilling J., Martinez R., Plowman G.D., Schlessinger J. Identification of a novel contactin-associated transmembrane receptor with multiple domains implicated in protein-protein interactions. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:978–988 . doi: 10.1093/emboj/16.5.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponniah S., Wang D.Z.M., Lim K.L., Pallen C.J. Targeted disruption of the tyrosine phosphatase PTPα leads to constitutive downregulation of the kinases Src and Fyn. Curr. Biol. 1999;9:535–538 . doi: 10.1016/s0960-9822(99)80238-3. [DOI] [PubMed] [Google Scholar]
- Ranscht B., Dours M.T. Sequence of contactin, a 130-kD glycoprotein concentrated in areas of interneuronal contact, defines a new member of the immunoglobulin supergene family in the nervous system. J. Cell Biol. 1988;107:1561–1573 . doi: 10.1083/jcb.107.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T., Lustig M., Nativ M., Hemperly J.J., Schlessinger J., Peles E., Grumet M. Induction of neurite outgrowth through contactin and Nr-CAM by extracellular regions of glial receptor tyrosine phosphatase β. J. Cell Biol. 1997;136:907–918 . doi: 10.1083/jcb.136.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J., Muranjan M., Sap J. Receptor protein tyrosine phosphatase α activates Src-family kinases and controls integrin-mediated responses in fibroblasts. Curr. Biol. 1999;9:505–511 . doi: 10.1016/s0960-9822(99)80234-6. [DOI] [PubMed] [Google Scholar]
- Vaughan L. Signalling by GPI-anchored molecules in the nervous system. Semin. Neurosci. 1996;8:397–403 . [Google Scholar]
- Wang Y., Pallen C.J. The receptor-like protein tyrosine phosphatase HPTPα has two active catalytic domains with distinct substrate specificities. EMBO (Eur. Mol. Biol. Organ.) J. 1991;10:3231–3237 . doi: 10.1002/j.1460-2075.1991.tb04886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X.M., Wang Y., Pallen C.J. Cell transformation and activation of pp60c-src by overexpression of a protein tyrosine phosphatase. Nature. 1992;359:336–339 . doi: 10.1038/359336a0. [DOI] [PubMed] [Google Scholar]
- Zisch A.H., D'Alessandri L., Ranscht B., Falchetto R., Winterhalter K.H., Vaughan L. Neuronal cell adhesion molecule contactin/F11 binds to tenascin via its immunoglobulin-like domains. J. Cell Biol. 1992;119:203–213 . doi: 10.1083/jcb.119.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisch A.H., D'Alessandri L., Amrein K., Ranscht B., Winterhalter K.H., Vaughan L. The glypiated neuronal cell adhesion molecule contactin/F11 complexes with src-family protein tyrosine kinase fyn. Mol. Cell Neurosci. 1995;6:263–279. doi: 10.1006/mcne.1995.1021. [DOI] [PubMed] [Google Scholar]