Abstract
The mechanisms of agonist-induced Ca2+ spikes have been investigated using a caged inositol 1,4,5-trisphosphate (IP3) and a low-affinity Ca2+ indicator, BTC, in pancreatic acinar cells. Rapid photolysis of caged IP3 was able to reproduce acetylcholine (ACh)-induced three forms of Ca2+ spikes: local Ca2+ spikes and submicromolar (<1 μM) and micromolar (1–15 μM) global Ca2+ spikes (Ca2+ waves). These observations indicate that subcellular gradients of IP3 sensitivity underlie all forms of ACh-induced Ca2+ spikes, and that the amplitude and extent of Ca2+ spikes are determined by the concentration of IP3. IP3-induced local Ca2+ spikes exhibited similar time courses to those generated by ACh, supporting a role for Ca2+-induced Ca2+ release in local Ca2+ spikes. In contrast, IP3- induced global Ca2+ spikes were consistently faster than those evoked with ACh at all concentrations of IP3 and ACh, suggesting that production of IP3 via phospholipase C was slow and limited the spread of the Ca2+ spikes. Indeed, gradual photolysis of caged IP3 reproduced ACh-induced slow Ca2+ spikes. Thus, local and global Ca2+ spikes involve distinct mechanisms, and the kinetics of global Ca2+ spikes depends on that of IP3 production particularly in those cells such as acinar cells where heterogeneity in IP3 sensitivity plays critical role.
Keywords: Ca2+ waves, caged-IP3, Ca2+ spikes, secretion, inositol trisphosphate
Agonist receptors induce the release of Ca2+ from intracellular stores and thereby generate Ca2+ spikes, waves, or oscillations that play important roles in many cellular functions (Berridge 1993; Petersen et al. 1994; Clapham 1995). It is thought that positive feedback effects of Ca2+ on Ca2+-release channels, including both inositol 1,4,5-trisphosphate (IP3)1 (Iino 1989; Bezprozvanny et al. 1991) and ryanodine receptors (Endo et al. 1970), result in Ca2+-induced Ca2+ release (CICR) and contribute to the generation of such Ca2+ responses. Indeed, local Ca2+ release events induced by IP3, such as puffs (Callamaras et al. 1998) and local Ca2+ spikes (Kasai et al. 1993; Thorn et al. 1993), are likely attributable to CICR mechanisms at IP3 receptors, because they can be induced at constant concentrations of IP3 (Wakui et al. 1989). However, it has remained unclear whether the generation of global Ca2+ spikes is also explained by CICR mechanisms (Bootman et al. 1997).
Pancreatic acinar cells represent an ideal system for investigating the mechanisms of agonist-induced generation of Ca2+ spikes. First, agonist-induced increases in the cytosolic concentration of Ca2+ ([Ca2+]i) in these cells are mostly attributable to the generation of IP3 from phosphatidylinositol 4,5-bisphosphate in a reaction catalyzed by phospholipase C (PLC) (Petersen 1992). Second, Ca2+ release channels are heterogeneously distributed along the polarized intracellular structures (Kasai et al. 1993), resulting in a fixed pattern of Ca2+ spike spread. The spikes are always initiated at the trigger zone, the apical pole of the secretory granule–containing region of the cell (Kasai and Augustine 1990; Nathanson et al. 1992; Toescu et al. 1992). Thus, the functioning of distinct Ca2+-release channels can be directly visualized. And third, agonists induce multiple forms of Ca2+ spikes in a dose-dependent manner; they can be local or global (Kasai et al. 1993; Thorn et al. 1993). Increases in [Ca2+]i remain restricted to a discrete area or expand to entire cells in the local and global Ca2+ spikes, respectively. The global Ca2+ spikes further manifest at submicromolar or micromolar concentrations of Ca2+ (Ito et al. 1997). The existence of multiple forms of Ca2+ spikes in the acinar cells enables us to investigate their mechanisms in the same experimental conditions.
We have now characterized the Ca2+ spikes induced by spatially uniform and rapid increases in [IP3]i, generated by photolysis of caged IP3, and compared them with the Ca2+ spikes induced by a natural stimulus, acetylcholine (ACh). If CICR mechanisms play a dominant role in ACh-induced Ca2+ spikes, then the time course of such spikes should resemble that of those induced by IP3. We found that this was indeed the case for local Ca2+ spikes, but not for global Ca2+ spikes. Ca2+ imaging was performed with a low-affinity Ca2+ indicator, benzothiazole coumarin (BTC), that minimizes the effects of changes in intrinsic Ca2+ buffering in the cells and allowed us to quantify large increases in [Ca2+]i without the problem of dye saturation (Ito et al. 1997; Kasai and Takahashi 1999).
Materials and Methods
Preparation of Acinar Cells
Acinar cells were dissociated from the pancreas of 5–7-wk-old mice by enzymatic treatment as described (Ito et al. 1997). For electrophysiological recording, the cells were dispersed in a small chamber in a solution (Sol A) containing 140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM Hepes-NaOH (pH 7.4), and 10 mM glucose. ACh (Wako) was dissolved in Sol A and applied to cells through a glass pipette. Ca2+ indicators, fluo-3 or BTC (Molecular Probes), were dissolved in a solution (basic internal solution) containing 120 mM cesium glutamate, 5 mM CsCl, 50 mM Hepes-CsOH (pH 7.2), 1 mM ATP, 0.2 mM GTP, and 2 mM MgCl2, and were then loaded into cells at a concentration of 200 μM by the patch clamp method. Caged IP3 [d-myo-inositol 1,4,5-trisphosphate, P4(5)-1-(2-nitrophenyl)-ethyl ester; Calbiochem-Novabiochem] or caged GPIP2 [1-(α-glycerophosphoryl)-d-myo-inositol 4,5-bisphosphate, P4(5)-1-(2-nitrophenyl)-ethyl ester; Calbiochem-Novabiochem] was also added to the basic internal solution. Osmolarities of the external and internal solutions were estimated to be ∼310 mOsM after addition of all chemicals (Semi-Micro Osmometer; Knauer). All experiments were performed under yellow light illumination (FL40S-Y-F; National) at room temperature (22–25°C).
Ca2+ Imaging
Confocal Ca2+ imaging was performed as described (Kasai et al. 1993), with the exception that fluo-3 was used as the Ca2+ indicator. Fluorescence from patch-clamped acinar cells was detected with a confocal laser scanning microscope (MRC-600; Bio-Rad) attached to an inverted microscope (IMT-2; Olympus) with an objective lens (DApo 40× UV/340 oil; Olympus). Fluo-3 was excited with an argon laser at 488 nm, and [Ca2+]i was calculated from the ratio of fluorescence values during stimulation (F) to that obtained before stimulation (F 0) according to the equation
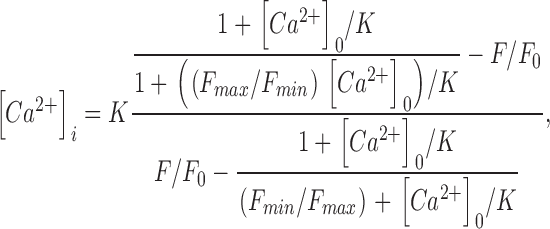 |
1 |
where K and [Ca2+]0 were assumed to be 0.39 and 0.1 μM, respectively. Values of F max/F min were estimated in vivo by assuming that the maximal [Ca2+]i achieved in the presence of ACh (10 μM) was 10 μM (see Fig. 6A and Fig. B). The mean value of F max/F min thus obtained was 6.5 and was used to calibrate local Ca2+ spikes induced with a low concentration of IP3 (see Fig. 1 A).
Figure 6.
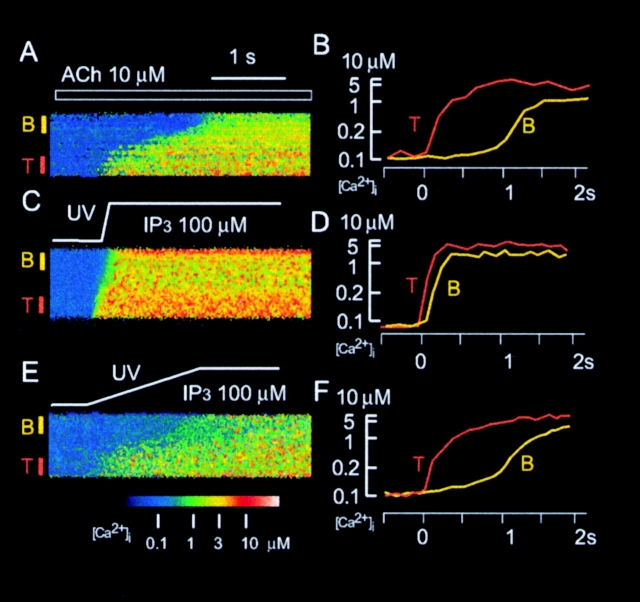
Line-scan analysis of global Ca2+ spikes induced by ACh or caged IP3. (A, C, and E) Increases in [Ca2+]i recorded with the line-scan mode of the confocal microscope and with fluo-3 as the Ca2+ indicator. The trigger zone and basal area of the acinar cells are denoted by T and B, respectively. The cells were stimulated either with 10 μM ACh (A), or by rapid (C) or slow (E) photolysis (indicated by tracings above images) of 100 μM caged IP3. (B, D, and F) Time courses of [Ca2+]i in the trigger zones and basal areas for the cells shown in A, C, and E, respectively.
Figure 1.
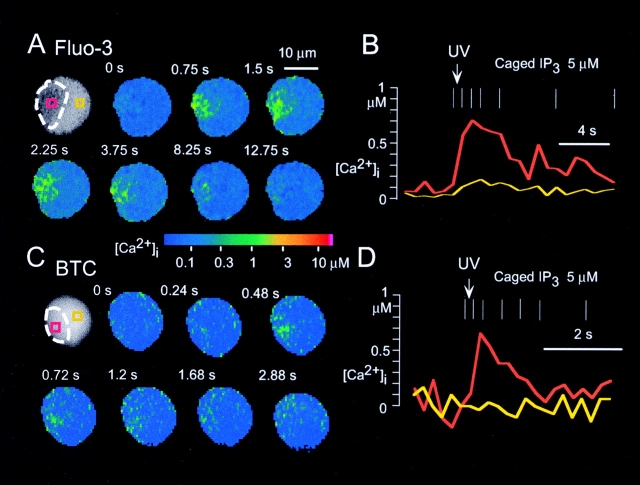
Local Ca2+ spikes induced by photolysis of caged IP3 in pancreatic acinar cells. Local increases in [Ca2+]i were induced by photolysis of 5 μM caged IP3. (A) Ca2+ images obtained with a confocal microscope and the high-affinity Ca2+ indicator fluo-3. (B) Time courses of [Ca2+]i within the rectangles shown in A. (C) Ca2+ images obtained with a cooled CCD camera and the low-affinity Ca2+ indicator BTC. (D) Time courses of [Ca2+]i within the rectangles shown in C. Dashed white lines in the black and white photographs shown in A and C indicate the secretory granule region of the cell. Vertical white bars in B and D indicate the times when the images shown in A and C were obtained; the arrow indicates the time of photolysis of caged IP3 induced by ultraviolet (UV) irradiation.
Ca2+ imaging with a cooled CCD camera was performed as described (Ito et al. 1997). In brief, a recording chamber was placed on an inverted microscope (IX; Olympus) and observed through an objective lens (DApo 40× UV/340 oil). The [Ca2+]i was measured with the Ca2+ indicator BTC. Monochromatic beams with wavelengths of 430 or 480 nm were isolated from light emitted by a xenon lamp with the use of a polychromator (T.I.L.L. Photonics), and were fed into one port of a light guide (IX-RFA caged; Olympus). The light was reflected by a dichroic mirror (DM500) placed beneath the objective lens, and fluorescent light emitted from the cells was captured with a cooled CCD camera system (T.I.L.L. Photonics) fixed at the side port of the microscope. The duration of image acquisition was 0.12 s, and the pairs of images were acquired every 0.24 s. [Ca2+]i was estimated from BTC fluorescence as described (Ito et al. 1997). Calibration constants for BTC were R max = 2.0 and K Bβ = 112. To obtain Ca2+ images from BTC fluorescence, we first estimated the distribution of R min in individual cells by averaging several frames of the resting distribution of R. This procedure was used to compensate for small heterogeneity in R min within a cell, and to reduce noise levels, particularly at [Ca2+]i values of <1 μM. The mean value of R min (m[R min]) was ∼0.55. Distributions of ΔR were then calculated by subtracting the distribution of R min from that of R. From ΔR, [Ca2+]i was estimated as K Bβ·ΔR/(R max – m[R min] –ΔR).
The [Ca2+]i in Ca2+ images was represented by pseudocolor coding, where 0.1, 0.3, 1, 3, and 10 μM were expressed as blue, sky blue, green, yellow, and red, respectively (Fig. 1, Fig. 2, Fig. 3, and Fig. 6).
Figure 2.
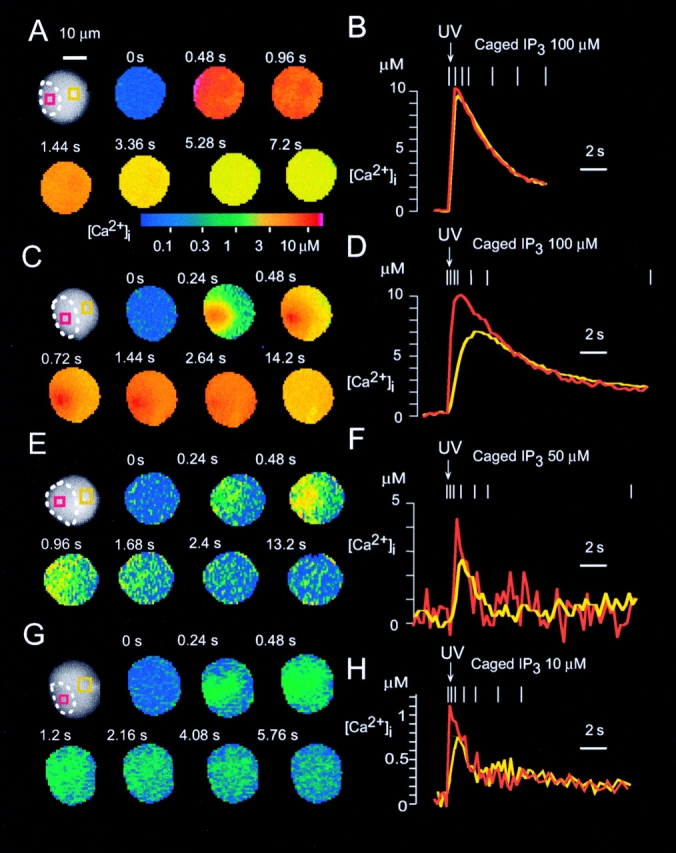
Global Ca2+ spikes or Ca2+ waves induced by photolysis of caged IP3. (A and B) Homogeneous increase in [Ca2+]i induced by photolysis of 100 μM caged IP3. (C–H) Ca2+ waves induced by photolysis of 100 μM (C and D), 50 μM (E and F), or 10 μM (G and H) caged IP3. Ca2+ images were obtained with a cooled CCD camera and BTC. Data are presented as described in the legend to Fig. 1.
Figure 3.
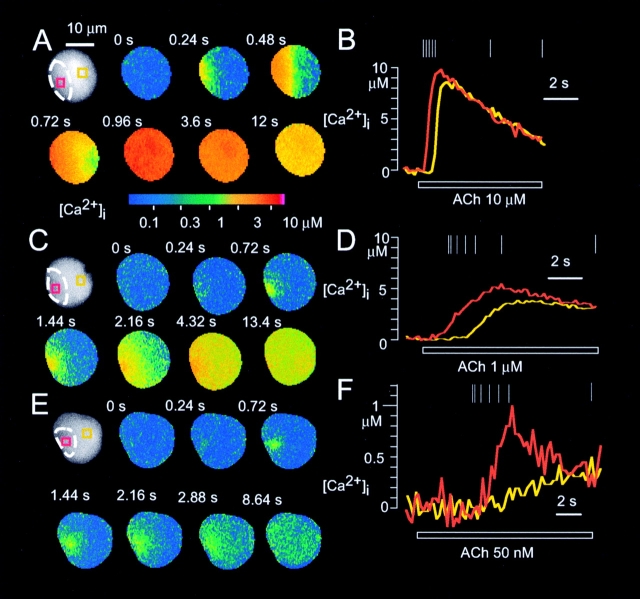
ACh-induced global Ca2+ spikes. Ca2+ waves induced by exposure of acinar cells to ACh at concentrations of 10 μM (A and B), 1 μM (C and D), or 50 nM (E and F) were imaged with a cooled CCD camera and BTC. The horizontal bars in (B), (D), and (F) indicate the duration of exposure to ACh.
Photolysis of Caged IP3
We used a mercury lamp (IX-RFC or IMT-2-RFC; Olympus) as an actinic light source for photolysis of caged IP3. Light from the mercury lamp was filtered through a 360-nm band-pass filter and fed into the second port of the light guide (IX-RFA caged or IMT-2-RFC caged; Olympus). Incorporation of a dichroic mirror (DM400) allowed the light guide to accommodate two light sources, one for photolysis of IP3 and the other for excitation of the Ca2+ indicator. Illumination from the actinic light was gated through an electric shutter (Copal). We estimated that irradiation for 125 ms was necessary and sufficient for full activation of caged IP3. For this calibration experiment, the irradiation was restricted to a recorded cell and not applied to a patch pipette to facilitate recovery of [IP3]i through the pipette, and photolysis was intermittently applied to the same cells. We found that Ca2+ responses depended on the duration of the irradiation, and reached the maximal response at 125 ms. In most experiments, we therefore set the duration of the opening of the shutter at 125 ms to achieve complete photolysis of caged IP3, and the irradiation was applied to whole objective field including the tip of patch pipette to maintain [IP3]i constant as long as possible. In some experiments, a neutral density filter (10, 20, or 50%) was used to reduce the light intensity, in which case the concentration of photolyzed IP3 was obtained by multiplying the concentration of caged IP3 introduced into the cells by the relative light intensity. Only those data obtained from the first photolysis were used to avoid complications of preceding Ca2+ spikes.
Results
IP3-Induced Local Ca2+ Spikes
We first investigated whether homogeneous and constant increases in [IP3]i could produce local Ca2+ spikes in the secretory granule area of pancreatic acinar cells similar to those induced by ACh. Photolysis of caged IP3 was induced 2–5 min after the establishment of whole-cell perfusion, at which time the concentration of IP3 in the cell should be equilibrated with that in the patch pipette. We monitored [Ca2+]i with a confocal microscope and a high-affinity Ca2+ indicator dye, fluo-3. Local increases in [Ca2+]i confined to small spots within the secretory granule area were detected immediately after photolysis of 5 μM caged IP3 (Fig. 1 A). The spatial pattern of the IP3-induced local Ca2+ spikes was similar to those induced by ACh (n = 7, data not shown; Kasai et al. 1993). The result is in accord with previous studies in which IP3 was microinjected into the cells (Kasai et al. 1993; Thorn et al. 1993).
The time course of local Ca2+ spikes induced by photolysis of caged IP3 (Fig. 1A and Fig. B) also was similar to that of ACh-induced local Ca2+ spikes (Kasai et al. 1993). We believe that IP3-induced Ca2+ spikes per se do not cause IP3 production, because, in the absence of receptor stimulation, increases in [Ca2+]i alone could not give rise to the Ca2+ gradients characteristics of IP3-induced Ca2+ spikes (Toescu et al. 1992; Maruyama et al. 1993). Thus, we believe that [IP3]i stays constant during IP3-induced Ca2+ spikes, and that local Ca2+ spikes were mediated by CICR mechanisms as reported (Wakui et al. 1989; Thorn et al. 1996). The increases in [Ca2+]i were always transient in the experiments described in this study. The transient nature of the responses is likely attributable to desensitization of IP3 receptors, given that photolyzed caged IP3 was continuously perfused from the patch pipette and that a metabolically stable analogue of caged IP3, caged GPIP2, also induced transient increases in [Ca2+]i (n = 5, data not shown). Concentrations of caged IP3 of <1 μM did not trigger detectable increases in [Ca2+]i.
The local Ca2+ spikes also could be detected with the use of the low-affinity Ca2+ indicator BTC and a cooled CCD (charge-coupled device) camera (Fig. 1C and Fig. D). A focal and transient increase in [Ca2+]i of ∼0.5 μM was detected in the trigger zone in response to photolysis of caged IP3 (n = 5). The increases in [Ca2+]i were confirmed by the appearance of Ca2+-dependent Cl− currents (data not shown). The detection of local Ca2+ spikes with BTC allowed us to make a direct comparison with their properties with those of global Ca2+ spikes recorded with BTC.
IP3-Induced Global Ca2+ Spikes
We next examined the effects of rapid photolysis of larger concentrations of IP3 (10–100 μM). Ratiometric Ca2+ imaging with BTC was used for reliable estimation of amplitudes and time courses of changes in [Ca2+]i persisting for >20 s. Because of substantial cell-to-cell variability in the responses, these experiments were performed with a large number of cells (n = 41). Photolysis of 100 μM caged IP3 often resulted in large increases in [Ca2+]i throughout the cell that were apparent within 0.24 s (Fig. 2A and Fig. B), the earliest time at which an image was collected by the CCD camera. The Ca2+ indicator (BTC) was not saturated with Ca2+ at these concentrations (Fig. 2 A), and it can therefore be concluded that the increases in [Ca2+]i were relatively homogeneous and exceeded 10 μM throughout the cell. Thus, the capacity for Ca2+ release appeared to be distributed homogeneously throughout the cell. The abundance of IP3 receptors in the basal area was also supported by the previous observation that IP3 injection could directly trigger Ca2+ release in the basal area (Fig. 6 C of Kasai et al. 1993).
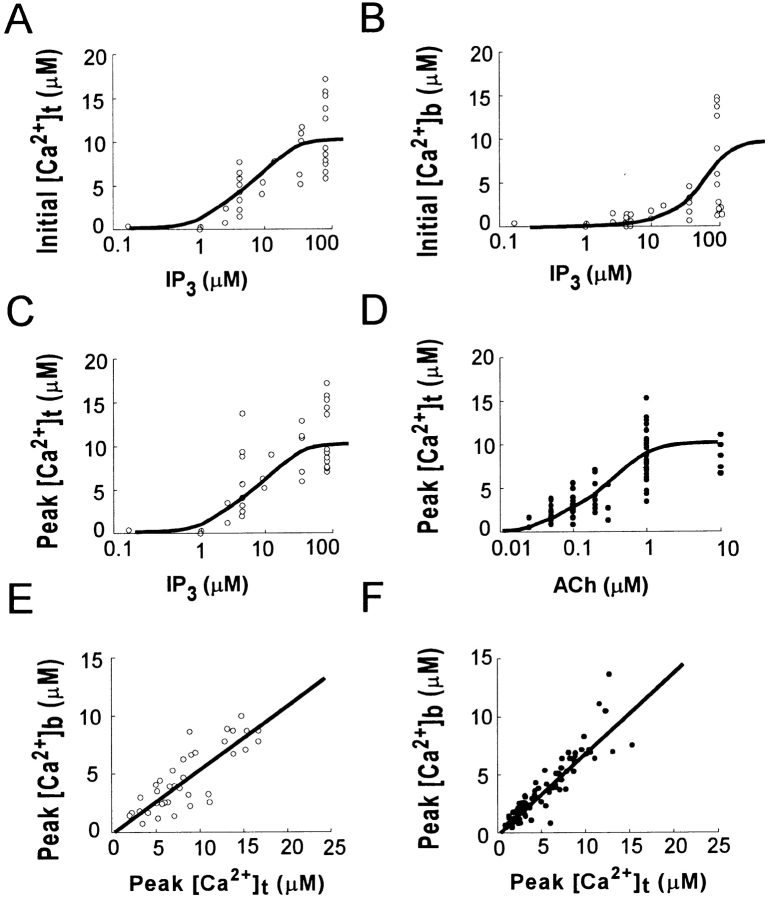
Photolysis of caged IP3 at concentrations between 10 and 100 μM induced Ca2+ spikes that were initiated at the trigger zone (Fig. 2C, Fig. E, and Fig. G) as in the case with ACh-induced Ca2+ spikes. In fact, Ca2+ concentrations immediately (0.24 s) after photolysis of caged IP3 were always larger in the trigger zone than in the basal area (Fig. 2D, Fig. F, and Fig. H). Furthermore, the initial Ca2+ concentrations in the trigger zone (initial [Ca2+]t) and the basal area (initial [Ca2+]b) depended on [IP3]i with median effective concentrations of 5 and 50 μM, respectively (Fig. 4A and Fig. B). These data suggest that IP3 receptors in the basal area were ∼10 times less sensitive to IP3 than those in the trigger zone. Gradual increases in [Ca2+]i were detected throughout the cells after photolysis of caged IP3, suggesting positive feedback effect of Ca2+ on Ca2+ release channels.
Figure 4.
Dose dependence of amplitudes of global Ca2+ spikes induced by caged IP3 or ACh. (A and B) Semilog plots of [Ca2+]i immediately (0.24 s) after photolysis of caged IP3 in the trigger zone ([Ca2+]t) (A) and in the basal area ([Ca2+]b) (B), respectively. The smooth curves were drawn assuming median effective concentrations of 5 and 50 μM, respectively. (C and D) Semilog plots of peak [Ca2+]i in the trigger zone ([Ca2+]t) versus [IP3]i (C) or ACh concentration (D). The smooth curves were drawn assuming median effective concentrations of 10 and 0.5 μM for IP3 and ACh, respectively. (E and F) Correlations between peak [Ca2+]i in the trigger zone ([Ca2+]t) and that in the basal area ([Ca2+]b) for global Ca2+ spikes induced by IP3 or ACh, respectively. The regression coefficients are 0.57 and 0.63 for IP3 and ACh, respectively.
The peak amplitudes of the IP3-induced Ca2+ spikes also depended on [IP3]i (see Fig. 4 C), as those of ACh-induced Ca2+ spikes did on the concentration of ACh (see Fig. 4 D). The amplitudes of Ca2+ spikes ranged from micromolar, with concentrations of >10 μM in the trigger zone (Fig. 2 C and 3 A), to intermediate (∼5 μM; Fig. 2 E and 3 C), to submicromolar (<1 μM) (Fig. 2 G and 3 E). The amplitudes of the smallest global Ca2+ spikes generated by IP3 or ACh were <1 μM in most regions of the cell (Fig. 2 G and 3 E). The peak amplitudes of ACh-induced increases in [Ca2+]i in the trigger zone were always larger than those in the basal area (Fig. 3 and Fig. 4 F). This Ca2+ gradient was not due to the gradient of [IP3]i, because similar Ca2+ gradients were induced by homogeneous increases in [IP3]i induced by caged IP3 (Fig. 2 and Fig. 4 E). Thus, IP3 receptors in the basal area was less sensitive to IP3 than those in trigger zone even at the peak of Ca2+ spikes in the respective areas.
Time Courses of Global Ca2+ Spikes
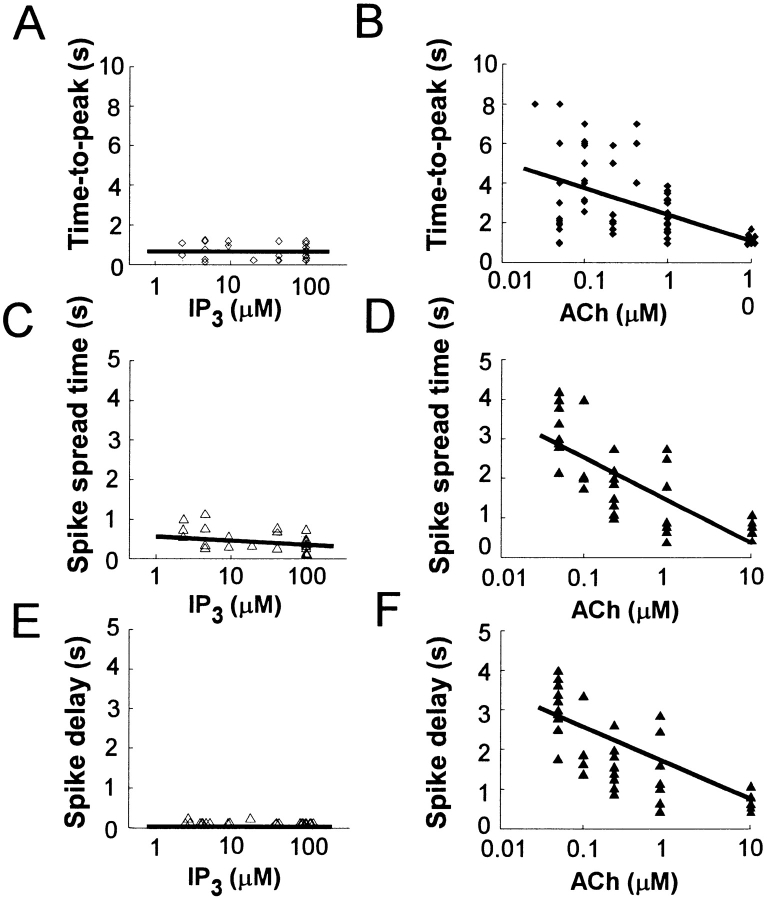
Marked differences were evident in the time courses of the global Ca2+ spikes induced by caged IP3 and of those induced by ACh (Fig. 5). First, the time-to-peak for Ca2+ spikes at the trigger zone induced by caged IP3 was <1 s in most experiments, and was independent of [IP3]i (Fig. 5 A). In contrast, the time-to-peak for ACh-induced global Ca2+ spikes was >1 s in most experiments, and decreased as the concentration of ACh increased (Fig. 5 B). These data indicate that [IP3]i increases gradually during ACh stimulation, and that the rate of this increase is dependent on ACh concentration.
Figure 5.
Dose dependence of time courses of global Ca2+ spikes induced by caged IP3 or ACh. (A and B) Semilog plots of the time-to-peak of Ca2+ spikes in the trigger zone versus concentration of IP3 (A) or ACh (B). (C and D) Semilog plots of spike spread time versus concentration of IP3 (C) or ACh (D). (E and F) Semilog plots of spike delay time versus concentration of IP3 (E) or ACh (F). Correlation coefficients are 0.066 (P > 0.1), 0.45 (P < 0.001), 0.29 (P > 0.1), 0.63 (P < 0.001), 0.003 (P > 0.1), and 0.61 (P < 0.001) for A through F, respectively.
Second, the spread of Ca2+ spikes induced by caged IP3 was faster than that of those induced by ACh. To quantify the rate of spread of Ca2+ spikes (Ca2+ waves), we defined the spike spread time as the difference between the times at which the half-maximal [Ca2+]i was achieved in the trigger zone and in the basal area. The spread time for spikes induced by caged IP3 was <0.7 s in most experiments, and was independent of [IP3]i (P > 0.1; Fig. 5 C). In contrast, the spread time for ACh-induced Ca2+ spikes was >0.7 s in most experiments, and it decreased as the concentration of ACh increased (Fig. 5 D).
Finally, the onset of Ca2+ spikes in the basal area was always delayed relative to that of Ca2+ spikes in the trigger zone for cells stimulated with ACh (Fig. 3), whereas little delay was observed for Ca2+ spikes induced by caged IP3 (Fig. 2). We quantified the delay in the onset of Ca2+ spikes in the basal area by measuring the difference between the times at which [Ca2+]i reached 0.5 μM in the trigger zone and in the basal area. The spike delay ranged between 0 and 0.24 s for IP3-induced Ca2+ spikes (Fig. 5 E) and between 0.48 and 4 s for ACh-induced Ca2+ spikes (Fig. 5 F). Precise measurements of delay and spike spread times were not possible at high IP3 concentrations with our cooled CCD camera operating at an acquisition interval of 0.24 s.
Line-Scan Analysis of Global Ca2+ Spikes
Therefore, we applied the line-scan mode of confocal laser scanning microscopy to analyze, in more detail, the speed of Ca2+ spikes (Ca2+ waves) induced by large concentrations of ACh (10 μM) or IP3 (100 μM). We chose fluo-3 as the Ca2+ indicator for these experiments, because, unlike BTC, it was not excited by the ultraviolet light used for the activation of caged IP3 and therefore permitted visualization of Ca2+ spikes during photolysis. The Ca2+ spikes induced by 10 μM ACh traversed the acinar cells with the spike spread time of 0.9 ± 1 s (mean ± SD, n = 7) and the spike delay of 0.9 ± 0.9 s (Fig. 6A and Fig. B; Kasai et al. 1993), whereas those induced by 100 μM IP3 exhibited the mean spread time of 0.1 ± 0.3 s (n = 4) and the delay of 0.1 ± 0.3 s (Fig. 6C and Fig. D). These results were consistent with those obtained by two-dimensional imaging with BTC (Fig. 5). Thus, spread of ACh-induced Ca2+ spikes were consistently slower than those induced by rapid photolysis of caged IP3 at all concentrations of IP3 and ACh examined.
We postulated that the slow spread of ACh-induced Ca2+ spikes is due to slow generation of IP3 and to sequential activation of Ca2+-release channels with heterogeneous sensitivities for IP3. To test this hypothesis, we reduced the rate of photolysis of caged IP3 by decreasing the intensity of the actinic light source to 10% of its original value, so that the increase in [IP3]i occurred over a period of 1 s. As predicted from our hypothesis, the spike spread time of the resulting Ca2+ spikes was increased to 0.7 ± 0.3 s (n = 5; Fig. 6E and Fig. F). More importantly, the spike delay was also prolonged to 0.8 ± 0.3 s, similar to the spike delay for ACh-induced Ca2+ spikes (Fig. 6A and Fig. B). Thus, an artificial slow increase in [IP3]i was required to reproduce the time course of ACh-induced global Ca2+ spikes.
Discussion
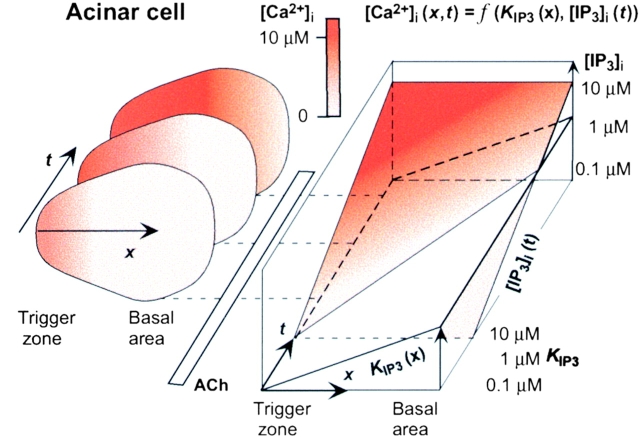
We have demonstrated that spatially homogeneous increases in [IP3]i can induce Ca2+ spikes in acinar cells that share most features of those induced by ACh, consistent with the role of IP3 as the Ca2+-mobilizing messenger for this neurotransmitter. Our data have also confirmed that subcellular gradients of IP3 sensitivities are important for the generation of all forms of Ca2+ spikes in these cells, and that IP3 is a long-range messenger and act as a global signal in those cells with diameters less than 20 μM (Allbritton et al. 1992; Kasai and Petersen 1994). Moreover, we have shown that the temporal profile of [IP3]i affects the kinetics of global Ca2+ spikes.
Control of Global Ca2+ Spikes by IP3 Production
The time courses of global Ca2+ spikes induced by instantaneous increases in [IP3]i were faster than those of ACh-induced Ca2+ spikes at all concentrations of IP3 and ACh examined. This observation indicates that ACh-induced activation of PLC results in a gradual increase in [IP3]i, and that the kinetics of [IP3]i is a key determinant of the time course of global Ca2+ spikes. Thus, we propose a mechanism for the generation of Ca2+ spikes in which the time course of their spread reflects that of [IP3]i, and in which their extent and amplitude are determined by the maximal [IP3]i (Fig. 7). The control of Ca2+ spikes by IP3 production can explain simply the key properties of agonist-induced Ca2+ spikes in exocrine gland cells. First, the spread of Ca2+ spikes is relatively slow (5–15 μm/s; Kasai and Augustine 1990; Jaffe 1991; Toescu et al. 1992). Second, their extent and speed depend on agonist type and concentration (Fig. 5; Nathanson et al. 1992; Kasai et al. 1993; Thorn et al. 1993; Sjoedin et al. 1997; Pfeiffer et al. 1998). And finally, their amplitude varies over a large concentration range (0.5 to >10 μM) depending on the agonist concentration (Fig. 4). Thus, global Ca2+ spikes in acinar cells predominantly reflect global increases in [IP3]i, which are predicted to reach a maximum 1 to 8 s after the application of ACh (Fig. 5 B).
Figure 7.
Control of Ca2+ spikes by IP3 production in pancreatic acinar cells. Increases in [IP3]i can trigger spread of Ca2+ spikes (Ca2+ waves) by sequential activation of IP3 receptors, if there is a spatial gradient of IP3 sensitivity across the cell and if the rate of IP3 production is slower than that of the CICR-mediated Ca2+ spikes. For simplicity, if we assume that the CICR mechanism occurred instantaneously, the distribution of [Ca2+]i is expressed as a function of the IP3 sensitivity at a specific place, K IP3(x), and of [IP3]i at a specific time, [IP3]i (t). [Ca2+]i is determined by a balance between CICR-amplified Ca2+ release and Ca2+ removal mechanisms. When [IP3]i is small, local Ca2+ spikes result; when [IP3]i is large, Ca2+ spikes spread further, and generate global Ca2+ spikes in a dose-dependent manner. More rapid increases in [IP3]i result in a faster spread of Ca2+ spikes. Thus, kinetics of IP3 production play a key role in the amplitude, extent, and time course of Ca2+ spikes. The same mechanism is operative in the generation of Ca2+ spikes in those cells with heterogenous IP3 receptors, even though there is no spatial gradient in IP3 receptors.
Our data also support role of CICR mechanisms of Ca2+ release channels in global Ca2+ spikes, because gradual increases in [Ca2+]i were induced in response to rapid photolysis of caged IP3 (Fig. 2, C–H). However, these increases in [Ca2+]i were too fast (Fig. 5A, Fig. C, and Fig. E) to account for ACh-induced global Ca2+ spikes (Fig. 5B, Fig. D, and Fig. F). Thus, it is conceivable that the CICR mechanism locally generates Ca2+ spikes, and that the increases in [IP3]i control the spread of such Ca2+ spikes. Since gradual increases in [IP3]i determine the kinetics of global Ca2+ spikes, it is likely that the positive feedback effect of Ca2+ on PLC plays a role in the generation of global Ca2+ spikes and oscillation in acinar cells as suggested in other preparations (Meyer and Stryer 1988; Harootunian et al. 1991; Hirose et al. 1999). In contrast, local Ca2+ spikes appear to be mediated solely by CICR mechanisms, because they occurred at constant level of [IP3]i (Fig. 1; Wakui et al. 1989; Thorn et al. 1996).
Given that the production of IP3 by PLC is not instantaneous in any cell type, the resulting time-dependent increase in [IP3]i may be crucial to Ca2+ spikes in general. Moreover, long-range control of Ca2+ spike spread (Fig. 7) can be applied to cells in which gradients of IP3 sensitivity exist (Inagaki et al. 1991; Fay et al. 1995; Lefevre et al. 1995; Robb-Gaspers and Thomas 1995; Missiaen et al. 1996; Simpson et al. 1997; Callamaras et al. 1998; Yamamoto-Hino et al. 1998). Thus, mechanisms of Ca2+ spiking generally involve (a) PLC dependent long-range control (Fig. 7), (b) local CICR mechanisms (Berridge 1993; Petersen et al. 1994; Clapham 1995), and (c) heterogeneity in the Ca2+ release channels.
Types of IP3 Receptors
The control of global Ca2+ spikes by [IP3]i in pancreatic acinar cells is consistent with the previous observation that agonists and IP3 each mobilize Ca2+ in a dose-dependent manner (Muallem et al. 1989; Petersen et al. 1991a,Petersen et al. 1991b). Our data further demonstrate that such dose-dependent control involves heterogeneity in the Ca2+-release processes distributed in various subcellular regions and results in a wide range of [Ca2+]i (0.1 to >10 μM). The graded nature of Ca2+ spikes (Fig. 4C and Fig. D) may reflect the balance between Ca2+ release and clearance in vivo (van de Put et al. 1994).
It has reported that all three types of IP3 receptors were expressed in acinar cells (Lee et al. 1997). The presence of type-1 IP3 receptors may account for the initiation of Ca2+ spikes and oscillations in the trigger zone (Hagar et al. 1998; Miyakawa et al. 1999). The preferential localization of type-3 IP3 receptors in the trigger zone (Nathanson et al. 1994) is possibly responsible for the large increases in [Ca2+]i in this region, given the small inhibitory effect of Ca2+ on these receptors (Hagar et al. 1998). It is therefore suggested that the type-3 IP3 receptor plays a specific role in cellular processes such as exocytosis that require high [Ca2+]i (Ito et al. 1997; Kasai and Takahashi 1999). The Ca2+ release in the trigger zone exhibited a similar sensitivity (Fig. 4 A) to the type-3 IP3 receptors in vivo (Miyakawa et al. 1999). The reasons for 10 times lower IP3 sensitivity in the basal area (Fig. 4 B) remain to be clarified.
Acinar cells may differ from oocytes and smooth muscle cells in that the latter cell types express predominantly one type of IP3 receptor, and Ca2+ spikes in these cells occur in an all-or-nothing manner (Lechleiter and Clapham 1992; Parker and Ivorra 1993; Iino et al. 1993). Thus, the distributions of distinct IP3 receptors appear critical for Ca2+-dependent cellular functions.
Acknowledgments
We thank T. Kishimoto, A. Tachikawa, H. Maeda, and T. Nemoto for collaboration throughout the experiments, M. Iino and K. Hirose for helpful discussions, and M. Ogawa for technical assistance.
This work was supported by the Research for the Future program of the Japan Society for the Promotion of Science (JSPS), grants-in-aid from the Ministry of Education, Science, and Culture of Japan, a research grant from the Human Frontier Science Program, a grant from the Toyota Foundation, and CREST (Core Research for Evolutional Science and Technology) of the Japan Science and Technology Corporation (JST). K. Ito is a research fellow of JSPS, and is now at School of Life Science, Tokyo University of Pharmacy and Life Science, Hachiooji, Tokyo 192-0392.
Footnotes
1.used in this paper: ACh, acetylcholine; BTC, benzothiazole coumarin; [Ca2+]i, cytosolic Ca2+ concentration; CICR, Ca2+-induced Ca2+ release; IP3, inositol 1,4,5-trisphosphate; PLC, phospholipase C
References
- Allbritton N.L., Meyer T., Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992;258:1812–1815. doi: 10.1126/science.1465619. [DOI] [PubMed] [Google Scholar]
- Berridge M.J. Inositol trisphosphate and calcium signaling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Watras J., Ehrlich B.E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Bootman M.D., Berridge M.J., Lipp P. Cooking with calciumthe recipes for composing global signals from elementary events. Cell. 1997;91:367–373. doi: 10.1016/s0092-8674(00)80420-1. [DOI] [PubMed] [Google Scholar]
- Callamaras N., Marchant J.S., Sun X.P., Parker I. Activation and co-ordination of InsP3-mediated elementary Ca2+ events during global Ca2+ signals in Xenopus oocytes. J. Physiol. 1998;509:81–91. doi: 10.1111/j.1469-7793.1998.081bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D.E. Calcium signaling. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- Endo M., Tanaka M., Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 1970;228:34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- Fay F.S., Gilbert S.H., Brundage R.A. Calcium signaling during chemotaxis. Ciba Found. Symp. 1995;188:121–135. doi: 10.1002/9780470514696.ch8. [DOI] [PubMed] [Google Scholar]
- Hagar R.E., Burgstahler A.D., Nathanson M.H., Ehrlich B.E. Type III InsP(3) receptor channel stays open in the presence of increased calcium. Nature. 1998;396:81–84. doi: 10.1038/23954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harootunian A.T., Kao J.P., Paranjape S., Tsien R.Y. Generation of calcium oscillations in fibroblasts by positive feedback between calcium and IP3 . Science. 1991;251:75–78. doi: 10.1126/science.1986413. [DOI] [PubMed] [Google Scholar]
- Hirose K., Kadowaki S., Tanabe M., Takeshima H., Iino M. Spatiotemporal dynamics of inositol 1,4,5-trisphosphate that underlies complex Ca2+ mobilization patterns. Science. 1999;284:1527–1530. doi: 10.1126/science.284.5419.1527. [DOI] [PubMed] [Google Scholar]
- Iino M. Calcium-induced calcium release mechanism in guinea pig taenia caeci. J. Gen. Physiol. 1989;94:363–383. doi: 10.1085/jgp.94.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M., Yamazawa T., Miyashita Y., Endo M., Kasai H. Critical intracellular Ca2+ concentration for all-or-none Ca2+ spiking in single smooth muscle cells. EMBO (Eur. Mol. Biol. Organ.) J. 1993;12:5287–5291. doi: 10.1002/j.1460-2075.1993.tb06224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N., Fukui H., Ito S., Yamatodani A., Wada H. Single type-2 astrocytes show multiple independent sites of Ca2+ signaling in response to histamine. Proc. Natl. Acad. Sci. USA. 1991;88:4215–4219. doi: 10.1073/pnas.88.10.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Miyashita Y., Kasai H. Micromolar and submicromolar Ca2+ spikes regulating distinct cellular functions in pancreatic acinar cells. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:242–251. doi: 10.1093/emboj/16.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe L.F. The path of calcium in cytosolic calcium oscillationsa unifying hypothesis. Proc. Natl. Acad. Sci. USA. 1991;88:9883–9887. doi: 10.1073/pnas.88.21.9883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Augustine G.J. Cytosolic Ca2+ gradients triggering unidirectional fluid secretion from exocrine pancreas. Nature. 1990;348:735–738. doi: 10.1038/348735a0. [DOI] [PubMed] [Google Scholar]
- Kasai H., Petersen O.H. Spatial dynamics of second messengersIP3 and cAMP as long-range and associative messengers. Trends Neurosci. 1994;17:95–101. doi: 10.1016/0166-2236(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Kasai H., Takahashi N. Multiple kinetic components and the Ca2+ requirements of exocytosis. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1999;354:331–335. doi: 10.1098/rstb.1999.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Li Y., Miyashita Y. Subcellular distribution of Ca2+ release channels underlying Ca2+ waves and oscillations in exocrine pancreas. Cell. 1993;74:669–677. doi: 10.1016/0092-8674(93)90514-q. [DOI] [PubMed] [Google Scholar]
- Lechleiter J.D., Clapham D.E. Molecular mechanisms of intracellular calcium excitability in X. laevis oocytes. Cell. 1992;69:283–294. doi: 10.1016/0092-8674(92)90409-6. [DOI] [PubMed] [Google Scholar]
- Lee M.G., Xu X., Zeng W., Diaz J., Wojcikiewicz R.J.H., Kuo T.H., Wuytack F., Racymaekers L., Muallem S. Polarized expression of Ca2+ channels in pancreatic and salivary gland cells. J. Biol. Chem. 1997;272:15765–15770. doi: 10.1074/jbc.272.25.15765. [DOI] [PubMed] [Google Scholar]
- Lefevre B., Pesty A., Testart J. Cytoplasmic and nucleic calcium oscillations in immature mouse oocytesevidence of wave polarization by confocal imaging. Exp. Cell Res. 1995;218:166–173. doi: 10.1006/excr.1995.1144. [DOI] [PubMed] [Google Scholar]
- Maruyama Y., Inooka G., Li Y., Miyashita Y., Kasai H. Agonist-induced localized Ca2+ spikes directly triggering exocytotic secretion in exocrine pancreas. EMBO (Eur. Mol. Biol. Organ.) J. 1993;12:3017–3022. doi: 10.1002/j.1460-2075.1993.tb05970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T., Stryer L. Molecular model for receptor-stimulated calcium spiking. Proc. Natl. Acad. Sci. USA. 1988;85:5051–5055. doi: 10.1073/pnas.85.14.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missiaen L., Lemaire F.X., Parys J.B., De Smedt H., Sienaert I., Casteels R. Initiation sites for Ca2+ signals in endothelial cells. Pflugers Arch. 1996;431:318–324. doi: 10.1007/BF02207268. [DOI] [PubMed] [Google Scholar]
- Miyakawa T., Maeda A., Yamazawa T., Hirose K., Kurosaki T., Iino M. Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:1303–1308. doi: 10.1093/emboj/18.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muallem S., Pandol S.J., Beeker T.G. Hormone-evoked calcium release from intracellular stores is a quantal process. J. Biol. Chem. 1989;264:205–212. [PubMed] [Google Scholar]
- Nathanson M.H., Padfield P.J., O'Sullivan A.J., Burgstahler A.D., Jamieson J.D. Mechanism of Ca2+ wave propagation in pancreatic acinar cells. J. Biol. Chem. 1992;267:18118–18121. [PubMed] [Google Scholar]
- Nathanson M.H., Fallon M.B., Padfield P.J., Maranto A.R. Localization of the type 3 inositol 1,4,5-trisphosphate receptor in the Ca2+ wave trigger zone of pancreatic acinar cells. J. Biol. Chem. 1994;269:4693–4696. [PubMed] [Google Scholar]
- Parker I., Ivorra I. Confocal microfluorimetry of Ca2+ signals evoked in Xenopus oocytes by photoreleased inositol trisphosphate. J. Physiol. 1993;461:133–165. doi: 10.1113/jphysiol.1993.sp019506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O.H. Stimulus-secretion couplingcytoplasmic calcium signals and the control of ion channels in exocrine acinar cells. J. Physiol. 1992;448:1–51. doi: 10.1113/jphysiol.1992.sp019028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen C.C., Toescu E.C., Petersen O.H. Different patterns of receptor-activated cytoplasmic Ca2+ oscillations in single pancreatic acinar cellsdependence on receptor type, agonist concentration and intracellular Ca2+ buffering EMBO (Eur. Mol. Biol. Organ.) J. 10 1991. 527 533a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen C.C., Toescu E.C., Potter B.V., Petersen O.H. Inositol triphosphate produces different patterns of cytoplasmic Ca2+ spiking depending on its concentration FEBS Lett. 293 1991. 179 182b [DOI] [PubMed] [Google Scholar]
- Petersen O.H., Petersen C.C.H., Kasai H. Calcium and hormone action. Annu. Rev. Physiol. 1994;56:297–319. doi: 10.1146/annurev.ph.56.030194.001501. [DOI] [PubMed] [Google Scholar]
- Pfeiffer F., Sternfeld L., Schmid A., Schulz I. Control of Ca2+ wave propagation in mouse pancreatic acinar cells. Am. J. Physiol. 1998;274:C663–C672. doi: 10.1152/ajpcell.1998.274.3.c663. [DOI] [PubMed] [Google Scholar]
- Robb-Gaspers L.D., Thomas A.P. Coordination of Ca2+ signaling by intercellular propagation of Ca2+ waves in the intact liver. J. Biol. Chem. 1995;270:8102–8107. doi: 10.1074/jbc.270.14.8102. [DOI] [PubMed] [Google Scholar]
- Simpson P.B., Mehotra S., Lange G.D., Russell J.T. High density distribution of endoplasmic reticulum proteins and mitochondria at specialized Ca2+ release sites in oligodendrocyte processes. J. Biol. Chem. 1997;272:22654–22661. doi: 10.1074/jbc.272.36.22654. [DOI] [PubMed] [Google Scholar]
- Sjoedin L., Ito K., Miyashita Y., Kasai H. Cytoplasmic Ca2+ gradients evoked by acetylcholine and peptides in pancreatic acinar cells of the guinea pig. Pflugers Archiv. 1997;433:397–402. doi: 10.1007/s004240050294. [DOI] [PubMed] [Google Scholar]
- Thorn P., Lawrie A.M., Smith P.M., Gallacher D.V., Petersen O.H. Local and global Ca2+ oscillations in exocrine cells evoked by agonists and inositol trisphosphate. Cell. 1993;74:661–668. doi: 10.1016/0092-8674(93)90513-p. [DOI] [PubMed] [Google Scholar]
- Thorn P., Moreton R., Berridge M. Multiple, coordinate Ca2+-release events underlie the inositol trisphosphate-induced local Ca2+ spikes in mouse pancreatic acinar cells. EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:999–1003. [PMC free article] [PubMed] [Google Scholar]
- Toescu E.C., Lawrie A.M., Petersen O.H., Gallacher D.V. Spatial and temporal distribution of agonist-evoked cytoplasmic Ca2+ signals in exocrine cells analysed by digital image microscopy. EMBO (Eur. Mol. Biol. Organ.) J. 1992;11:1623–1629. doi: 10.1002/j.1460-2075.1992.tb05208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Put F.H., De Pont J.J., Willems P.H. Heterogeneity between intracellular Ca2+ stores as the underlying principle of quantal Ca2+ release by inositol 1,4,5-trisphosphate in permeabilized pancreatic acinar cells. J. Biol. Chem. 1994;269:12438–12443. [PubMed] [Google Scholar]
- Wakui M., Potter B.V., Petersen O.H. Pulsatile intracellular calcium release does not depend on fluctuations in inositol trisphosphate concentration. Nature. 1989;339:317–320. doi: 10.1038/339317a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto-Hino M., Miyawaki A., Segawa A., Adachi E., Yamashina S., Fujimoto T., Sugiyama T., Furuichi T., Hasegawa M., Mikoshiba K. Apical vesicles bearing inositol 1,4,5-trisphosphate receptors in the Ca2+ initiation site of ductal epithelium of submandibular gland. J. Cell Biol. 1998;141:135–142. doi: 10.1083/jcb.141.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]