Abstract
Skeletal muscle in vertebrates is derived from somites, epithelial structures of the paraxial mesoderm, yet many unrelated reports describe the occasional appearance of myogenic cells from tissues of nonsomite origin, suggesting either transdifferentiation or the persistence of a multipotent progenitor. Here, we show that clonable skeletal myogenic cells are present in the embryonic dorsal aorta of mouse embryos. This finding is based on a detailed clonal analysis of different tissue anlagen at various developmental stages. In vitro, these myogenic cells show the same morphology as satellite cells derived from adult skeletal muscle, and express a number of myogenic and endothelial markers. Surprisingly, the latter are also expressed by adult satellite cells. Furthermore, it is possible to clone myogenic cells from limbs of mutant c-Met−/− embryos, which lack appendicular muscles, but have a normal vascular system. Upon transplantation, aorta-derived myogenic cells participate in postnatal muscle growth and regeneration, and fuse with resident satellite cells.
The potential of the vascular system to generate skeletal muscle cells may explain observations of nonsomite skeletal myogenesis and raises the possibility that a subset of satellite cells may derive from the vascular system.
Keywords: myogenesis, satellite cells, endothelial cells, multipotent progenitors, vascular–endothelial cadherin
It is generally accepted that all skeletal muscle cells of the vertebrate body derive from the dorsal domain of somites, epithelial structures that form in a cranio–caudal sequence from the paraxial mesoderm, flanking the axial neural tube on both sides ( Christ and Ordhal 1995). This evidence largely derives from classic chick–quail transplantation experiments, and has been confirmed by in vitro explant culture in both birds and mammals (reviewed in Cossu et al. 1996a).
Skeletal myogenesis begins soon after onset of somitogenesis and continues throughout development and postnatal growth. Adult muscle retains proliferative potential due to the presence of quiescent satellite cells. Cells in the somites are first instructed to become myogenic by signals emanating from neighboring tissues, such as notochord, neural tube, and dorsal ectoderm ( Cossu et al. 1996a). Once committed, a subset of dorsal epithelial somite cells will begin migrating beneath the dermomyotome to form the myotome, the first skeletal muscle structure, initially composed of terminally differentiated postmitotic, mononucleate cells ( Denetclaw et al. 1997; Kahane et al. 1998). Cells of the dermomyotome, exposed to the same signals, enter other mesodermal lineages, such as dermis or endothelium, or remain undifferentiated as progenitors of successive generations of myogenic cells that will later give rise to primary and secondary fibers, as well as to satellite cells.
Most experiments addressing the origin of skeletal muscle have analyzed only the embryonic stages of development. It has been assumed that skeletal muscle cells that form during fetal and postnatal life share the same origin, although experimental proof for this is lacking.
However, much evidence has accumulated for in vivo or in vitro myogenic differentiation of cells of nonsomite origin during late fetal and postnatal development (reviewed in Cossu 1997), when satellite cells are considered to be the only remaining myogenic progenitors ( Bischoff 1994). Whether or not satellite cells originate exclusively from somites also remains an open question.
We recently reported that some myogenic cells that participate in skeletal muscle regeneration originate in the bone marrow and are delivered to the muscle through the blood circulation ( Ferrari et al. 1998). This prompted us to define the embryological origin of these myogenic cells, as well as of satellite cells, but lack of specific markers has hampered this work. Lineage studies for skeletal muscle are also complicated by the multinucleate nature of the differentiated tissue. However, it is possible to clone mammalian satellite cells that, in the mouse, show a typical round-shaped morphology during proliferation, clearly different from spindle-shaped myoblasts ( Cossu et al. 1987). Upon serum withdrawal, these cells will elongate and then fuse into multinucleate contracting myotubes.
By extending this clonal analysis to various early embryonic structures, we now report that the highest yield in myogenic clones with a morphology typical of satellite cells and expressing a number of myogenic and endothelial markers was obtained from dorsal aorta, a tissue not previously known to give rise to skeletal myogenic cells. These results have implications for current interpretations of fetal and postnatal myogenesis.
Materials and Methods
Transgenic Mouse Lines
Experiments were performed on MLC3F-nlacZ, Myf-5-nlacZ, c-Met double tyrosine mutant, and Splotch mouse lines ( Kelly et al. 1995; Maina et al. 1996; Tajbakhsh et al. 1996, Tajbakhsh et al. 1997).
Cell and Organ Cultures
Embryos were dissected and embryonic structures were isolated as described ( Cossu et al. 1996b). The tissues were then either separated into a single cell suspension by gentle pipetting or cultured as explants ( Cusella-De Angelis et al. 1994) for periods ranging from 3–7 d. At the end of the culture period, tissues were either stained for β-galactosidase activity, immunofluorescence, or used for reverse transcriptase (RT)1 -PCR. Cultures were grown as described ( Cossu et al. 1996b). Northern Blot analysis and RT-PCR were performed as described ( Ferrari et al. 1997). Oligos for Pax3 amplification were 5′-TGT GGA ATA GAC GTG GGC TGG TA-3′ and 5′-AGG AGG CGG ATC TAG AAA GGA AG-3′; for MyoD: 5′- CAC TAC AGT GGC GAC TCA GAC GCG-3′, nt 730–753, 5′-CCT GGA CTC GCG CAC CGC CTC ACT-3′, nt 873–850.
Immunocytochemistry
The antibodies used against MyoD, myosin heavy chains, and c-Met have been described ( Bader et al. 1982; Tajbakhsh et al. 1994; Koishi et al. 1995; Maina et al. 1996). The antibodies against M-cadherin and MNF were donated by A. Starzyski-Powitz (Humangenetik fur Biologen, Goethe-Universitat, Frankfurt, Germany) and R. Bussel-Duby (University of Texas SW Medical Center, Dallas, TX), respectively ( Irintchev et al. 1994; Garry et al. 1997). The antibodies against vascular-endothelial (VE) -cadherin, CD34, and PECAM were donated by E. Dejana and C. Gherlanda (Istituto di Ricerche Farmacologiche Mario Negri, Milan, Italy). Antibodies against VEGR2, αM-integrin (MAC-1), β3-integrin, and von Willebrand (Santa Cruz and Pharmingen) were donated by A. Stoppacciaro (Dipartimento di Medicinal Sperimentale e Patologia, Universita ‘La Sapienza,’ Rome, Italy). Immunocytochemistry on tissue sections and cultured cells was carried out as described ( Tajbakhsh et al. 1994).
Transplantation Studies
Genetically labeled cells were injected into the regenerating Tibialis anterior (TA) of SCID/bg mice as described ( Ferrari et al. 1998). Fetal limbs were isolated from E16–17 wild-type (wt) embryos and, after removal of the skin, were transplanted subcutaneously into newborn (P1–2) MLC3F-nlacZ mice ( Lagrand et al. 1997). Alternatively, freshly dissected dorsal aortas from E9 MLC3F-nlacZ embryos were transplanted into the TA of newborn (P4–5) SCID/bg mice. At different periods after transplantation, the mice were killed, the transplanted and the contralateral TA muscles or the transplanted fetal limb were recovered, and stained for β-galactosidase activity or cryostat-sectioned and processed for immunofluorescence.
Results
Clonal Analysis of Myogenic Cells
Mouse satellite cells grown in culture under clonal conditions appear as round-shaped cells expressing myogenic markers, such as MyoD, desmin, and c-Met, and differentiate into multinucleated myotubes upon mitogen deprivation. Satellite cells can be cloned from skeletal muscle of postnatal mice and of fetuses older than E15, but not from earlier stages ( Cossu et al. 1987). We found that when limbs from E13 or E11, or even forelimbs from E9.5 (20–24 somites) embryos were precultured as explants for three, five, or seven days, respectively, and then successively dissociated to a single cell suspension and cultured at low density, myogenic clones with the phenotype of satellite cells appeared (one example is shown in Fig. 2 A). Thus, cells competent to generate satellite cell-like clones must enter the limb field as early as the first migratory population of somite-derived myoblasts, but seem not to acquire this competence until later developmental stages and upon maintenance of interaction with other limb cells.
Figure 2.
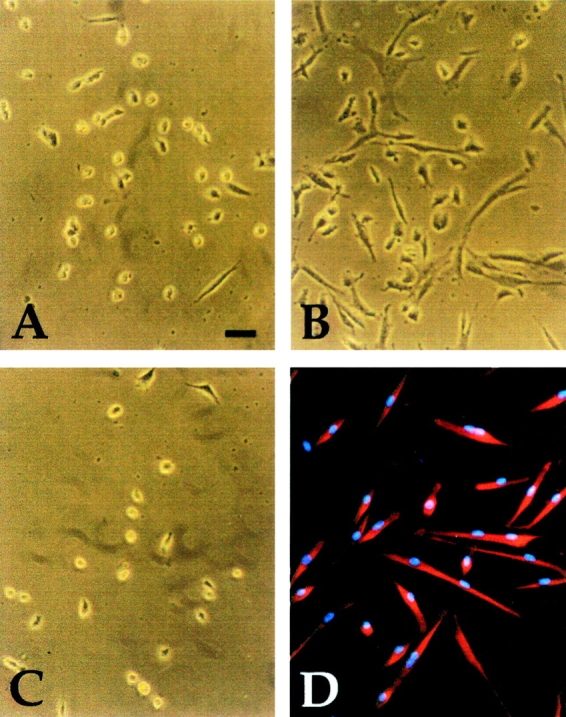
Morphology of typical clones derived from explant cultures of E9.5 limb bud (A), somites (B), and dorsal aorta (C), cultured in growth medium. Immunofluorescence analysis with antisarcomeric myosin antibody of a clone from dorsal aorta, after three days of culture in differentiation medium is shown in D. Bar, 15 μm.
Myogenic Clones Are Derived from Dorsal Aorta
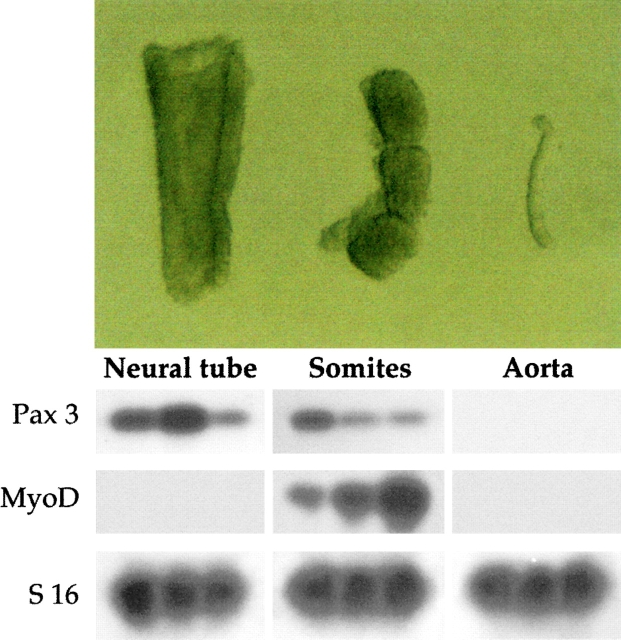
We set out to investigate, by the explant preculture method described above, the possible presence of satellite cell-like cells in different tissue anlagen of the mouse embryo. We cultured newly formed somites or segmental plates, neural tube, dorsal ectoderm, lateral mesoderm, dorsal aorta (from the corresponding body level), and heart. Careful digestion with pancreatin-trypsin allowed clean dissection of the epithelial somites from both the neural tube/notochord complex axial and lateral mesoderm ( Fig. 1, top). Possible contamination not detected by microscopy was ruled out by RT-PCR analysis, which did not reveal expression of the medial markers Pax3 and MyoD in the dorsal aorta or in other lateral structures ( Fig. 1, bottom, and data not shown). After one week, half of the explants were stained for the expression of myosin heavy chains and, as expected, only cultures from somites, limb buds, and heart contained hundreds of positive cells. Virtually no myosin positive cells were present in cultures from other tissues (not shown). The rest of these explants were dissociated to single cell suspensions and cloned by limited dilution under conditions that favor clonal growth of satellite cells. Fig. 2 A shows the typical morphology of a satellite cell-like clone derived from precultured E9.5 forelimb bud explants, indistinguishable from a clone directly derived from older limbs (not shown). Unexpectedly, the vast majority of clones with this morphology came from explants of dorsal aorta ( Fig. 2 C). In contrast, most clones derived from precultured somites had a fibroblast-like morphology ( Fig. 2 B). After shifting the clonal cultures to differentiation medium, all round-shaped satellite cell-like, but not the fibroblast-like, clones differentiated into myosin positive cells ( Fig. 2 D).
Figure 1.
Top, Morphology of embryonic structures isolated from E9.5 mouse embryos after pancreatin digestion. Bottom, RT-PCR revealed the medial markers MyoD and Pax3 were expressed in dissected somites (different ratio of Pax3 to MyoD in different lanes depends upon isolation of somites at different cranio–caudal level); Pax3, but not MyoD, in the neural tube; and none of the markers was detectable in dissected dorsal aorta.
Quantitative analysis showed that a high proportion of satellite cell-like clones (defined by morphology and myosin expression) were derived from explants of dorsal aorta ( Fig. 3 A). These precultured explants gave rise to an average of five times more clones than the somites, whereas the latter contained ten times more cells for the same segment length. Thus, each segment of dorsal aorta can give rise to 50 satellite cell-like clones on a per cell basis, versus one clone originating from somites. Very few clones of satellite cell-like cells originated from lateral mesoderm and neural tube, and none from ectoderm and heart.
Figure 3.
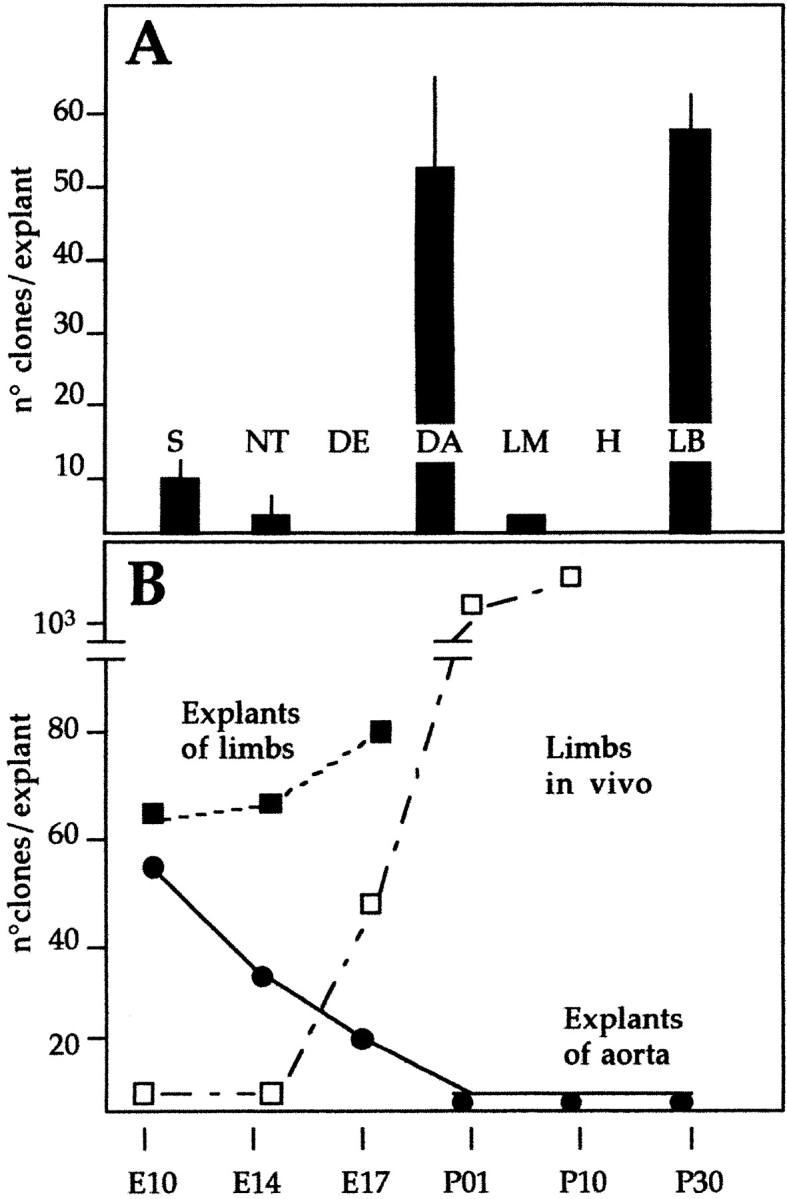
A, Quantitative analysis of satellite cell-like clones (shown in Fig. 1) from explant cultures of different anlagen of E9.5 embryos. Each bar is the average of at least three separate experiments, each performed in triplicate. B, Time course of the appearance of satellite cell-like clones from explants of vessels (___) or limb buds (- - -) at successive periods of development. The time course of the appearance of satellite cell-like clones, directly cloned from limb buds in vivo is also shown (– · –).
As reported above, many satellite cell-like clones were obtained from cultures of forelimb bud from 20–24 somite embryos. At these stages the limb bud already contains both myoblasts and vascular endothelial cells. Thus, all the tissues that gave rise to satellite cell-like clones contain endothelial cells. The only tissue that, despite the presence of abundant endothelium (endocardium), never gave rise to satellite cell-like clones was heart. This may reflect unique tissue interactions that occur during endocardium specification ( Sugi and Markwald 1996) and may result in a more restricted developmental potential, as compared with noncardiac endothelium.
We next investigated whether the aorta would maintain the potential of generating clones of satellite cell-like cells during later developmental stages. Fig. 3 B shows that this potential declines during development, but at E17 is still approximately one third of the maximal value obtained at E9.5. Postnatal or adult aorta, as well as smaller vessels dissected from limb muscle, did not give rise to satellite cell-like clones, either directly or after explant culture (not shown). Forelimbs from the same stage were used as positive controls.
Endothelial Markers Are Expressed in Myogenic Cells from Aorta and in Satellite Cells
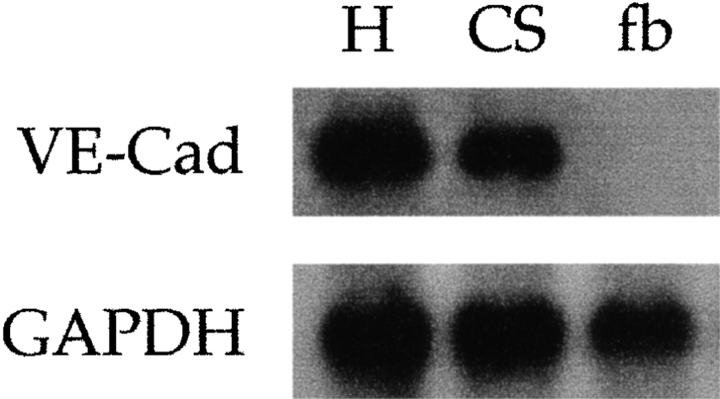
These novel results led us to compare the phenotype of these clonable satellite cell-like cells with that of adult satellite cells and fetal myoblasts. Table and Fig. 4 show that most known myogenic markers (MyoD, Myf-5, desmin, MNF, c-Met, and M-cadherin) were expressed by clones of dorsal aorta, as well as by postnatal muscle satellite cells and fetal myoblasts. Unexpectedly, vascular-endothelial markers, such as VE-cadherin, VEGF-R2, αM-integrin, β3 integrin, P-selectin, smooth α-actin, and PECAM were also expressed by the first two cell types, while fetal myoblasts did not express VE-cadherin, P-selectin and β3 integrin (all clones were negative for von Willebrand factor). Fig. 5 shows clones of aorta-derived myogenic cells ( Fig. 5 A) and of adult satellite cells ( Fig. 5 B) that all coexpress VE-cadherin on the surface and MyoD in the nucleus. Clones from dorsal aorta also expressed CD34, but only during the first two days in vitro. Muscle fibroblasts (used as controls) were negative for all of these markers. Thus, clones from the dorsal aorta were indistinguishable from clones of satellite cells in terms of differential expression of any of 15 different markers analyzed. Expression of VE-cadherin was confirmed by Northern blot analysis in satellite cells ( Fig. 6). It was unexpected, and is notable that satellite cells derived from adult muscle express a number of endothelial markers.
Table 1.
Expression of Different Markers by Aorta-derived and Other Mesodermal Cells
| Cell type: | Aorta | Satellite | Fetal myoblast | Fibroblast |
|---|---|---|---|---|
| MyoD | + | + | + | − |
| Myf-5 | ± | ± | ± | − |
| Desmin | + | + | + | − |
| Smooth α-actin | + | + | + | − |
| MNF | + | + | + | − |
| c-Met | + | + | + | − |
| M-Cad | + | + | + | − |
| VE-Cad | + | + | − | − |
| VEGF-R2 | + | + | + | − |
| P-Selectin (CD62P) | + | + | − | − |
| αM-Integrin (CD11b) | + | + | −/+ | − |
| β3-Integrin (CD61) | + | + | − | − |
| PECAM (CD31) | −/+ | −/+ | −/+ | − |
| von Willebrand | − | − | ND | − |
| CD34 | +/− | +/− | − | − |
Aorta-cells were cloned from seven day-old organ cultures of dorsal aorta from E9.5 day embryos. Immunofluorescence was carried out on at least ten individual clones of each cell type in three separate cultures. Satellite cells were cloned from hind limb skeletal muscles of P10 mice. Fetal myoblasts and fibroblasts were cultured as described ( Cossu et al. 1987). Myoblasts were identified by coexpression of MyoD or desmin.
+, Indicates that >95% of the cells in the clone expressed a high level of the antigen (examples shown in Fig. 3) during the phase of clonal growth. ±, Indicates that ∼10–20% of the cells in most clones expressed Myf-5, revealed by β-galactosidase staining of clones derived from Myf-5/nLacZ embryos. −/+, Indicates that virtually all the cells of a clone expressed high levels of the antigen only after fusion. +/−, Indicates expression of the antigen only during the first days of clonal growth.
Figure 4.
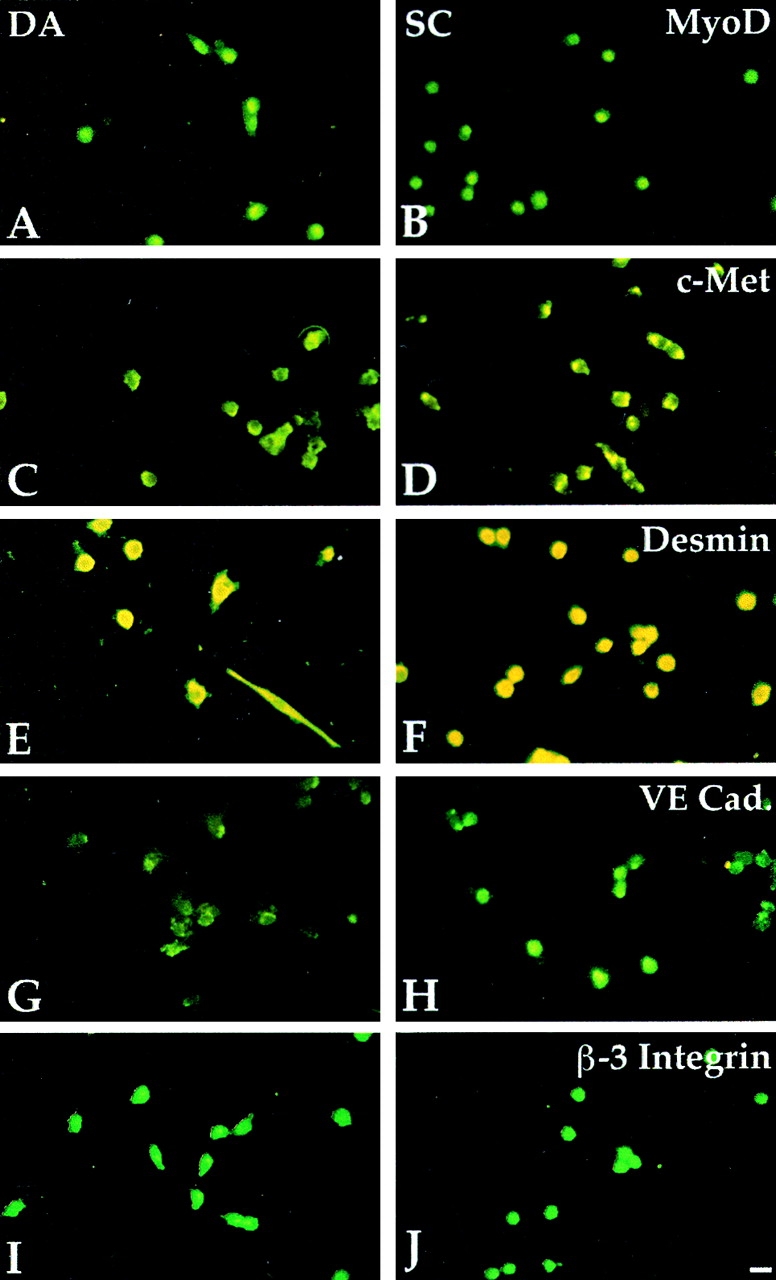
Myogenic and endothelial markers are expressed in aorta-derived myogenic cells and in satellite cells. Immunofluorescence analysis with antibodies against MyoD (A and B), c-Met (C and D), Desmin (E and F), VE-cadherin (G and H), and β-3 integrin (I and J) of clones derived from E9.5 dorsal aorta (A, C, E, G, and I) or P10 satellite cells (B, D, F, H, and J). Bar, 10 μm.
Figure 5.
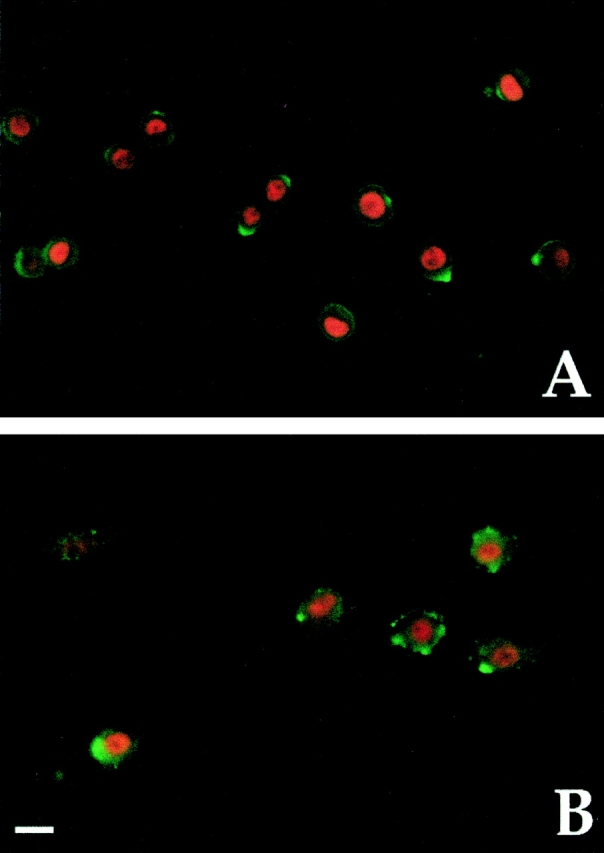
MyoD and VE-cadherin are coexpressed in aorta-derived myogenic cells and in satellite cells. Double immunofluorescence analysis with antibodies against MyoD (red) and VE-cadherin (green) of clones derived from E9.5 dorsal aorta (A) and of adult satellite cells (B). Bar, 10 μm.
Figure 6.
The message for VE-cadherin is expressed in adult satellite cells. Northern blot analysis of VE-cadherin expression in E 9.5 embryonic hearts (H), adult satellite cells (CS), and primary fibroblasts (fb).
Myogenic Clones Can Be Derived from the Limb Buds of c-Met Null Embryos
In embryos null for c-Met, or expressing a Met receptor unable to transduce the HGF signal (MetD), myoblasts fail to emigrate from the somite and to colonize the limb bud ( Bladt et al. 1995; Maina et al. 1996). Although devoid of myogenic cells, the developing limbs of these mutants have normal blood vessels, as shown in Fig. 7A and Fig. B. Forelimb buds from MetD homozygous embryos were cultured and then dissociated into single cell suspensions and grown as clones. Satellite cell-like clones emerged from forelimbs of homozygous MetD embryos (∼50% of those obtained from heterozygous or wt siblings). This demonstrates the presence of clonogenic satellite cell-like cells that are not derived from somitic myoblasts. The clones obtained from mutant embryos were notably smaller (averaging 4 to 6 cells) than those derived from the heterozygous siblings (averaging 20 to 60 cells), and expressed high levels of MyoD ( Fig. 7F and Fig. G). With time in culture, some of these clones disappeared and occasionally fragmented chromatin was observed, but the surviving clones differentiated into myosin-positive mono- or binucleate muscle cells.
Figure 7.
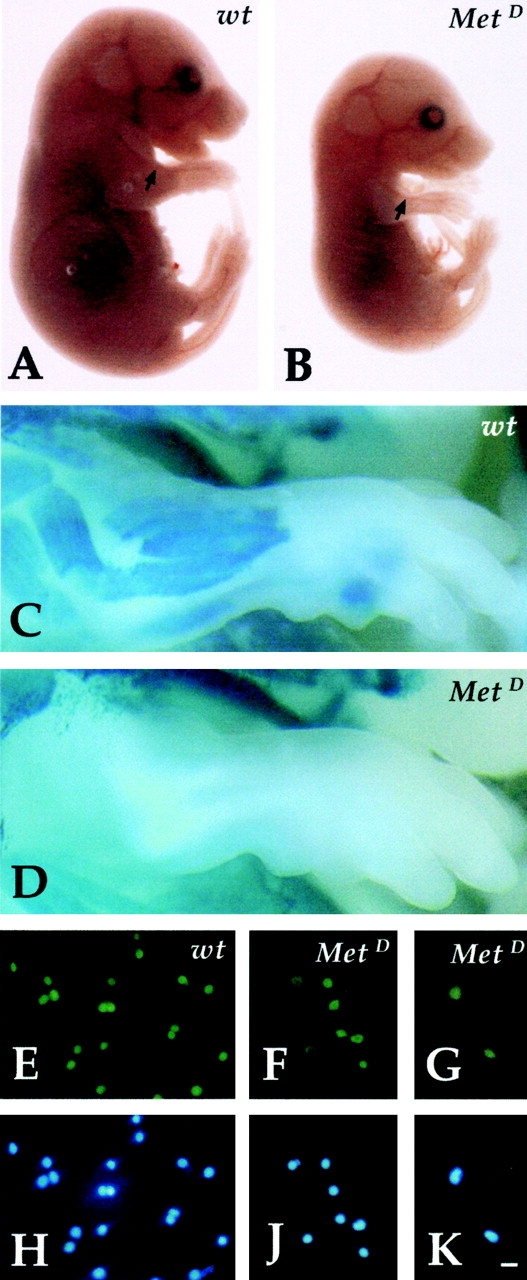
Myogenic clones are present in the limbs of c-Met–deficient embryos. Phase-contrast microscopy of wt (A) and MetD (B) E14 embryos showing normal vasculature in mutant embryos (arrow). β-galactosidase staining of wt (C) and MetD (D) crossed to MLC3F-nLacZ embryos showing complete absence of muscle (β-gal+) cells in the limb of E15 mutant embryos. Immunofluorescence analysis with antibodies against MyoD of clones derived from E13 limb buds of wt (E) or MetD (F and G) embryos. Nuclear staining (Hoechst) is shown in H, J, and K. Bar, 10 μm.
We thus conclude that at least a fraction of satellite cell-like myogenic progenitors are present in the limbs of MetD mutant embryos, which are colonized by endothelial cells, but not by somite-derived myoblasts. Their growth is impaired, likely because they cannot respond to SF/HGF, and eventually apoptosis may occur. Still, they represent a considerable fraction (∼50%) of those obtained from wt embryos. Similar results were also obtained using limb buds of Splotch embryos as a source of myogenic cells (not shown). Splotch mice are defective in the Pax-3 gene and their phenotype overlaps with the Met null in terms of lack of migration of somite-derived precursors in the limbs ( Bober et al. 1994; Goulding et al. 1994).
In Vivo Myogenic Potential of Cells from Dorsal Aorta
We tested the potential of transplanted aorta-derived satellite cell-like cells to participate in in vivo perinatal growth and regeneration of skeletal muscle, in three different sets of experiments.
In the first experiment, a cell suspension from cultured explants of E9.5 embryonic aorta of MLC3F-nLacZ transgenic mice, in which transgene expression is restricted to heart and skeletal muscle ( Kelly et al. 1995), was mixed with a tenfold excess of satellite cells from wt P10 mice. Part of this suspension was grown in culture. Fig. 8 A shows a myotube containing one β-galactosidase positive (β-gal+) nucleus which, because of the genetic label, must be derived from the dorsal aorta explant. The rest of the mixed cell suspension was injected into a regenerating TA muscle of SCID/bg mice, where they gave rise to small clusters of several β-gal+ nuclei within regenerating fibers, surrounded by a laminin-positive basal membrane ( Fig. 8B and Fig. C). Thus, cells derived from the dorsal aorta can fuse with satellite cells in vitro and participate in skeletal muscle regeneration in vivo. All these results show that myogenic cells derived from the aorta cannot be distinguished from bona fide satellite cells.
Figure 8.
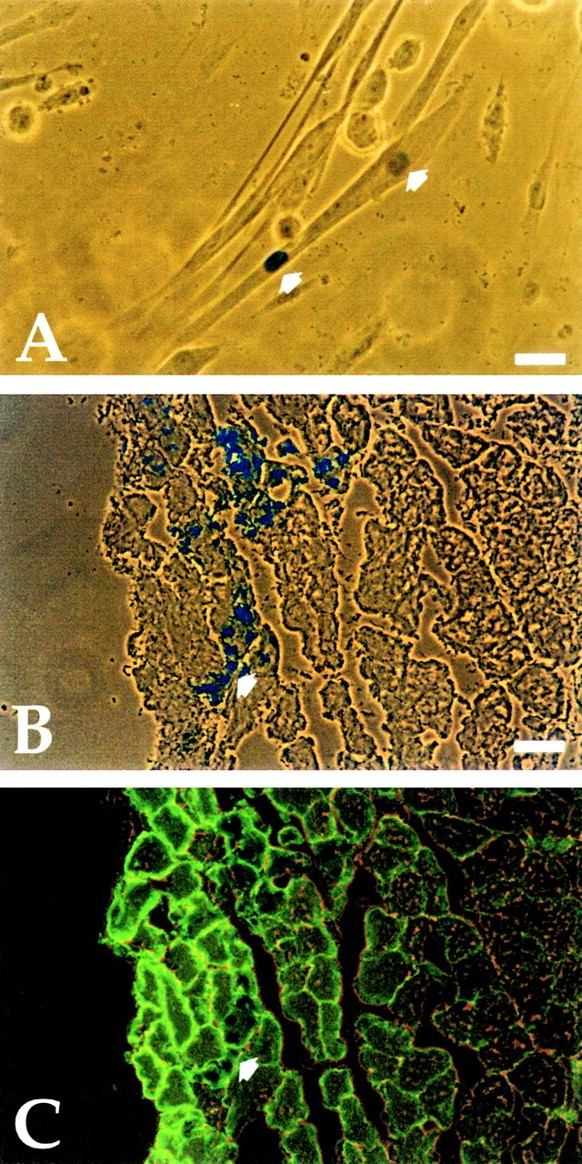
Aorta-derived myogenic cells undergo myogenesis in vitro and in vivo. A, Cocultures of clones from E9.5 dorsal aorta from MLC3F-nLacZ embryos and P10 wt satellite cells. Arrows indicate one β-gal+ and one β-gal− nucleus within the same myotube. Bar, 10 μm. B, Cross-section of a regenerating TA of a SCID/bg injected with pooled clones of satellite cell-like clones, showing a cluster of β-gal+ nuclei (arrow) within small regenerating fibers, labeled with an antibody against laminin in C. Bar, 25 μm.
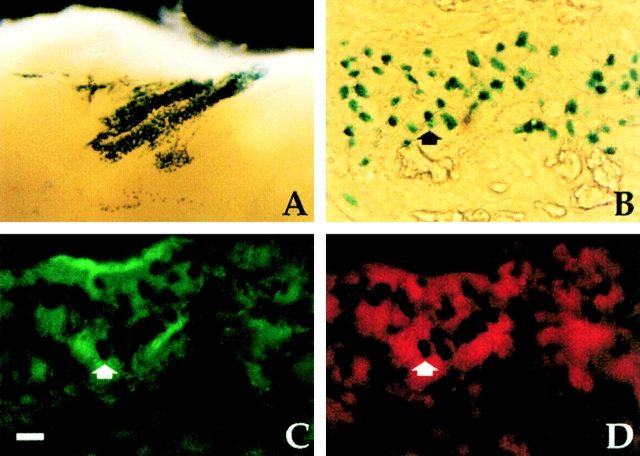
For the second experiment, to test whether myogenic cells derived from the vasculature may also contribute to normal development of skeletal muscle, we transplanted E16 fetal limbs from wt embryos under the skin of a newborn (P2) MLC3F-nLacZ transgenic mouse ( Lagrand et al. 1997). After one week, the transplanted limbs had grown and were vascularized by the host. Whole-mount staining revealed the presence of many β-gal+ nuclei clustered in the area where the vessels had penetrated the transplant ( Fig. 9 A). Sections of the same samples revealed β-gal+ nuclei within myosin-positive fibers adjacent to VE-cadherin–positive vessels ( Fig. 9, B–D).
Figure 9.
Host-derived myogenic cells are present in fetal limbs transplanted under the skin of newborn MLC3F-nLacZ transgenic mice and vascularized by the host. A, Whole-mount stain reveals a cluster of β-gal+ nuclei in the transplanted limb. B–D, Cross-section of the same sample stained for β-galactosidase activity revealed the β-gal+ nuclei (B) inside myosin positive muscle fibers (D) adjacent to VE-cadherin positive vessels (C). Bar, 25 μm.
In the third experiment, isolated dorsal aortas from E9 MLC3F-nLacZ transgenic embryos were transplanted into the TA on one side of newborn (P 4–5) SCID/bg mice. After two weeks, the TA anterior contained a cluster of fibers with many β-gal+ nuclei in the area of the transplant ( Fig. 10 A). However, the untreated contralateral TA was also found to contain many β-gal+ nuclei dispersed throughout the whole muscle ( Fig. 10 B), indicating that these cells had reached the developing fibers through the circulation. In contrast, when somites from the same embryos were similarly transplanted into the developing TA muscle of newborn immune-deficient mice, many β-gal+ nuclei were observed surrounding a core of cartilage (presumably from the sclerotome), but no β-gal+ nuclei were found in other muscles (data not shown).
Figure 10.
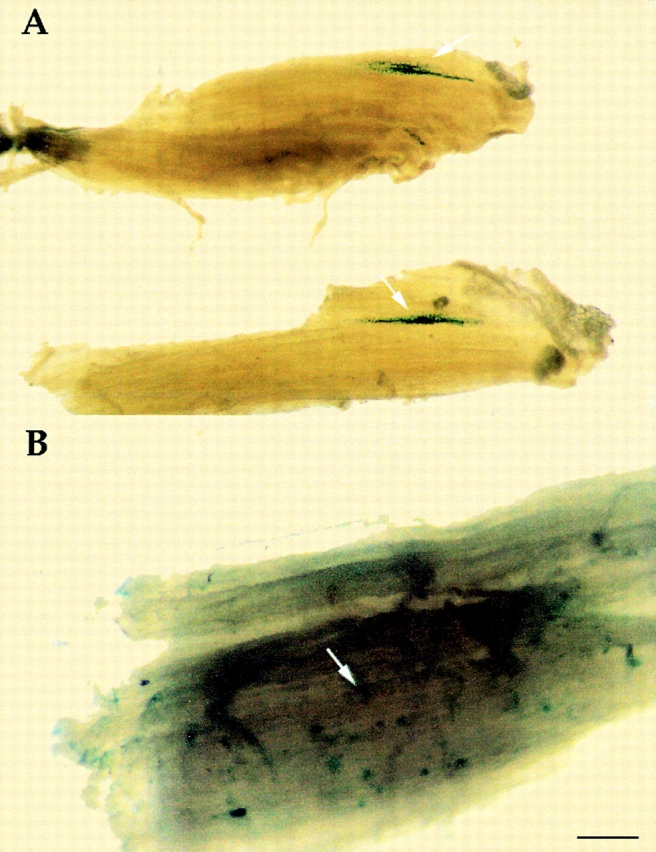
Aorta-derived myogenic cells contribute to growing fibers and also circulate. A, Whole-mount stain reveals clusters of β-gal+ nuclei (arrow) in the TA of P14 SCID/bg mice, two weeks after transplantation of embryonic aortas from E9 MLC3F-nLacZ embryos. B, Whole-mount stain of the contralateral TA of the same SCID/bg reveals several β-gal+ nuclei (arrow) dispersed throughout the whole muscle. Bar, 50 μm.
Discussion
Skeletal Myogenic Cells from the Embryonic Vasculature
Our data show that cells derived from embryonic vessels can give rise to skeletal myogenic cells. There is no skeletal muscle surrounding the developing vessels in the mouse embryo. In vertebrates, somitic muscles derive from dorsal mesoderm, whereas the intraembryonic vasculature mainly derives from ventral splancnic mesoderm ( Flamme and Risau 1992), although the dorsal portion of the aorta is derived from the paraxial mesoderm and is not endowed with hemopoietic capacity ( Pardanaud et al. 1996).
In explant cultures, we found that dorsal aorta did not contain differentiated muscle cells, which typically appear in similar cultures of somitic or limb tissues. However, when dorsal aortas were first explant-cultured for a week, and then dissociated to single cell suspensions and cloned under conditions that favor expansion of adult satellite cells, many myogenic clones appeared, undistinguishable from bona fide satellite cells. The fact that such clones could not be obtained directly from the aorta suggests a requirement for growth factors provided by neighboring cells during the incubation as organ culture, and absent in our culture medium.
The myogenic clones express all the early myogenic markers considered, including M-cadherin, MNF, and desmin. Virtually 100% of these cells express MyoD, while only a minority of them express Myf-5. Although the majority of postnatal satellite cells, upon clonal expansion in vitro or activation in vivo, express both MyoD and Myf5 ( Cornelison and Wold 1997; Cooper et al. 1999), in the MyoD knockout mouse regeneration is impaired via an effect on the satellite cell population ( Megeney et al. 1996).
The unexpected coexpression of a number of endothelial and myogenic markers in cells derived from the dorsal aorta suggests that these myogenic cells may be derived from true endothelial cells or from a common precursor. It is interesting to note that endothelial cells appear to be the only cell type capable of generating immortal clones in early postimplantation mouse embryos ( Hatzopoulos et al. 1998) and that circulating endothelial progenitor cells can be isolated from adult human blood ( Asahara et al. 1997). These endothelial progenitors typically express CD34. Aorta-derived myogenic clones only express CD34 at the beginning of clonal expansion, suggesting early loss of this antigen upon entry in the myogenic pathway. Other endothelial markers, such as VE-cadherin, are lost upon terminal differentiation, but are coexpressed with MyoD throughout clonal expansion.
To our surprise, endothelial markers were also coexpressed with MyoD in bona fide satellite cells, a puzzling observation for cells presumed to be of somitic origin. The problem of the embryonic origin of satellite cells has never been directly approached, except in a study that was inconclusive due to technical difficulties in identifying quail nuclei in chick–quail chimeras at the ultrastructural level ( Armand et al. 1983). It is interesting to note that, unlike postnatal satellite cells, fetal myoblasts were found not to express VE-cadherin, β3-integrin, or P-selectin, in keeping with the possibility that several phenotypically distinct populations of myogenic cells may appear sequentially during muscle histogenesis ( Cossu and Molinaro 1987).
Our data suggest that, differently from embryonic and fetal myoblasts derived from somites, satellite cells may derive, at least in part, from progenitors in the dorsal aorta. Coexpression of endothelial and myogenic markers supports this hypothesis. In normal development, endothelial cells first migrate into the limb bud from the lateral edge of newly formed somites and are soon followed by myogenic cells ( Solursh et al. 1987). In contrast, only endothelial cells (but not myogenic precursors) enter the limb bud of embryos mutant for c-Met or Pax3. The embryos do not survive until the stage when satellite cells appear in vivo. However, explant cultures from limb buds of these embryos yielded a significant number of satellite cell-like clones (∼50% of wt), supporting the idea that they are the progeny of endothelial cells, rather than myoblasts. Interestingly, all the cells of these clones express MyoD, do not grow to more than four to eight cells, and, for the most part, eventually die, but the few surviving undergo terminal muscle differentiation. Met signaling in response to SF/HGF thus appears to be required for the clonal growth of these newly identified myogenic cells.
Myogenic Progenitors from the Dorsal Aorta Contribute to Postnatal Muscle Growth and Regeneration
Expression of myogenic markers in vitro suggests a potential for in vivo myogenic differentiation. Our results indicate that these myogenic cells do participate in both postnatal muscle growth and regeneration. When directly injected into the regenerating TA of an immune-deficient mouse, genetically labeled nuclei of cells from the dorsal aorta are incorporated into newly formed muscle fibers, much as bona fide satellite cells. Indeed, skin fibroblasts also are incorporated into regenerating fibers when similarly injected, although at an extremely low frequency ( Gibson et al. 1995). Thus, while regenerating muscle must be a rich source of signals recruiting competent cells to myogenesis, it is also possible that within a population of skin fibroblasts, there may be a small fraction of still multipotent progenitors ( Bianco and Cossu 1999) and it is only this fraction that is capable of differentiating in vivo.
When a fetal limb is transplanted under the skin of a transgenic MLC3F-nlacZ newborn mouse, it is vascularized by the host. These limbs contain β-gal+ nuclei, usually clustered in the area of the vessel in-growth, suggesting that they were associated with it, rather than deriving from neighboring host muscle. Finally, when an embryonic dorsal aorta is transplanted into the growing TA of a newborn immune-deficient mouse, it gives rise to many β-gal+ nuclei clustered in the area of the transplant. This does not happen if the aorta is grown in vitro, indicating that signals from the surrounding developing skeletal muscle recruit some of the aorta cells (endothelium and/or pericytes) to myogenesis. Remarkably, several dispersed β-gal+ nuclei are also present in the contralateral untreated TA, indicating that these myogenic cells must have reached this site through the circulation, much as happens for the adult progenitors in bone marrow ( Ferrari et al. 1998). Possible contamination from adjacent somitic tissue is ruled out because when somites are similarly transplanted, they give rise to differentiated muscle cells only in the area of transplant.
Transdifferentiation or Multipotentiality?
Our data may simply represent one example of transdifferentiation leading to the formation of skeletal muscle cells, as reported for the esophagus ( Patapoutian et al. 1995), the neural tube ( Tajbakhsh et al. 1994), the kidney ( Mayer and Leinwand 1997), or mesenchymal cells from bone marrow ( Grigoriadis et al. 1988) and dermis ( Salvatori et al. 1995). We propose a different explanation: that, at least in this case and in the case of mesenchymal cells, multipotent progenitors may be present in the endothelium (or closely associated cells). When invading developing muscle anlagen, these progenitors will be subject to a muscle field, and thus will adopt a satellite cell fate. When the vessels develop inside a different tissue, these cells may adopt the specific fate of that tissue, and contribute to its histogenesis. The only tissue in which these progenitors remain demonstrable may be the bone marrow, and this would explain our recent observation that cells from the bone marrow can contribute new myogenic cells to regenerating skeletal muscle ( Ferrari et al. 1998). Multipotent mesenchymal cells, capable of producing osteoblasts, chondroblasts, adipocytes, and even skeletal muscle, have long been known to be present in the bone marrow ( Caplan 1991; Prockop 1997). We do not know whether the cells we describe in embryonic vessels represent the progenitors of multipotent mesenchymal cells or a separate lineage with at least part of the same developmental potential. Preliminary observations suggest that clones of dorsal aorta can give rise to osteoblast-like cells in the presence of BMP-2. Indeed, multipotentiality is preserved, even in adult muscle satellite cells, as shown by the fact that BMP2 can switch them to an osteogenic fate ( Katagiri et al. 1994).
In vivo work will clarify whether the contribution of aorta-derived myogenic cells is quantitatively relevant during fetal and/or postnatal growth and regeneration of skeletal muscle.
Acknowledgments
We thank M. Buckingham, E. Dejana, F. Mavilio, P. Rigby, and S. Tajbakhsh for helpful discussion and critical reading of the manuscript. We also thank R. Bussel-Duby, E. Dejana, D. Fischman, R. Kelly, J. Harris, A. Stoppacciaro, A. Starzyski-Powitz, S. Tajbakhsh, and W. Wright for the gift of antibodies, plasmids, and mice. We are grateful to Bob Milne for his final revision of the manuscript and to F. Maina for providing 7A and B.
This work was supported by grants from Telethon, European Community, Fondazione Cenci Bolognetti, Agenzia Spaziale Italiana, and Ministero dell'Università e della Ricerca Scientifica e Technologica.
Footnotes
1.used in this paper: β-gal+, β-galactosidase positive; MetD, Met receptor unable to transduce the HGF signal; RT, reverse transcriptase; TA, Tibialis anterior; VE, vascular–endothelial; wt, wild-type
Dr. Berghella's present address is Department of Biology, California Institute of Technology, Pasadena, CA 91125.
References
- Armand O., Boutineau A.M., Mauger A., Pautou M.P., Kieny M. Origin of satellite cells in avian skeletal muscles. Arch. Anat. Microsc. Morphol. Exp. 1983;72:163–181 . [PubMed] [Google Scholar]
- Asahara T., Murohara T., Sullivan A., Silver M., van der Zee R., Li T., Witzenbichler B., Schatteman G., Isner J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967 . doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Bader D., Masaki T., Fischman D.A. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J. Cell Biol. 1982;95:763–770 . doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P., Cossu G. Uno, nessuno e centomilasearching for the identity of mesodermal progenitors. Exp. Cell Res. 1999;251:257–263 . doi: 10.1006/excr.1999.4592. [DOI] [PubMed] [Google Scholar]
- Bischoff R. The satellite cell and muscle regeneration. In: Engel A.G., Franzini-Armstrong C., editors. Myology. 2nd ed. McGraw-Hill; NY : 1994. pp. 97–133. [Google Scholar]
- Bladt F., Rethmacher D., Isenmann S., Aguzzi A., Birchmaier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–771 . doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- Bober E., Franz T., Arnold H.H., Gruss P., Tremblay P. Pax-3 is required for the development of limb musclesa possible role for the migration of dermomyotomal muscle progenitor cells. Development. 1994;120:603–612 . doi: 10.1242/dev.120.3.603. [DOI] [PubMed] [Google Scholar]
- Caplan A. Mesenchymal stem cells. J. Orthopaed. Res. 1991;169:641–650 . doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- Christ B., Ordhal C.P. Early stages of chick somite development. Anat. Embryol. 1995;191:381–396 . doi: 10.1007/BF00304424. [DOI] [PubMed] [Google Scholar]
- Cooper R.N., Tajbakhsh S., Mouly V., Cossu G., Buckingham M., Butler-Browne G.L. Two distinct pathways of satellite cell activation in regenerating mouse skeletal muscle. J. Cell Sci. 1999;112:2895–2901 . doi: 10.1242/jcs.112.17.2895. [DOI] [PubMed] [Google Scholar]
- Cornelison D.D.W., Wold B.J. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev. Biol. 1997;191:270–283 . doi: 10.1006/dbio.1997.8721. [DOI] [PubMed] [Google Scholar]
- Cossu G. Unorthodox myogenesispossible developmental significance and implications for tissue histogenesis and regeneration. Histol. Histopathol. 1997;12:755–760 . [PubMed] [Google Scholar]
- Cossu G., Molinaro M. Cell heterogeneity in the myogenic lineage. Curr. Topics Dev. Biol. 1987;23:185–208 . doi: 10.1016/s0070-2153(08)60625-0. [DOI] [PubMed] [Google Scholar]
- Cossu G., Eusebi F., Grassi F., Wanke E. Acetylcholine receptor channels are present in undifferentiated satellite cells but not in embryonic myoblasts in culture. Dev. Biol. 1987;123:43–50 . doi: 10.1016/0012-1606(87)90425-8. [DOI] [PubMed] [Google Scholar]
- Cossu G., Tajbakhsh S., Buckingham M. How is myogenesis initiated in the embryo? Trends Genet. 12 1996. 218 223a [DOI] [PubMed] [Google Scholar]
- Cossu G., Kelly R., Tajbakhsh S., Di Donna S., Vivarelli E., Buckingham M. Activation of different myogenic pathwaysMyf-5 is induced by the neural tube and MyoD by the dorsal ectoderm in mouse paraxial mesoderm Development. 122 1996. 429 437b [DOI] [PubMed] [Google Scholar]
- Cusella-De Angelis M.G., Molinari S., Ledonne A., Coletta M., Vivarelli E., Bouche M., Molinaro M., Ferrari S., Cossu G. Differential response of embryonic and fetal myoblasts to TGFβa possible regulatory mechanism of skeletal muscle histogenesis. Development. 1994;120:925–933 . doi: 10.1242/dev.120.4.925. [DOI] [PubMed] [Google Scholar]
- Denetclaw W.F., Christ B., Ordhal C.P. Location and growth of epaxial myotome precursor cells. Development. 1997;124:1601–1610 . doi: 10.1242/dev.124.8.1601. [DOI] [PubMed] [Google Scholar]
- Ferrari G., Cusella-De Angelis M.G., Coletta M., Stornaioulo A., Paolucci E., Cossu G., Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530 . doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- Ferrari S., Molinari S., Melchionna R., Cusella-De Angelis M.G., Battini R., Kelly R., Cossu G. Absence of MEF2 binding to the A/T-rich element in the muscle creatine kinase (MCK) enhancer correlates with lack of early expression of the MCK gene in embryonic mammalian muscle. Cell Growth Differ. 1997;8:23–34 . [PubMed] [Google Scholar]
- Flamme I., Risau W. Induction of vasculogenesis and hematopoiesis in vitro. Development. 1992;116:435–439 . doi: 10.1242/dev.116.2.435. [DOI] [PubMed] [Google Scholar]
- Garry D.J., Yang Q., Bassel-Duby R., Williams R.S. Persistent expression of MNF identifies myogenic stem cells in postnatal muscle. Dev. Biol. 1997;188:280–294 . doi: 10.1006/dbio.1997.8657. [DOI] [PubMed] [Google Scholar]
- Gibson A.J., Karasinski J., Relvas J., Moss J., Sherratt T.G., Strong P.N., Watt D.J. Dermal fibroblasts convert to a myogenic lineage in mdx mouse muscle. J. Cell Sci. 1995;108:207–214 . doi: 10.1242/jcs.108.1.207. [DOI] [PubMed] [Google Scholar]
- Goulding M., Lumsden A., Paquette A.J. Regulation of Pax-3 expression in the dermomyotome and its role in muscle development. Development. 1994;120:957–971 . doi: 10.1242/dev.120.4.957. [DOI] [PubMed] [Google Scholar]
- Grigoriadis A.E., Heersche J., Aubin J.E. Differentiation of muscle, fat, cartilage, and bone from progenitor cells present in a bone-derived clonal cell populationeffect of dexamethasone. J. Cell Biol. 1988;106:2139–2151 . doi: 10.1083/jcb.106.6.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzopoulos A.K., Folkman J., Vasile E., Eiselen G.K., Rosenberg R.D. Isolation and characterization of endothelial progenitor cells from mouse embryos. Development. 1998;125:1457–1468 . doi: 10.1242/dev.125.8.1457. [DOI] [PubMed] [Google Scholar]
- Irintchev A., Zeschnigk M., Starzinski-Powitz A., Wernig A. Expression pattern of M-cadherin in normal, denervated, and regenerating mouse muscles. Dev. Dyn. 1994;199:326–337 . doi: 10.1002/aja.1001990407. [DOI] [PubMed] [Google Scholar]
- Kahane N., Cinnamon Y., Kalcheim C. The origin and fate of pioneer myotomal cells in the avian embryo. Mech. Dev. 1998;74:59–73 . doi: 10.1016/s0925-4773(98)00066-5. [DOI] [PubMed] [Google Scholar]
- Katagiri T., Yamaguchi A., Komaki M., Abe E., Takahashi N., Ikeda T., Rosen V., Wozney J.M., Fujisawa-Sehara A., Suda T. Bone morphogenetic protein-2 coverts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J. Cell Biol. 1994;127:1755–1766 . doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R., Alonso S., Tajbakhsh S., Cossu G., Buckingham M. Myosin light chain 3F regulatory sequences confer regionalised cardiac and skeletal muscle expression in transgenic mice. J. Cell Biol. 1995;129:383–396 . doi: 10.1083/jcb.129.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koishi K., Zhang M., McLennan I.S., Harris A.J. MyoD protein accumulates in satellite cells and is neurally regulated in regenerating myotubes and skeletal muscle fibers. Dev. Dyn. 1995;202:244–254 . doi: 10.1002/aja.1002020304. [DOI] [PubMed] [Google Scholar]
- Lagrand F., Khazaal I., Peuchmaur M., Fenneteau O., Cave H., Rohrlich P., Vilmer E., Peault B. Long-term malignant hematopoiesis in human acute leukemia bone marrow biopsies implanted in severe combined immunodeficiency mice. Blood. 1997;90:2001–2009 . [PubMed] [Google Scholar]
- Maina F., Casagrande F., Audero E., Simeone A., Comoglio P.M., Klein R., Ponzetto C. Uncoupling of Grb2 from the Met receptor in vivo reveals complex roles in muscle development. Cell. 1996;87:531–542 . doi: 10.1016/s0092-8674(00)81372-0. [DOI] [PubMed] [Google Scholar]
- Mayer D.C., Leinwand L.A. Sarcomeric gene expression and contractility in myofibroblasts. J. Cell Biol. 1997;139:1477–1484 . doi: 10.1083/jcb.139.6.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megeney L.A., Kabkar B., Garrett K., Anderson J., Rudnicki M.A. MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev. 1996;10:1173–1183 . doi: 10.1101/gad.10.10.1173. [DOI] [PubMed] [Google Scholar]
- Pardanaud L., Luton D., Prigent M., Bourcheix L.M., Catala M., Dieterlen-Lievre F. Two distinct endothelial lineages in ontogeny, one of them related to hemopoiesis. Development. 1996;122:1363–1371 . doi: 10.1242/dev.122.5.1363. [DOI] [PubMed] [Google Scholar]
- Patapoutian A., Wold B.J., Wagner R. Evidence for developmentally programmed transdifferentiation in mouse esophageal muscle. Science. 1995;270:1818–1821 . doi: 10.1126/science.270.5243.1818. [DOI] [PubMed] [Google Scholar]
- Prockop D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74 . doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Salvatori G., Lattanzi L., Coletta M., Aguanno S., Vivarelli E., Kelly R., Ferrari G., Harris A.J., Mavilio F., Molinaro M., Cossu G. Myogenic conversion of mammalian fibroblasts induced by differentiating muscle cells. J. Cell Sci. 1995;108:2733–2739 . doi: 10.1242/jcs.108.8.2733. [DOI] [PubMed] [Google Scholar]
- Solursh M., Drake C., Meier S. The migration of myogenic cells from the somites at the wing level in avian embryos. Dev. Biol. 1987;121:389–396 . doi: 10.1016/0012-1606(87)90175-8. [DOI] [PubMed] [Google Scholar]
- Sugi Y., Markwald R.R. Formation and early morphogenesis of endocardial endothelial precursor cells and the role of endoderm. Dev. Biol. 1996;175:66–83 . doi: 10.1006/dbio.1996.0096. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S., Vivarelli E., Cusella-De Angelis M.G., Rocancourt D., Buckingham M., Cossu G. A population of myogenic cells derived from the mouse neural tube. Neuron. 1994;13:813–821 . doi: 10.1016/0896-6273(94)90248-8. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S., Rocancourt D., Buckingham M. Muscle progenitor cells failing to respond to positional cues adopt non-myogenic fates in Myf-5 null mice. Nature. 1996;384:266–270 . doi: 10.1038/384266a0. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S., Rocancourt D., Cossu G., Buckingham M. Redefining the genetic hierarchies controlling skeletal myogenesisPax-3 and Myf-5 act upstream of MyoD. Cell. 1997;89:127–138. doi: 10.1016/s0092-8674(00)80189-0. [DOI] [PubMed] [Google Scholar]