Abstract
Tetraspanins (or proteins from the transmembrane 4 superfamily, TM4SF) form membrane complexes with integrin receptors and are implicated in integrin-mediated cell migration. Here we characterized cellular localization, structural composition, and signaling properties of α3β1–TM4SF adhesion complexes. Double-immunofluorescence staining showed that various TM4SF proteins, including CD9, CD63, CD81, CD82, and CD151 are colocalized within dot-like structures that are particularly abundant at the cell periphery. Differential extraction in conjunction with chemical cross-linking indicated that the cell surface fraction of α3β1–TM4SF protein complexes may not be directly linked to the cytoskeleton. However, in cells treated with cytochalasin B α3β1–TM4SF protein complexes are relocated into intracellular vesicles suggesting that actin cytoskeleton plays an important role in the distribution of tetraspanins into adhesion structures. Talin and MARCKS are partially codistributed with TM4SF proteins, whereas vinculin is not detected within the tetraspanin-containing adhesion structures. Attachment of serum-starved cells to the immobilized anti-TM4SF mAbs induced dephosphorylation of focal adhesion kinase (FAK). On the other hand, clustering of tetraspanins in cells attached to collagen enhanced tyrosine phosphorylation of FAK. Furthermore, ectopic expression of CD9 in fibrosarcoma cells affected adhesion-induced tyrosine phosphorylation of FAK, that correlated with the reorganization of the cortical actin cytoskeleton. These results show that tetraspanins can modulate integrin signaling, and point to a mechanism by which TM4SF proteins regulate cell motility.
Keywords: integrin, tetraspanin, adhesion complexes, signaling, cytoskeleton
Tetraspanins, or tetraspan proteins, are a large family (transmembrane 4 superfamily [TM4SF]1) of ubiquitously expressed membrane proteins that are implicated in a number of basic biological phenomena, including cell proliferation, cell migration, and tumor cell invasion (Hemler et al. 1996; Maecker et al. 1997). Although the biochemical function(s) of TM4SF proteins remains undefined, it had been proposed that the proteins may have an important role in the assembly of signaling complexes that also include other transmembrane proteins, such as CD4 and CD8 on T cells, CD21–CD19 complex on B cells, HB-EGF on epithelial cells (Matsumoto et al. 1993; Higashiyama et al. 1995; Imai et al. 1995).
Notably, TM4SF proteins form membrane complexes with adhesion receptors from the integrin family (Hemler et al. 1996; Maecker et al. 1997). In contrast to cell lineage specific associations involving tetraspanins, integrin–TM4SF protein complexes are more common and have been detected on different cell types (Slupsky et al. 1989; Rubinstein et al. 1994; Berditchevski et al. 1995, Berditchevski et al. 1996; Nakamura et al. 1995; Hadjiargyrou et al. 1996; Jones et al. 1996; Mannion et al. 1996; Radford et al. 1996; Berditchevski et al. 1997a; Tachibana et al. 1997; Claas et al. 1998; Yáñez-Mó et al. 1998). Recent data has clearly demonstrated that the function of integrin–TM4SF protein complexes is particularly relevant to cell migration. For example, ectopic expression of CD9 in various cell lines either reduces (Ikeyama et al. 1993) or enhances (Shaw et al. 1995) chemotactic migration. Similarly, when introduced into melanoma cells, CD63 suppresses cell motility on various ECM substrates (Radford et al. 1997). Other studies have shown that mAbs to CD9, CD53, CD81, CD82, and CD151 inhibit integrin-mediated cell migration (Miyake et al. 1991; Domanico et al. 1997; Lagaudriere-Gesbert et al. 1997; Yáñez-Mó et al. 1998). In spite of the phenomenological evidence, it remains unclear which cellular processes during migration are affected by TM4SF proteins. In attached cells TM4SF proteins are concentrated within punctate adhesion structures (Nakamura et al. 1995; Berditchevski et al. 1997b) that morphologically resemble Rac-dependent peripheral focal complexes (Nobes and Hall 1995) and point contacts (Nermut et al. 1991; Tawil et al. 1993). It has been suggested that focal complexes and point contacts, which contain different structural elements, may have distinct functions in cell migration where the former help to stabilize adhesive interactions at the cell front (Nobes and Hall 1995), whereas the latter are important for a cell surface recycling of adhesion receptors (Tawil et al. 1993). Whether or not tetraspanins are indeed integrated into either types of the adhesion structures remains unknown. Recent results indicate that tetraspanins may either directly affect integrin-mediated cell attachment (Yáñez-Mó et al. 1998) or be involved in post-adhesion signaling (Shaw et al. 1995). On the other hand, the fact that TM4SF proteins were detected on intracellular vesicles (Peters et al. 1991; Hamamoto et al. 1994; Berditchevski et al. 1997b) suggests that they may have a role in integrin trafficking. Thus, obtaining detailed information regarding a structural organization of integrin–TM4SF adhesion complexes may provide an important insight into the function of tetraspanins as modulators of cell motility.
In this study, we have characterized the properties of integrin–TM4SF protein complexes, particularly, in relation to different types of cell–ECM adhesion structures (e.g., focal adhesions, focal contacts, and point contacts). Our data indicate that TM4SF proteins are mostly excluded from the vinculin-containing adhesion complexes (both focal adhesions and focal complexes), but are coclustered with α3β1 integrin within peripheral adhesion structures, some of which contain talin and MARCKS. Furthermore, we have demonstrated that TM4SF proteins may contribute to integrin-mediated signaling.
Materials and Methods
Cell Lines
Human breast carcinoma cell line, MDA-MB-231, was purchased from American Type Culture Collection and maintained in L-15 medium Leibovitz supplemented with 15% fetal calf serum. Human fibrosarcoma cells, HT1080, were grown in DME supplemented with 10% fetal calf serum. HT1080/zeo cells were generated by transfection of pZeoSV (Invitrogen) into HT1080 cells. HT1080-CD9 cells were generated as follows: HindIII-XbaI fragment of CD9 cDNA (Berditchevski et al. 1996) was subcloned into pZeoSV cut with HindIII and SpeI sites, and the resulting plasmid, pZeoSV-CD9, was introduced into HT1080 cells by electroporation. Zeocin-resistant clones in each transfection experiment were pooled together and cells expressing CD9 (HT1080/CD9) were additionally enriched by fluorescent cell sorting using the mAb ALB-6.
Antibodies
The anti-TM4SF mAbs used were C9-BB, anti-CD9 (Tachibana et al. 1997); 6H1, anti-CD63 (Berditchevski et al. 1995); 5C11, anti-CD151 (Berditchevski et al. 1997a). The anti-integrin mAbs used were A2-2E10, anti-α2 (Berditchevski et al. 1995); A3-X8 and A3-IIF5, anti-α3 (Weitzman et al. 1993), A5-PUJ2, anti-α5 (Berditchevski et al. 1995); A6-ELE, anti-α6 (Berditchevski et al. 1995); and TS2/16, anti-β1 (Hemler et al. 1983). Monoclonal anti-CD9 antibody ALB6 was generously provided by Dr. C. Boucheix and Dr. E. Rubinstein (INSERM U268, Villejuif, France). Monoclonal anti-CD63 antibody RUU-SP 2.28 was provided by Dr. K. Nieuwenhuis (University Hospital, Utrecht, Netherlands). Monoclonal anti-CD151 antibodies 14A2.H1 and 11B1.G4 were from Dr. L. Ashman (Institute of Medical and Veterinary Science, Adelaide, Australia). Monoclonal anti-CD81 antibody M38 and anti-CD82 antibody C33 were generous gifts from Dr. O. Yoshie (Shionogi Institute, Osaka, Japan). Monoclonal anti-CD82 antibody 4F9 and polyclonal anti-FAK sera were from Dr. C. Morimoto (Dana-Farber Cancer Institute, Boston). Rabbit polyclonal sera against the cytoplasmic tail of α3 integrin subunit was a generous gift from Dr. F. Watt (ICRF, London, UK). Monoclonal anti-MARCKS antibody, 2F12, was from Dr. P. Blackshear (National Institute of Environmental Health Sciences, Durham, NC). Anti-CD9 mAb, BU16, were purchased from The Binding Site; anti-CD81 mAb, JS64, were purchased from Serotech. Anti-CD44 mAb, clone F10-44-2, was purchased from Novosastra Laboratories Ltd. Anti-vinculin mAb, clone hVIN-1, and anti-talin mAb, clone 8d6, were from Sigma. Other antibodies used were W6/32, anti–MHC class I (Barnstable et al. 1978), and P3, the negative control antibody (Lemke et al. 1978).
Adhesion Assay
A standard static adhesion assay (30–35 min) was carried out as previously described (Weitzman et al. 1993). When the effect of mAbs on adhesion was studied, cells were preincubated with mAbs at 4°C for 30 min and then aliquoted into 96-well plates precoated with ECM substrates (10 μg/ml). Laminin-5–containing ECM was prepared from the confluent culture of A431 cells as previously described (Weitzman et al. 1993).
Immunoblotting
In the experiments studying the solubility of membrane proteins, cells were plated on ECM-coated dishes for 1.5–2 h in serum-free DME. Membrane proteins were solubilized for 10 min at 4°C into 0.2% Triton X-100/PBS (or for 20 min into 1% Tween 20/PBS) supplemented with a cocktail of protease inhibitors, and then insoluble material was precipitated at 12,000 rpm for 10 min at 4°C. Tween and Triton lysates were appropriately supplemented with 4× Laemmli buffer and treated for 5 min at 95°C. Detergent-insoluble proteins were reextracted into Laemmli loading buffer at 95°C for 10 min. Proteins were separated in 10% SDS-PAGE, and, after transferring to nitrocellulose membrane, were probed with appropriate primary Ab. Protein bands were visualized with HRPO-conjugated goat anti–rabbit or goat anti–mouse Ab (both from Sigma) using ECL reagent (Amersham).
In studies of FAK tyrosine phosphorylation, cells were plated on bacteriological dishes precoated with either ECM proteins (collagen, laminin) at 10 μg/ml or mAbs at 5 μg/ml for 1 h. In some experiments collagen (2.5 μg/ml) was coimmobilized on bacteriological dishes together with purified goat anti–mouse IgG Ab (10 μg/ml) overnight at 4°C. The dishes were subsequently blocked with heat-denatured BSA for 2 h at 37°C and then incubated with an appropriate mAb (15 μg/ml) for 3 h at 37°C. Cells were solubilized in 1% Triton X-100 lysis buffer for 1 h at 4°C, and FAK was immunoprecipitated by using polyclonal Ab immobilized on protein A–agarose beads. Immunoprecipitated material was eluted from the beads into Laemmli loading buffer and resolved in 10% SDS-PAGE. After transferring to nitrocellulose membrane, proteins were probed with Ab as described above.
Immunofluorescent Staining
For immunofluorescence analyzes cells were plated on glass coverslips coated with ECM ligands for 1–2 h. When spread, cells were fixed for 7–10 min with 2% paraformaldehyde in PBS, containing 5% sucrose and 2 mM MgCl2, and then treated with 1% Brij 98 in PBS for 2 min. In some experiments, fixed cells were permeabilized with 0.5% CHAPS in PBS for 2 min, or 0.25% Triton X-100 for 1 min. Coverslips were blocked for 1 h with 20% heat-inactivated normal goat serum, HI-NGS, in PBS. Cells were then stained with primary mAbs diluted in 20% HI-NGS in PBS. Staining was subsequently visualized with FITC-conjugated goat anti–mouse serum (Sigma Chemical Co.) before the coverslips were mounted with FluorSave (Calbiochem-Novabiochem), and immunofluorescence was examined using a Zeiss Axioscop. Serial Z-sections (0.2 μΜ) of stained cells captured with Coolview CCD camera (Photonic Sciences) were digitally saved by using Biovision software package (Bio-Rad Laboratories). The images were further processed by using a digital deconvolution module of the OpenLab software package (Improvision). For colocalization experiments, paraformaldehyde-fixed and permeabilized cells were first incubated with a combination of mouse mAbs, and then visualized with a combination of isotype specific goat anti–mouse sera coupled to FITC (anti-IgG2A; anti-IgG2B) and to Rhodamine (anti-IgG1).
Immunoelectronmicroscopy
Cells were plated on 20-mm Termoplax coverslips precoated with laminin-5–containing ECM for 1 h in serum-free media. Paraformaldehyde-treated cells were stained with primary mAb as described above, and subsequently incubated with rabbit anti–mouse IgG conjugated with 10-nm gold particles (Amersham International). After washing, the stained cells were additionally fixed with 0.2% glutaraldehyde for 2 h at room temperature. Samples were dehydrated and embedded in Lowicryl HM20 resin. Ultrathin sections were taken from the embedded sample and collected on nickel grids. The sections were stained in 30% uranyl acetate in 100% methanol for 5 min, washed in water, and dried. The sections were then analyzed using Jeol 1200 EX transmission electron microscope.
Immunoprecipitation
Cells (2–3 × 106) were plated on ECM coated surface for 2 h in serum-free DMEM. Cells were washed 3 times with PBS and lysed in immunoprecipitation buffer (1% Brij 98 in PBS, containing 2 mM MgCl2, 2 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin) for 1 h at 4°C. Insoluble materials were pelleted at 12,000 rpm for 10 min, and the cell lysates were precleared by incubation with agarose beads, conjugated with goat anti–mouse antibodies for 30 min at 4°C (Sigma). Immune complexes were collected onto the agarose beads that were prebound with appropriate mAb, followed by four washes with the immunoprecipitation buffer. In some experiments Brij 98 in the immunoprecipitation buffer was substituted for Brij 96, CHAPS, or Triton X-100 (all used at final concentration 1%). Immune complexes were eluted from beads with Laemmli sample buffer and resolved by 8–12% SDS-PAGE. In cross-linking experiments, spread MDA-MB-231 cells were washed with a cross-linking buffer (2 mM MgCl2, 5 mM KCl, 5 mM glucose, 140 mM NaCl, 20 mM Hepes, pH 7.4), and subsequently treated with 0.4 mM 3′3′-Dithiobis(sulfosuccinimidyl)propionate (Pierce & Warriner) in a cross-linking buffer for 20 min at room temperature. After additional washes with PBS, the cells were scraped into a lysis buffer containing 1% Tween 20, and immunoprecipitation was carried out as described above, except that immune complexes collected on the beads were washed with a lysis buffer supplemented with 0.1% SDS.
Flow Cytometry
Cells were incubated with saturating concentrations of primary mouse mAbs for 45 min at 4°C, washed twice, and then labeled with fluorescein isothiocyanate (FITC)-conjugated goat anti–mouse immunoglobulin. Stained cells were analyzed on a FACScan® (Becton Dickinson).
Results
TM4SF Proteins Are Colocalized within Adhesion Complexes
We have previously found that in human fibrosarcoma cells, HT1080, two TM4SF proteins, CD63 and CD81, are localized at the cell periphery and on intracellular vesicles (Berditchevski et al. 1997b). To examine whether other TM4SF proteins could be detected at the similar cellular locations we carried out immunofluorescence staining experiments using human mammary carcinoma cells, MDA-MB-231. In addition to CD63 and CD81, these cells abundantly express three other TM4SF proteins including CD9, CD82, and CD151/PETA-3, and, therefore, represent a good model system for a comparative study. To obtain detailed information on the distribution of TM4SF proteins, immunofluorescence images of the stained cells were first captured using CCD camera, and then analyzed by using the OpenLab computer software package (see Materials and Methods for details). When spread on laminin-5–containing ECM in serum-free media all TM4SF proteins were clustered within dot-like structures that are abundant at the plane of cell attachment, both at and out of the cell periphery (Fig. 1A, Fig. E, Fig. I, and Fig. M). Notably, peripheral distribution was particularly prominent for CD81 and CD82 (Fig. 1E and Fig. I). In addition, dot-like staining was detected above the plane of the cell attachment, suggesting that the TM4SF proteins may be localized on intracellular vesicles or in small aggregates at the apical surface of the cells (Fig. 1, B–D, F–H, and N–P). Likewise, prominent peripheral staining of tetraspanins was observed when cells were plated on collagen (Fig. 2, A–D) and fibronectin (not shown). To exclude a possibility of artefactual results caused by using a detergent during the staining procedure, we carried out the experiments on nonpermeabilized MDA-MB-231 cells plated on laminin-5–containing ECM (Fig. 2, E–H). As with the detergent-treated cells, strong staining was observed at the cell periphery with an additional fraction of tetraspanins present at the apical surface (compare Fig. 1 and Fig. 2, E–H). In addition, clustering of tetraspanins at the cell periphery was detected when we studied the distribution of tetraspanins in other cell types, including melanoma cells, endothelial cells, and rhabdomyosarcoma cells (not shown), suggesting that localization within punctate adhesion structures is a general property of TM4SF proteins.
Figure 1.
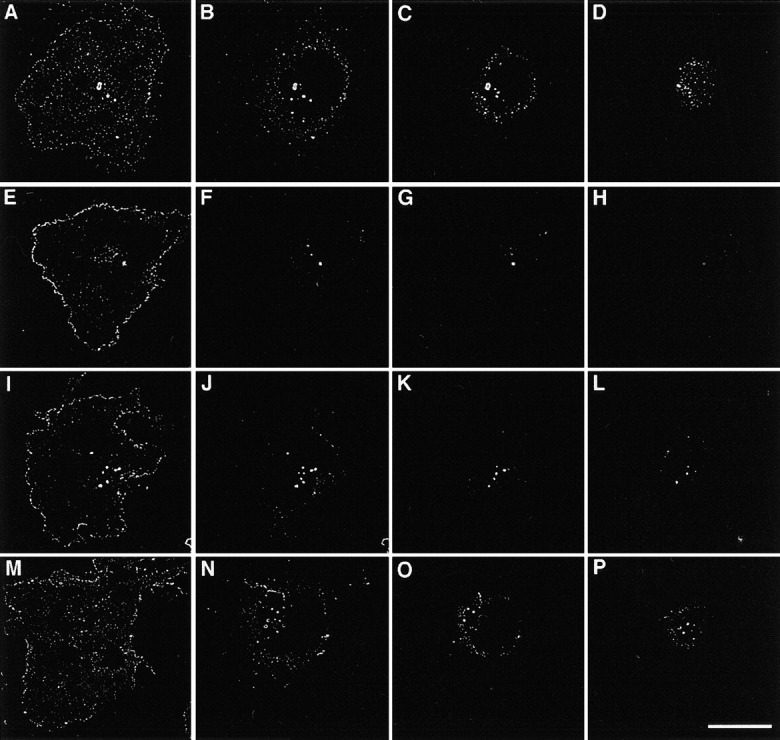
Distribution of TM4SF proteins in cells plated on laminin-5–containing ECM. MDA-MB-231 cells were plated in serum-free DMEM on glass coverslips coated with laminin-5–containing ECM. After 1 h cells were fixed with 2% paraformaldehyde, and permeabilized with 1% Brij 98. Indirect immunofluorescence staining was carried out using mAb C9-BB to CD9 (A–D), M38 to CD81 (E–H), M104 to CD82 (I–L), and 5C11 to CD151 (M–P). Staining was visualized using FITC-conjugated goat anti–mouse IgG. Serial images were captured by CCD camera at various focal planes: A, E, I, M, at the plane of cell–ECM contacts; B, F, J, N, 1 μM above the plane of the attachment; C, G, K, O, 2 μM above the plane of the attachment; D, H, L, P, 3.5 μM above the plane of the attachment. Captured images were processed using the OpenLab software package and the deconvolution module. Bar, 10 μM.
Figure 2.
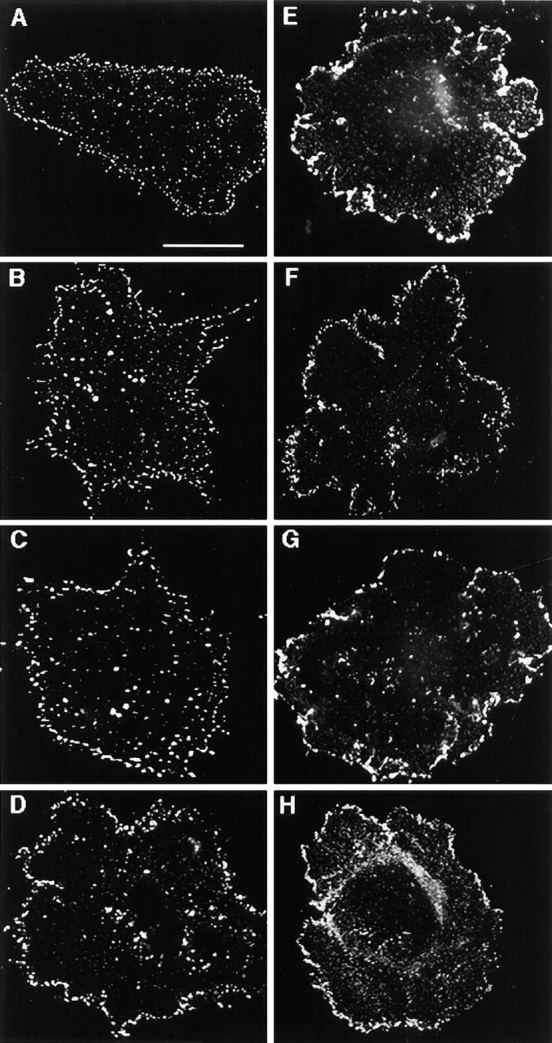
Distribution of tetraspanins in cells plated on collagen. MDA-MB-231 cells were plated in serum-free DMEM on glass coverslips coated with collagen (A–D) or laminin-5–containing ECM (E–H). After 1 h cells were fixed with 2% paraformaldehyde, and either permeabilized with 1% Brij 98 (A–D) or left untreated (E–H). Indirect immunofluorescence staining was carried out using mAb C9-BB to CD9 (A and E), M38 to CD81 (B and F), M104 to CD82 (C and G), and 5C11 to CD151 (D and H). Staining was visualized using FITC-conjugated goat anti–mouse IgG. Images A–D focused on cell–ECM contacts were captured and processed by using the OpenLab software package. Images E–H were photographed using Nikon photocamera attached to the microscope. Bar, 5 μM.
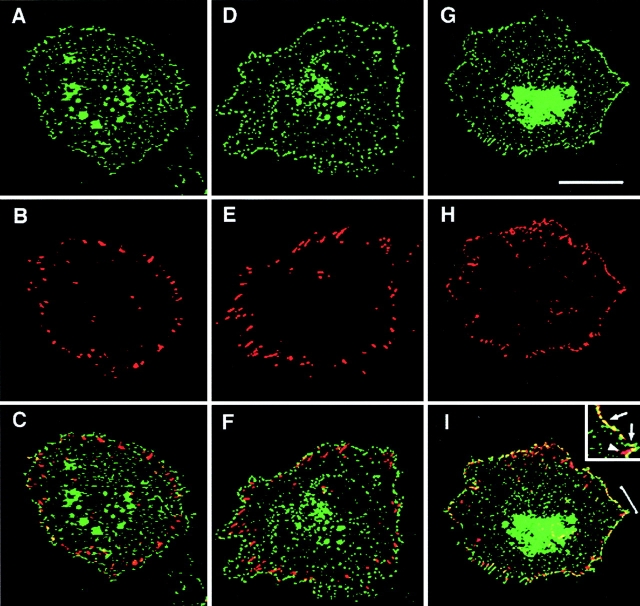
To examine whether different TM4SF proteins are colocalized with each other within adhesion structures we carried out double-labeling immunofluorescent experiments. Fig. 3 shows staining of MDA-MB-231 cells with three combinations of anti-TM4SF mAbs, CD9-CD63, CD81-CD151, and CD82-CD151. In these, and in the experiments where other combinations of anti-TM4SF mAbs were used, we observed notable colocalization of the proteins within dot-like adhesion complexes that was particularly prominent at the cell periphery (Fig. 3C, Fig. F, and Fig. I). In another set of experiments we have demonstrated that α3β1 integrin is colocalized with TM4SF proteins within these adhesion structures (Fig. 3, J–L). Similarly, tetraspanins were codistributed with one another and with α3β1 integrin in cells plated on collagen and fibronectin (data not shown). Abundance of α3β1–TM4SF protein complexes at the cell periphery was further confirmed by immunoelectron microscopy (Fig. 4A and Fig. B). Colocalization of TM4SF proteins with α3β1 integrin agrees with the immunoprecipitation data that have shown that in MDA-MB-231 cells this integrin is associated with four different tetraspanins, including CD9, CD81, CD82, and CD151 (Fig. 5 A). In contrast, mAbs recognizing α2β1 or α5β1 integrins did not coimmunoprecipitated these tetraspanins (Fig. 5 A).
Figure 3.
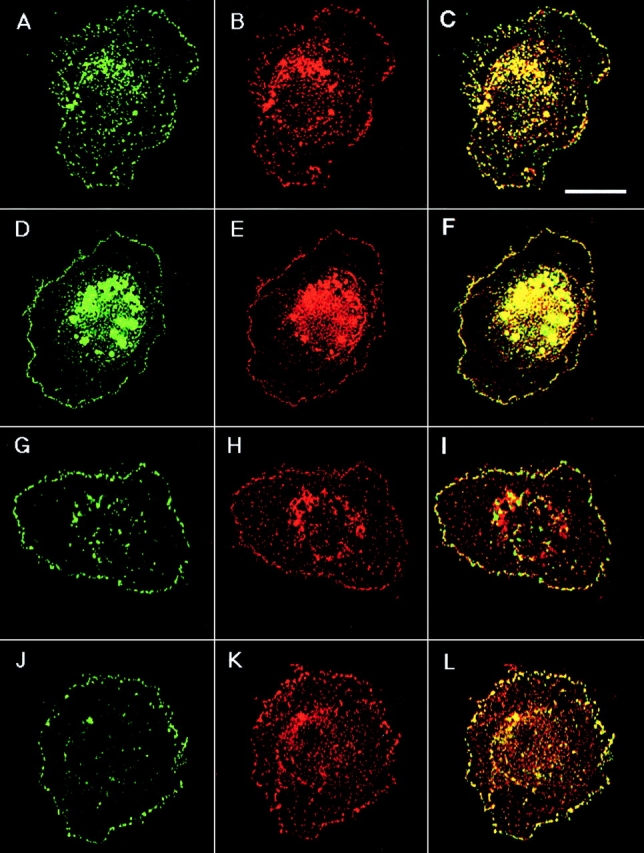
Colocalization of TM4SF proteins and α3β1 integrin within dot-like adhesion complexes. MDA-MB-231 cells were prepared for double-immunofluorescence staining as described in the legend to Fig. 1. Codistribution of TM4SF proteins was studied by using mAbs C33/anti-CD82 and 5C11/anti-CD151 (A and B, respectively), mAbs 6H1/anti-CD63 and BU16/anti-CD9 (D and E, respectively), and mAbs JS64/anti-CD81 and 5C11/anti-CD151 (G and H, respectively). Localization of α3β1 integrin within TM4SF protein–containing adhesion complexes was studied by using mixtures of anti-α3 (A3-X8/A3-IVA5) and anti-TM4SF (JS64/C33) mAbs (J and K, respectively). Staining was visualized using Rhodamine-conjugated goat anti–mouse IgG1 and FITC-conjugated goat anti–mouse IgG2a antibodies. Images focused on cell–ECM contacts were captured, processed and superimposed (C, F, I, and L) by using the OpenLab software package. Bar, 5 μM.
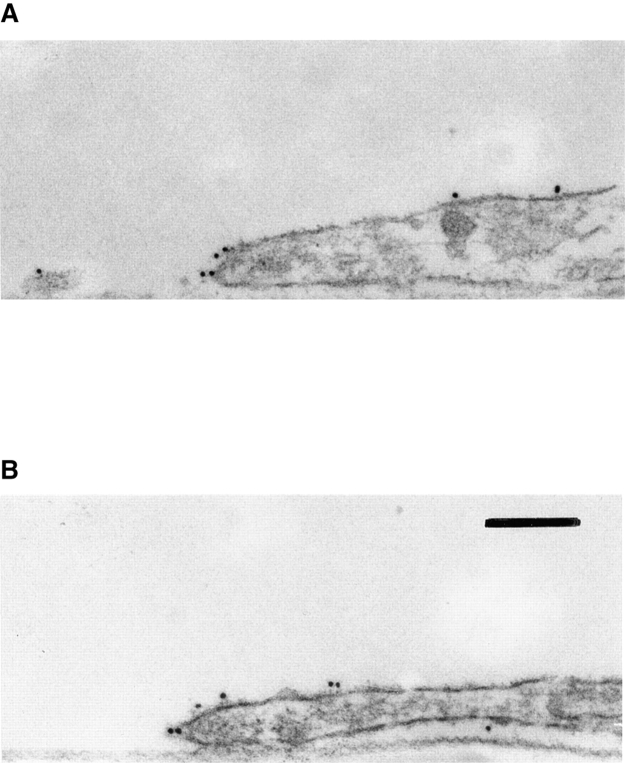
Figure 4.
Localization of α3β1 and CD81 in the peripheral adhesion complexes by immunoelectron microscopy. MDA-MB-231 cells were fixed with 2% paraformaldehyde and stained with mAb P1B5 to α3 integrin subunit (A) and JS64 to CD81 (B). Staining was visualized by using goat anti–mouse IgG Ab coupled to 10-nm gold particles. Bar, 0.2 μM.
Figure 5.
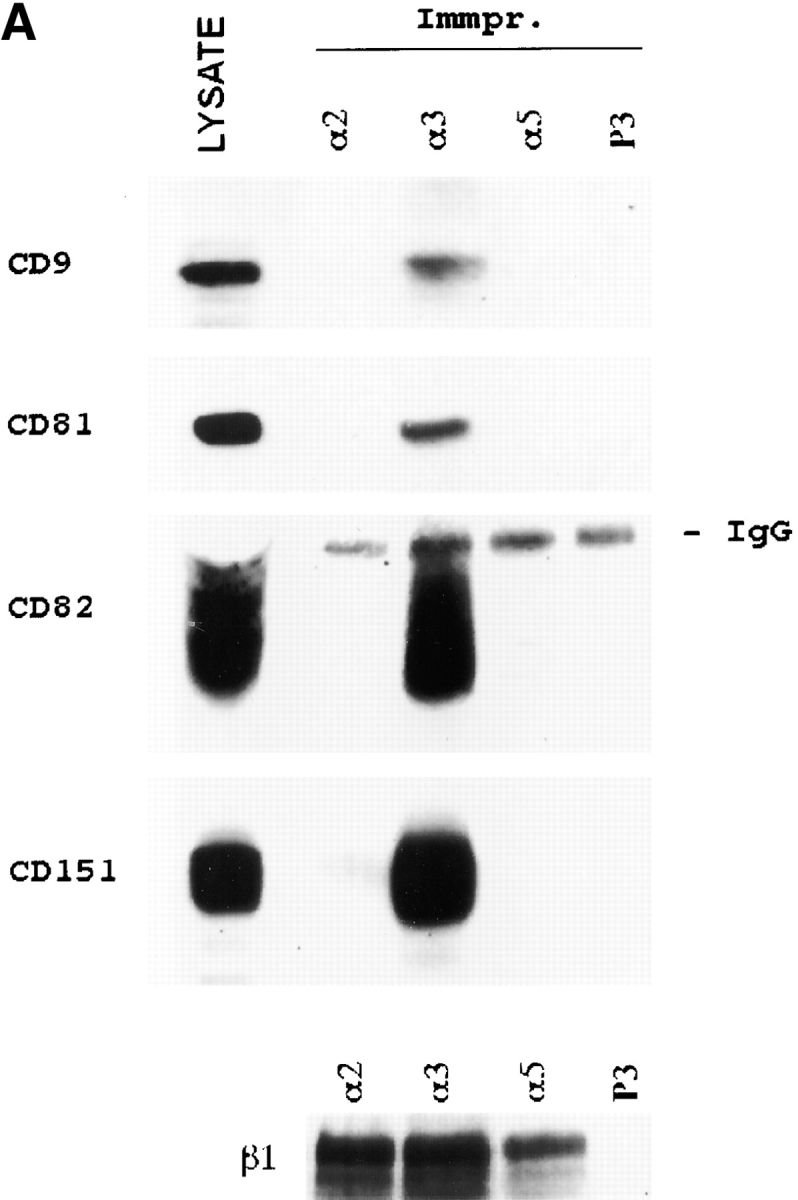
Specific association of tetraspanins with α3β1 integrin in MDA-MB-231 cells, and a role of α3β1/tetraspanin protein complexes in cell adhesion. (A) MDA-MB-231 cells were lysed in the buffer containing 1% Brij 96, and protein complexes were immunoprecipitated from the cellular lysate using specific anti-integrin mAbs (A3-X8 to α3β1, A2-7C6 to α2β1, and A5-PUJ2 to α5β1). Immunoprecipitated proteins were separated in 10% PAGE, and transferred to a nitrocellulose membrane. The membrane was probed with anti-tetraspanin mAbs (C9-BB to CD9, JS64 to CD81, C33 to CD82, and 11G1.B4 to CD151), or polyclonal sera to β1 integrin subunit. The mAb P3 was used as a negative control. (B) BCECF-AM–labeled MDA-MB-231 cells were preincubated with anti-integrin (15 μg/ml) or a mixture of anti-TM4SF mAbs (used at 10 μg/ml each) and tested for adhesion to 96-well microtiter plate coated with collagen type I (10 μg/ml) or laminin-5–containing ECM as described in Materials and Methods. The mAbs used were A3-IVA5 to α3; A2-IIE10 to α2; G0H3 to α6; C9-BB, anti-CD9; 6H1, anti-CD63; M38 and JS64, anti-CD81; M104, anti-CD82, 5C11, anti-CD151. The results are presented as a ratio of relative fluorescence values obtained for cells attached in the presence of mAbs to the value of untreated cells.
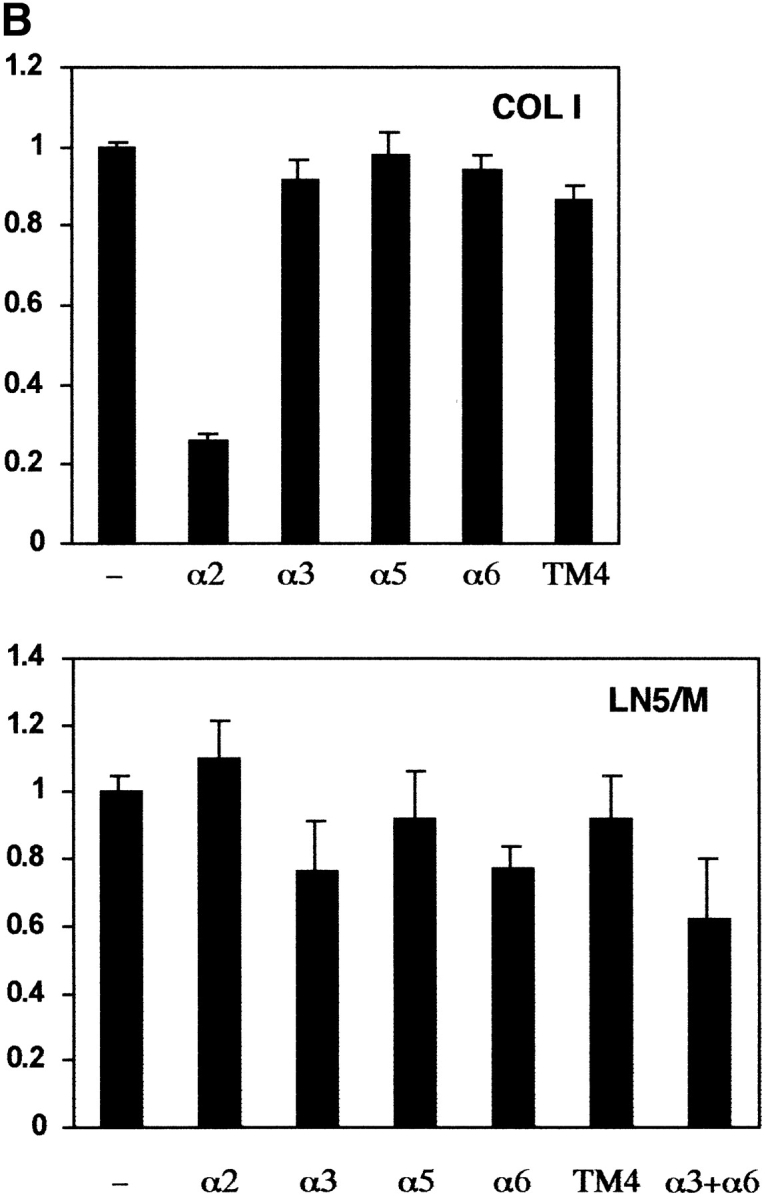
Previous reports suggested that TM4SF proteins may affect integrin-mediated cell adhesion (Yáñez-Mó et al. 1998). Thus, we examined the effect of anti-tetraspanin mAbs on adhesion of MDA-MB-231 cells to various ECM ligands. In these experiments we found that mAbs to TM4SF proteins tested either separately or in combination with each other did not influence adhesion of MDA-MB-231 cells to collagen I, laminin-5–containing matrix (Fig. 5 B) and fibronectin (data not shown). Interestingly, even when combined, blocking mAbs to α3- and α6-integrin subunits had only a partial inhibitory effect on adhesion MDA-MB-231 cells to laminin-5–containing matrix, suggesting that cellular interactions with the substrate involve other integrin receptors (e.g., α1β1 or α2β1 integrins). Collectively, these experiments indicate that α3β1–tetraspanin peripheral adhesion complexes are not directly involved in mediating strong cell–ECM attachment that is tested in static adhesion assays.
TM4SF Proteins Are Absent from Focal Adhesions and Point Contacts
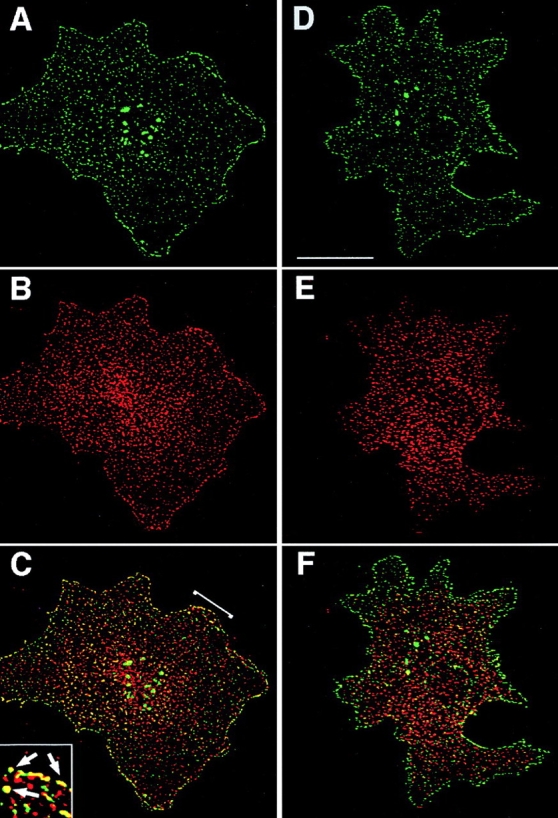
To analyze the composition of TM4SF-containing adhesion complexes further, we studied colocalization of tetraspanins with a number of cytoplasmic proteins, which are typically associated with various adhesion structures. In these experiments cells were labeled with a cocktail of anti-TM4SF mAbs to visualize all various TM4SF-containing protein complexes. Initial experiments were carried out using serum-starved MDA-MB-231 cells. Fig. 6 illustrates that under serum-free conditions vinculin was found in dot-like adhesion structures at the peripheral locations as well as in rear focal adhesions (Fig. 6 B). Notably, only a few of the vinculin-containing punctate adhesion structures and none of the focal adhesions included TM4SF proteins (Fig. 6A and Fig. C). Because of its established role in the assembly of various types of adhesion complexes we investigated whether fetal calf serum can facilitate colocalization of tetraspanins with vinculin. As expected, in the presence of fetal calf serum the number of focal adhesions was increased, but the treatment failed to direct TM4SF proteins into vinculin-containing adhesion structures (Fig. 6, D–F). Similar results were obtained when we examined the codistribution of tetraspanins with paxillin and VASP (data not shown). In contrast, under the serum-free conditions a substantial number of tetraspanin-containing peripheral adhesion structures included talin (Fig. 6, G–I). More strikingly, another actin binding protein, MARCKS, was colocalized with tetraspanins in the peripheral and in some of the centrally located adhesion complexes (Fig. 7, A–C).
Figure 6.
Colocalization of tetraspanins with vinculin and talin. MDA-MB-231 cells were plated in serum-free DMEM (A–C and G–I) or in DMEM supplemented with 10% FCS (D–F) on glass coverslips coated with laminin-5–containing ECM. After 1 h cells were fixed with 2% paraformaldehyde and permeabilized with 1% Brij 98. Codistribution of TM4SF proteins with vinculin (A–C and D–F) was studied in double-immunofluorescence staining experiments by using mAb to vinculin, hVIN-1 (B and E), and a mixture of mAbs to TM4SF proteins, BU16/JS64/C33/RUU.SP.2.28 (A and D). Staining was visualized using Rhodamine-conjugated goat anti–mouse IgG1 and FITC-conjugated goat anti–mouse IgG2a antibodies. Colocalization of TM4SF proteins with talin (G–I) was studied by using mouse mAb to talin, 8d6 (H), and a mixture of mAbs to TM4SF proteins, BU16/JS64/C33/RUU.SP.2.28 (G). Staining was visualized using Rhodamine-conjugated goat anti–mouse IgG1 and FITC-conjugated goat anti–mouse IgG2a and IgG2b antibodies. Images focused on cell–ECM contacts were captured, processed, and superimposed (C, E, I) by using the OpenLab software package. Inset shows digitally enlarged area of the colocalization of TM4SF proteins with talin in punctate adhesion structures (arrows), and absence of tetraspanins from focal adhesions (arrow head). Bar, 5 μM.
Figure 7.
TM4SF proteins colocalize with MARCKS but not adaptin. MDA-MB-231 cells were prepared for double-immunofluorescence staining as described in the legend to Fig. 1. Codistribution of TM4SF proteins with MARCKS (A–C) and adaptin (D–F) was studied by using mAb 2F12/MARCKS (B) or 100/2/adaptin (E) in combination with a mixture of mAbs to TM4SF proteins, BU16/JS64/C33 (A and D). Staining was visualized using Rhodamine-conjugated goat anti–mouse IgG1 and FITC-conjugated goat anti–mouse IgG2a antibodies. Images focused on cell–ECM contacts were captured, processed, and superimposed (C and F) by using the OpenLab software package. Inset shows digitally enlarged area of colocalization of TM4SF proteins with MARCKS. Bar, 5 μM.
Finally, we investigated whether integrin–TM4SF adhesion complexes contain components of clathrin coated structures, that are known to be associated with point contacts (Nermut et al. 1991; Tawil et al. 1993). As shown in Fig. 7 (D–F), adaptin was excluded from most of the peripheral tetraspanin-containing adhesion complexes and centrally located dots. Taken together, these results suggest that (a) tetraspanins are likely to function outside of focal contacts, adhesion structures that mediate strong cell attachment; and (b) TM4SF proteins may be associated with a distinct type of adhesion contacts some of which contain structural elements of focal complexes.
Peripheral Localization of TM4SF Proteins Is Dependent on Intact Actin Cytoskeleton
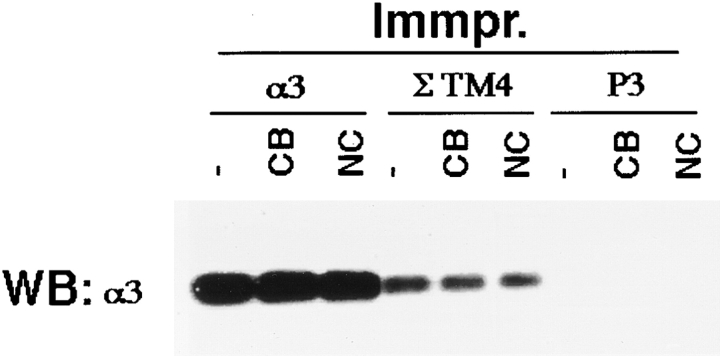
Assembly of various types of adhesion complexes is intimately linked to the reorganization of cytoskeleton (Nobes and Hall 1995; Bershadsky et al. 1996). To determine the role played by the cytoskeleton in the peripheral localization of TM4SF proteins, we analyzed the distribution of α3β1–TM4SF protein complexes in MDA-MB-231 cells treated with cytochalasin B or nocodazole, drugs that induce destabilization of actin filaments and depolymerization of microtubules, respectively. Cytochalasin B induced the collapse of lamellipodia in most cells with few residual protrusions left extending from the rounded cell bodies. Fig. 8 illustrates that in cytochalasin-treated cells both α3β1 integrin and TM4SF proteins were found predominantly on intracellular vesicles (Fig. 8, A–C). In contrast, in cells treated with nocodazole, α3β1–TM4SF protein complexes were not only retained at the cell periphery, but, in some cells, were redistributed into large peripheral clusters (Fig. 8, D–F). Immunoprecipitation experiments and subsequent densitometric measurements showed that the amount of α3β1 integrin coimmunoprecipitated with TM4SF proteins from both nocodazole- and cytochalasin-treated cells was only slightly decreased (by 45 and 20%, respectively) comparing to the control sample (Fig. 9, lanes 4–6). Thus, we have concluded that both actin cytoskeleton and microtubules may affect cellular distribution of α3β1–TM4SF protein complexes.
Figure 8.
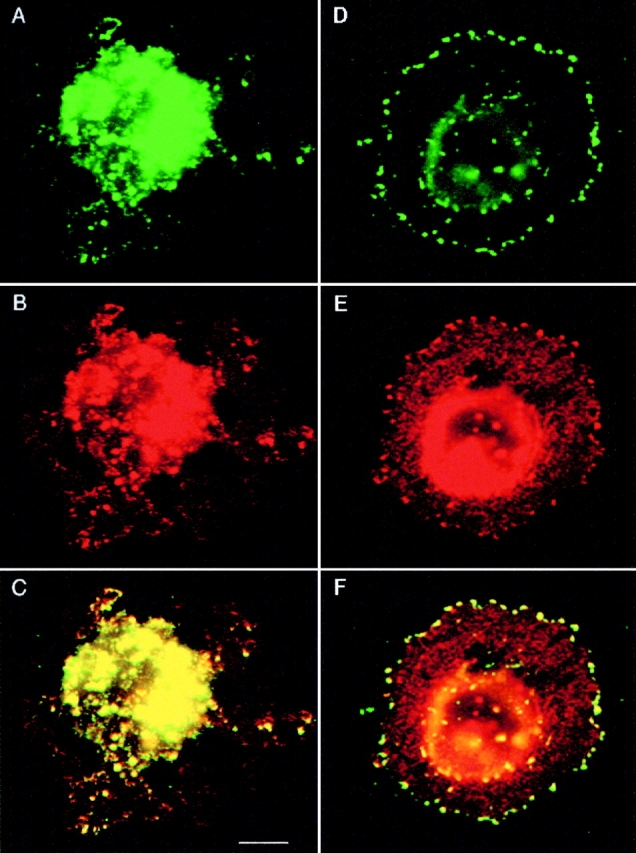
A role of cytoskeleton in cellular distribution of α3β1–TM4SF protein complexes. MDA-MB-231 cells were first plated in serum-free DMEM on glass coverslips coated with laminin-5–containing ECM for 1 h, and then treated with cytochalasin B at 10 μg/ml (A–C) or nocodazole at 20 μg/ml (D–F) for 30 min. Cells were prepared for double-immunofluorescence experiments as described in the legend to Fig. 1. Cellular localization of α3β1–TM4SF protein complexes was studied with mAb to α3 (B and E) or with a mixture of mAbs to TM4SF proteins (A and D). Staining was visualized using Rhodamine-conjugated goat anti–mouse IgG1 and FITC-conjugated goat anti–mouse IgG2a and anti–mouse IgG2b antibodies. The mAbs were: A3-X8 anti-α3; BU16, anti-CD9; RUU.SP.2.28, anti-CD63; JS64, anti-CD81; C33, anti-CD82. Note, α3β1 and TM4SF proteins are colocalized on intracellular vesicles (C), and in the peripheral adhesion clusters (F). Bar, 2.5 μM.
Figure 9.
A role of cytoskeleton in association of TM4SF proteins with α3β1 integrin. MDA-MB-231 cells spread on laminin-5–containing ECM were treated for 30 min with cytochalasin B at 10 μg/ml (CD), and nocodazole at 20 μg/ml (NC), or left untreated (−), and subsequently lysed in buffer containing 1% Brij 98. Protein complexes were immunoprecipitated from cell lysates with mAb to α3 (lanes 1–3) or a mixture of mAbs to TM4SF proteins (lanes 4–6). Proteins were eluted from beads in Laemmli-loading buffer containing 3% β-mercaptoethanol and resolved in 11% PAGE. Presence of α3β1 integrin in the immunoprecipitates was detected by Western immunoblotting with polyclonal Ab to α3 integrin subunit. The mAb A3-X8 was used to immunoprecipitate α3β1 integrin; a mixture of the following mAbs was used to immunoprecipitate TM4SF protein complexes: BU16 and C9-BB, anti-CD9; 6H1, anti-CD63; M38 and JS64, anti-CD81; M104, anti-CD82.
The α3β1–TM4SF Protein Complexes Are Not Directly Linked to Cytoskeleton
During the course of immunofluorescence staining we noticed that in cells that were permeabilized with detergents other than Brij 98 (e.g., Triton X-100 or CHAPS), TM4SF proteins and α3β1 integrin could no longer be detected at the peripheral locations (not shown). These results suggested to us that the linkage between α3β1–tetraspanin protein complexes and the cytoskeleton is weak. To test this hypothesis we studied the solubility of TM4SF proteins in Triton X-100. As shown in Fig. 10 A, TM4SF proteins and α3β1 integrin were detected mostly in Triton-soluble fraction. Importantly, similar results were obtained regardless of whether MDA-MB-231 cells were plated on laminin-5–containing matrix or collagen I, suggesting that direct ligation of α3β1 integrin by its most avid ligand (laminin-5) does not affect solubility of α3β1–tetraspanin complexes. In contrast, ∼30–35% of CD44 was insoluble under the same experimental conditions. As not all α3β1 and TM4SF proteins presented on the cell surface are engaged in interactions with each other (Berditchevski et al. 1996), it is theoretically possible that 5–10% of the proteins that remain insoluble in Triton represent in fact these minor fractions of them that form the complexes. Thus, we searched for a detergent that would allow more even distribution of α3β1 and tetraspanins between soluble and insoluble fractions thus permitting to assess directly association of the complexes with the cytoskeleton. Of various detergent tested for this purpose, including CHAPS, Brij 96, Brij 98, and Tween 20, we found that the latter gives the most reproducible results when 20–50% of the total amounts of tetraspanins and α3β1 integrin could be solubilized from the membranes of MDA-MB-231 cells (Fig. 10 B). To assess the solubility of the cell surface fraction of α3β1–TM4SF protein complexes directly, we pretreated intact MDA-MB-231 cells with a membrane-impermeable chemical cross-linker before solubilization with Tween. Subsequently, we purified the complexes from the Tween-soluble fraction and from the pellet (that was reextracted with Tween containing 0.1% SDS) by immunoprecipitation using a mixture of anti-tetraspanin mAbs, and compared the amounts of α3β1 integrin present in the immunoprecipitates. Importantly, all immunoprecipitation steps were carried out in the presence of 0.1% SDS to dissociate the intracellular complexes, which were inaccessible to the action of the cross-linker. As shown in Fig. 10 C, α3β1 integrin was almost exclusively detected in the Tween-soluble fraction. Together these results provide a strong support for the idea that the linkage of α3β1–TM4SF protein complexes to the cytoskeleton is weak.
Figure 10.
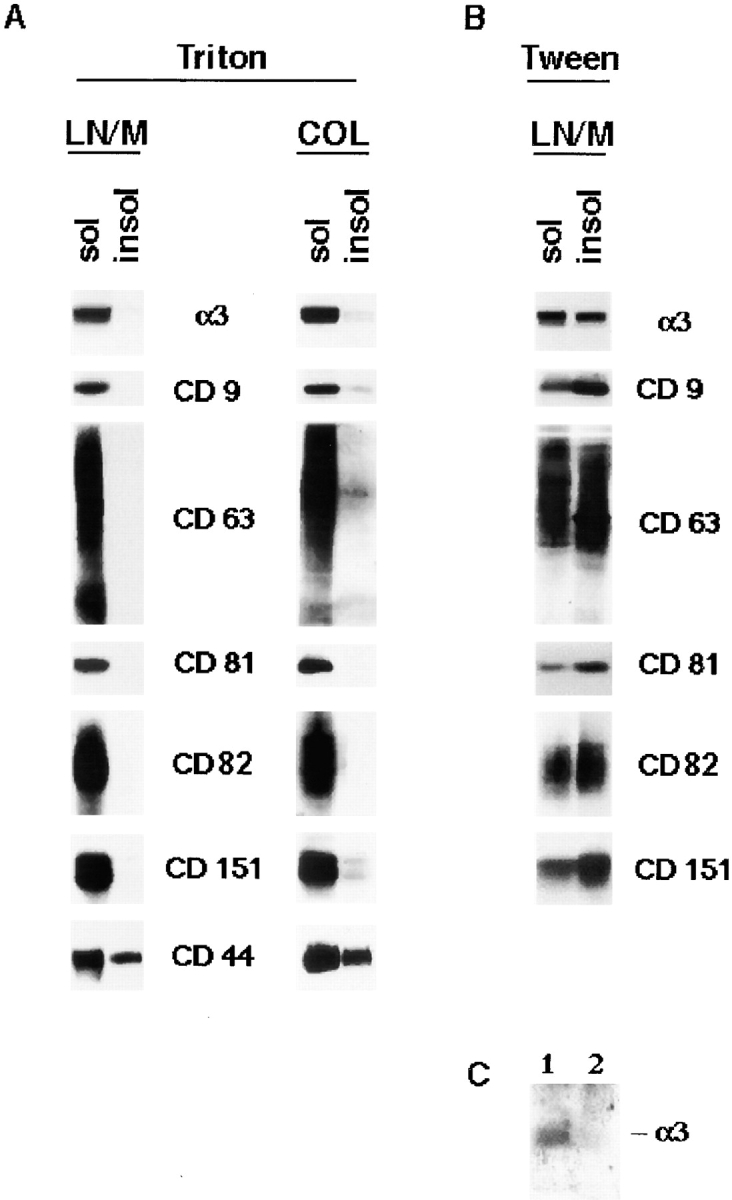
α3β1–TM4SF protein complexes are not directly linked to the cytoskeleton. MDA-MB-231 cells were plated on laminin-5–coated ECM in serum-free DMEM. After 1 h membrane proteins were extracted in buffer containing 0.2% Triton X-100 for 10 min (A) or 1% Tween-20 for 20 min followed by the extraction with 1% Triton X-100 for 30 min (B). Proteins were separated in 10% PAGE and detected using Western immunoblotting with the appropriate Ab. The Abs were as follows: polyclonal sera to the cytoplasmic domain of α3 integrin subunits, mAb C9-BB to CD9, mAb M38 to CD81, mAb C33 to CD82, mAb 11G1.B4 to CD151, mAb F10-44-2 to CD44. (C) MDA-MB-231 cells plated on laminin-5–coated ECM were first pretreated with a membrane-impermeable chemical cross-linker, DTSSP, and then lysed in buffer containing 1% Tween-20. Nonsolubilized material was reextracted with buffer containing both 1% Tween 20 and 1% Triton X-100. Subsequently, Tween- and Tween/Triton-lysates (lanes 1 and 2, respectively) were supplemented with SDS (0.1%), and α3β1–TM4SF protein complexes were immunoprecipitated with a mixture of anti-TM4SF mAbs as described in the legend to Fig. 7. Proteins were eluted from beads in Laemmli loading buffer containing 3% β-mercaptoethanol and resolved in 11% PAGE. The α3β1 integrin in the immunoprecipitates was detected by Western immunoblotting with polyclonal Ab to α3 integrin subunit.
A Role of Integrin–TM4SF Protein Complexes in Signaling
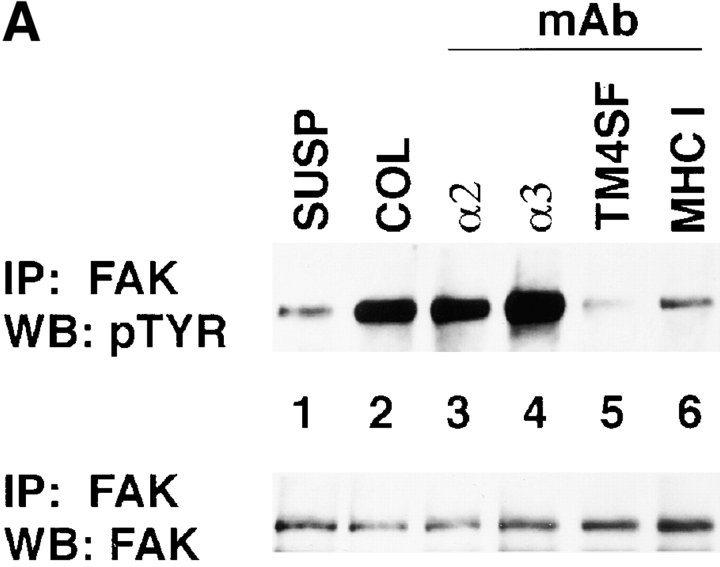
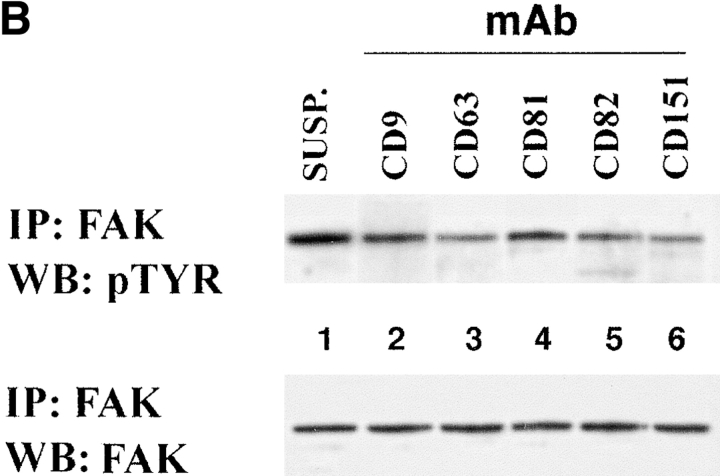
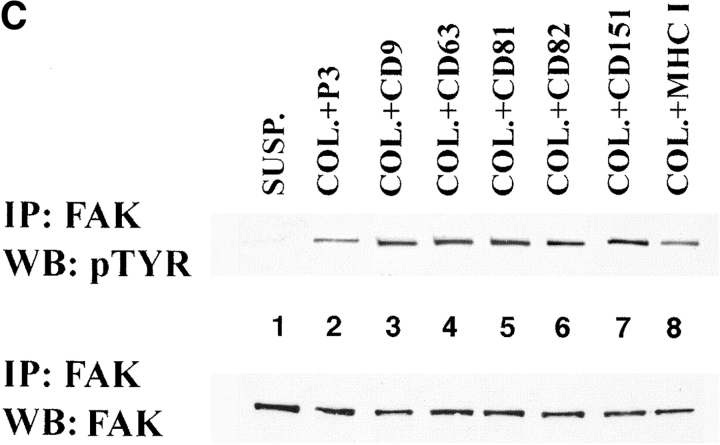
Three approaches were used to study a contribution of TM4SF proteins in integrin-mediated signaling. First, we examined phosphorylation of focal adhesion kinase induced by adhesion of MDA-MB-231 cells to immobilized anti-integrin and anti-TM4SF mAbs. After 1 h of incubation, serum-starved MDA-MB-231 cells appeared well spread on a control ECM substrate (collagen) and anti-integrin mAbs. In contrast, most cells plated on a mixture of anti-TM4SF or anti–MHC class I mAbs were rounded and developed only short projections. As expected, in cells attached to collagen and anti-integrin mAbs the level of tyrosine phosphorylation of FAK was 8–15 times higher of that in cells kept in suspension (Fig. 11 A, top, lanes 1–4). Surprisingly, tyrosine phosphorylation of FAK in cells attached to a mixture of anti-TM4SF mAbs (lane 5) was reduced by 2.5-fold comparing to suspended cells. Importantly, we found that tyrosine phosphorylation of FAK was essentially identical in suspended cells and in cells attached to anti–MHC class I mAb (Fig. 11 A, top, lane 6). In additional experiments we found that the levels of tyrosine phosphorylation of FAK were also lower in the cells plated on the separately immobilized anti-TM4SF mAbs (Fig. 11 B, top) with a stronger effect observed for the anti-CD63, -CD82, and -CD151 Ab. Control experiments confirmed that similar amounts of FAK were immunoprecipitated in each case (Fig. 11a and Fig. b, bottom panels). Second, we investigated the effect of the clustering of tetraspanins with mAbs on tyrosine phosphorylation of FAK in cells plated on ECM ligand. To this end, the MDA-MB-231 cells were plated on a mixed substrate that included collagen and anti-tetraspanin mAbs. To standardize the amounts of the immobilized mAb in each case, the immobilization of the substrates was carried out in two steps. First, the bacteriological dishes were incubated with a solution containing collagen and purified goat anti–mouse IgG Ab (see Material and Methods for details). Second, the anti-TM4SF or anti–MHC class I mAbs were captured on the dishes after an additional incubation at 37°C. As illustrated in Fig. 11 C, all tested anti-TM4SF mAbs (but not a control anti–MHC class I mAb) potentiated collagen-induced tyrosine phosphorylation of FAK.
Figure 11.
The effect of ligation of TM4SF proteins on FAK tyrosine phosphorylation in MDA-MB-231 cells. Serum-starved MDA-MB-231 cells were detached using EDTA and either left in suspension (lane 1 in A, B, and C) or replated for 1 h on bacteriological dishes coated with: (A) collagen (lane 2), anti-α2 (lane 3), anti-α3 (lane 4), anti-TM4SF (lane 5), anti–MHC class I (lane 6) mAbs; (B) anti-CD9 mAb (lane 2), and anti-CD63 mAb (lane 3), anti-CD81 mAb (lane 4), anti-CD82 mAb (lane 5), anti-CD151 mAb (lane 6); (C) collagen (lane 2), collagen and anti-CD9 mAb (lane 3), collagen and anti-CD63 mAb (lane 4), collagen and anti-CD81 mAb (lane 5), collagen and anti-CD82 mAb (lane 6), collagen and anti-CD151 mAb (lane 7), collagen and anti–MHC class I mAb (lane 8). Cells were lysed in buffer containing 1% Triton X-100, and FAK was immunoprecipitated using polyclonal Ab. The immunoprecipitates were divided into two equal aliquots, and tyrosine phosphorylation of FAK was analyzed by Western immunoblotting with mAb 4G10 (top), and with anti-FAK Ab (bottom). The immobilized mAbs were A2-IIE10 anti-α2 and A3-IVA5 anti-α3. The following anti-TM4SF mAbs were used either as a mixture (A) or separately (B and C): BU16 and C9-BB, anti-CD9; 6H1, anti-CD63; M38 and JS64, anti-CD81; M104, anti-CD82, 5C11-anti-CD151.
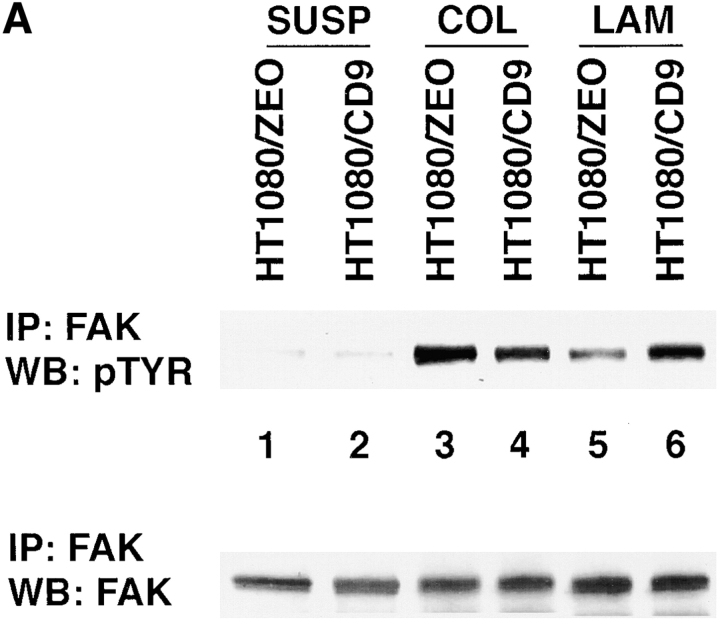
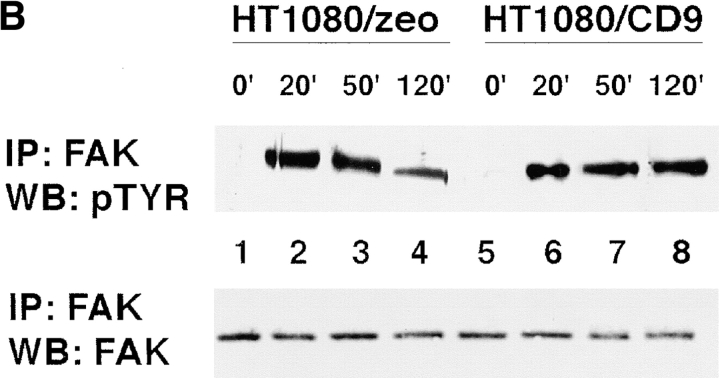
Finally, we investigated how the ectopic expression of TM4SF proteins affects ECM-stimulated tyrosine phosphorylation of FAK. For these experiments we used a newly developed pair of cell lines HT1080/zeo and HT1080/CD9. When analyzed by flow cytometry, expression levels of β1 integrins on HT1080/zeo and HT1080/CD9 cells were comparable (Table ). Furthermore, HT1080/zeo and HT1080/CD9 cells showed no differences in their ability to attach and spread on various ECM substrates, including collagen, fibronectin, and laminins (Fig. 12). Tyrosine phosphorylation of FAK was assessed in cells spread on collagen type I and laminin-1. As seen in Fig. 13 A, adhesion of HT1080/zeo cells to collagen caused the increase of FAK tyrosine phosphorylation by ∼17-fold (lanes 1 and 3), whereas in HT1080/CD9 cells the increase was somewhat less dramatic (∼8-fold, lanes 2 and 4). On the contrary, when attached to laminin-1 induction of tyrosine phosphorylation of FAK was stronger for HT080/CD9 cells (∼5.5- versus ∼3.5-fold for HT1080/zeo cells; Fig. 13 A, lanes 5 and 6). Time-course experiments with the cells plated on laminin have demonstrated that initial increase in tyrosine phosphorylation of FAK (20 min) was comparable in both cell lines (Fig. 13 B, lanes 2 and 6). However, by 2 h the differences became apparent: in HT1080/zeo cells the level of FAK phosphorylation has gradually decreased whereas no significant changes were observed in HT1080/CD9 cells (Fig. 13 B, lanes 4 and 8). Taken together, these results imply that signaling through integrin–TM4SF protein complexes may contribute to adhesion-dependent activation of FAK.
Table 1.
Expression of Integrins in HT1080/ZEO and HT1080/CD9 Cells
| HT1080/ZEO | HT1080/CD9 | |
|---|---|---|
| Neg. control | 0.37 | 0.32 |
| CD9 | 0.51 | 45.8 |
| α3β1 | 50.0 | 48.1 |
| α2β1 | 42.6 | 43.4 |
| α6β1 | 38.5 | 37.8 |
Cells were stained using mAbs to CD9 (C9-BB) and α3- (A3-X8), α2- (A2-7C6), and α6 (A6-ELE) subunits. mAb P3 was used as a negative control. Staining was analyzed by FACScan®. The numbers are mean of fluorescent intensity values.
Figure 12.
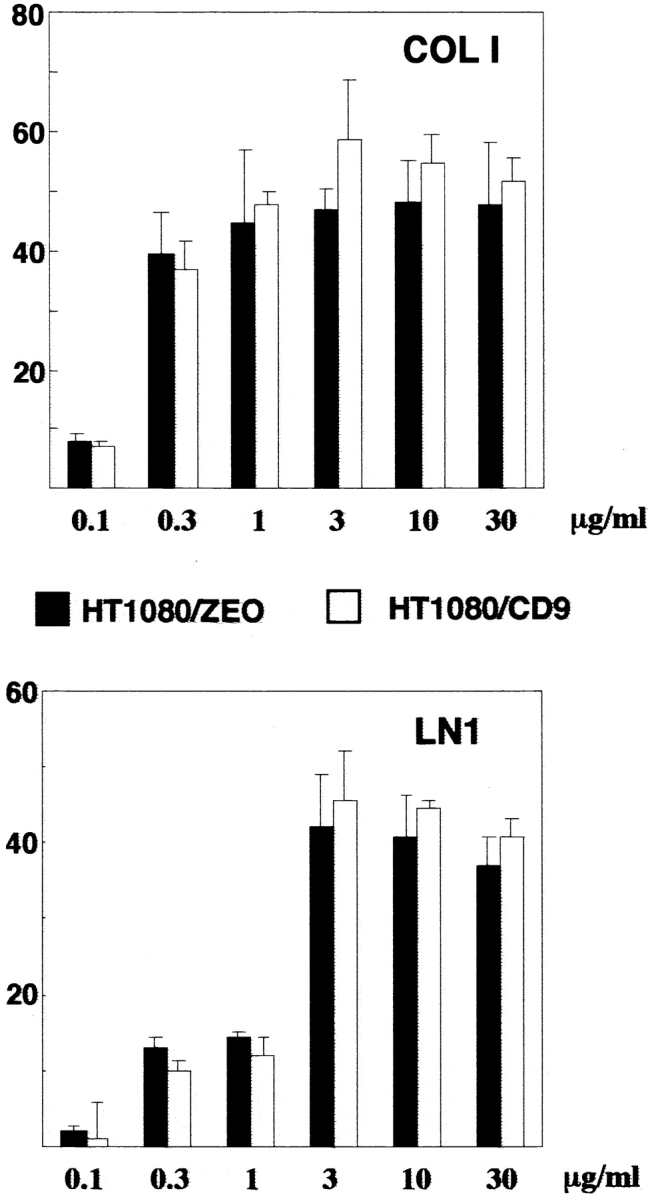
Ectopic expression of CD9 in HT1080 cells does not affect cell adhesion to collagen I and laminin 1. BCECF-AM–labeled cells tested for adhesion to 96-well microtiter plate coated with collagen type I or laminin-1 as described in Materials and Methods. The results are presented as percentage of attached cells.
Figure 13.
The effect of overexpression of CD9 on adhesion-dependent phosphorylation of FAK. (A) HT1080/zeo and HT1080/CD9 cells were serum-starved for 4 h in suspension before plating on collagen- (lanes 3 and 4) or laminin-coated (lanes 5 and 6) bacteriological dishes for 2 h at 37°C. Cells were lysed in buffer containing 1% Triton X-100, and FAK was immunoprecipitated using polyclonal Ab. Tyrosine phosphorylation of FAK was analyzed by using Western immunoblotting with mAb 4G10 (top). In the lower panel, the filter was stripped and reprobed with anti-FAK Ab. (B) Cells were prepared for the experiment as above before plating to laminin-coated dishes for various lengths of time. Immunoprecipitation of FAK and subsequent analysis of phosphorylation was carried out as described above.
Integrin–TM4SF Protein Complexes Are Involved in Reorganization of Actin Cytoskeleton
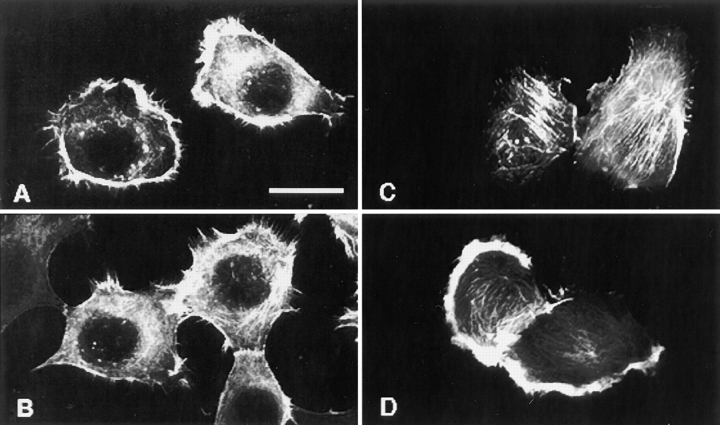
Activation of FAK is known to be dependent on the organization of actin cytoskeleton. Thus, we examined whether observed differences in phosphorylation of FAK in HT1080/zeo and HT1080/CD9 cells were related to the distribution of filamentous actin. No significant differences were found between the cell lines when we quantified the F-actin contents in the cells plated on collagen or laminin (not shown). Surprisingly, immunofluorescence staining with rhodamine-conjugated phalloidin revealed that in 60–70% of HT1080/zeo cells plated on collagen, actin filaments at the cortical areas were more abundant than in HT1080/CD9 cells (Fig. 14A and Fig. B), and opposite results were obtained when the cells were plated on laminin (Fig. 14C and Fig. D). Thus, although tetraspanins do not seem to have a role in integrin-induced actin polymerization, their function may be related to adhesion-dependent reorganization of actin filaments in the cortical areas.
Figure 14.
The effect of overexpression of CD9 on the organization of actin cytoskeleton. Serum-starved HT1080/zeo and HT1080/CD9 cells were plated on collagen- (A and B) or laminin-coated (C and D) glass coverslips for 1 h. Cells were fixed with paraformaldehyde and permeabilized with 0.25% CHAPS. Actin filaments were visualized with TRITC-conjugated phalloidin. Bar, 10 μM.
Discussion
Migrating cells interact with the extracellular matrix using various types of adhesion structures, including initial adhesion contacts that the cell makes at the periphery of extending protrusions, point contacts, focal complexes, and focal adhesions. Focal complexes and focal adhesions, that represent anchoring sites for actin filaments, confer strong cell–ECM interactions and, therefore, may play an important role in guiding cellular protrusions and generating traction forces. On the other hand, adhesive interactions that take place at the edge of cellular extensions are more dynamic: by using real-time video microscopy it was observed that the frontal edge of lamellipodial protrusions and tips of filopodia frequently undergo a few cycles of detachment and reattachment before the protrusions are firmly fixed on the substrate (Nobes and Hall 1995; Rabinovitz and Mercurio 1997). Based on the results presented in this study we propose that TM4SF proteins are structural components of these dynamic adhesion complexes. First, TM4SF proteins are specifically clustered with α3β1 integrins at the most distal parts of lamellipodial and filopodial extensions, an appropriate location for proteins that are involved in attachment-detachment cycles. Second, the link between α3β1–TM4SF protein complexes and cytoskeleton appears to be weak, suggesting that adhesive interactions mediated by the complexes are not constrained by the cytoskeletal proteins. Third, depending on receptor occupancy, integrin–TM4SF protein complexes may deliver functionally opposite signals, causing rapid reorganization of actin cytoskeleton at the periphery of cellular extensions, thus supporting dynamic interactions between the protrusions and a substrate.
Most cells simultaneously express more than one member of TM4SF and at least one tetraspanin-associated integrin (Hemler et al. 1996, Maecker et al. 1997) which together form a whole variety of integrin–TM4SF and TM4SF–TM4SF protein complexes on the plasma membrane. It has been suggested that these complexes may be linked to one another, forming a “tetraspan web” on the cell surface, and tetraspanins may have overlapping functions within this structural entity (Lagaudriere-Gesbert et al. 1997). On the other hand, recent data has clearly demonstrated that by changing the composition of the web it is possible to modulate its functional properties (Ikeyama et al. 1993; Dong et al. 1995; Shaw et al. 1995). Given this complexity it is important to understand what might be the functional contributions of each of the individual integrin-TM4SF associations in the adhesion-related phenomena. As the initial step to addressing this question we compared cellular distribution of different tetraspanins. The results presented in this study together with earlier data indicate that in different cell types various tetraspanins are clustered into adhesion complexes at the cell–ECM attachment sites. Notably, by carrying out detailed microscopic analysis, we demonstrated that TM4SF proteins are colocalized within punctate adhesion structures (particularly at the cell periphery), and, therefore, may indeed function as a network in adhesion-related events. This idea is further supported by the fact that mAbs to various tetraspanins induced similar modulatory effects when a signaling pathway leading to tyrosine phosphorylation of FAK was studied. It should be noted, however, that within more centrally located adhesion structures individual tetraspanins could be separated from one another, raising the possibility that these incomplete adhesion complexes may contribute to integrin signaling as separate functional units.
One of the main objectives of this study was to relate TM4SF-containing adhesion complexes to other types of adhesion structures (e.g., focal contacts/focal adhesions, focal complexes, and point contacts). In the previous report, we have found that CD63 and CD81 are clustered in punctate adhesion structures that are morphologically distinct from focal contacts (Berditchevski et al. 1997b). Moreover, the specific location of these adhesion structures at the cell periphery led us to suggest that tetraspanins are specific structural components of focal complexes. Indeed, our current findings confirmed that TM4SF proteins could not be detected in focal adhesions even when cells were treated with fetal calf serum or sphingosin monophosphate, two factors that promote their assembly. TM4SF proteins were also excluded from the vinculin-containing dot-like adhesion structures that resemble focal complexes. Similarly, we did not observe a colocalization of tetraspanins with paxillin and VASP, two proteins that are also associated with focal complexes and focal adhesions (not shown). On the other hand, some tetraspanin adhesion structures contained talin (Fig. 4) and FAK (Berditchevski, F., manuscript in preparation). As both talin and FAK could be recruited into assembling adhesion complexes before vinculin and paxillin (DePasquale and Izzard 1991; Miyamoto et al. 1995), we suggest that tetraspanins are present only in initial (talin-/FAK-negative) and early (talin-/FAK-positive) focal complexes, and dissociate from the complexes as other proteins (e.g., vinculin, paxillin, and VASP) join them. Alternatively, tetraspanin-containing complexes may represent a new class of adhesive structures that share some of their structural components with focal complexes. Finally, our data argue against the idea of the integrin/TM4SF adhesion complexes being equivalent to point contacts as only a few of them contain adaptin, an important component of clathrin coated structures that are associated with this type of adhesive contacts (Nermut et al. 1991; Tawil et al. 1993). Based on their cellular distribution and structural composition we propose that integrin/tetraspanin protein complexes do not regulate the attachment strength of immobile cells. Indeed, both antibody blocking experiments and comparative adhesion study using cell transfectants support this notion. Instead, integrin–tetraspanin protein complexes may regulate organization of cortical cytoskeleton at the peripheral attachment sites during the extension of lamellipodial and filopodial protrusions, thus affecting cell migration (also see below).
The cytoskeleton (both actin filaments and microtubules) appears to play an important role in the cellular distribution of the integrin–TM4SF protein complexes. One possibility is that the actin-based cell cortex is acting as a physical barrier to endocytosis of the complexes, a process that, in turn, may be dependent on the microtubular cytoskeleton. This would explain the apparent incompatibility of tetraspanins with focal adhesions: destabilization of actin filaments at the cell cortex that occurs while cell extends its protrusions will make integrin–TM4SF protein complexes more susceptible to internalization and therefore preclude their occurrence in focal adhesions. In this regard, colocalization of tetraspanins with MARCKS, a protein that is thought to promote actin dynamics at the cell periphery during cell spreading (Wiederkehr et al. 1997), may be a key factor that provides a functional link between integrin–TM4SF protein complexes and actin cytoskeleton, and, ultimately, regulates complex turnover on the cell surface.
Our results clearly demonstrate direct involvement of TM4SF protein complexes in integrin-dependent signaling. Tyrosine phosphorylation of FAK is a well established hallmark of outside-in integrin signaling that depends on the rearrangement of the actin cytoskeleton. Given the fact that the level of FAK tyrosine phosphorylation can be affected by tetraspanins (either via antibody clustering or by comparing adhesion-dependent responses using cell transfectants), we propose that TM4SF proteins are signaling modulators of integrin-mediated reorganization of the cortical actin cytoskeleton. In principal, ligated integrins can affect the actin cytoskeleton in two ways. First, certain cytoskeletal proteins are constitutively associated with integrin cytoplasmic tails, thus providing docking sites for the attachment of actin filaments. In this regard, the accumulation of talin and MARCKS (both actin-binding proteins) within integrin–TM4SF adhesion complexes may be sufficient for tetraspanins to sterically transmit their modulatory effect to the actin cytoskeleton. Although possible, the idea that integrin–TM4SF protein complexes play only a mechano-scaffolding role in the spatial organization of actin filaments is somewhat confronting the fact that a link between the complexes and the cytoskeleton is weak. On the other hand, the signaling scenario proposes that integrins may affect actin cytoskeleton by triggering distinct signaling pathways (for example, through activation of protein or/and phosphoinositide kinases) (Schwartz et al. 1995). Thus, it is possible that tetraspanins could modify/or intervene with integrin-associated signaling machinery. Indeed, we recently have found that distinct TM4SF proteins may differentially affect phosphoinositide kinase activity associated with α3β1 integrin (Berditchevski, F., unpublished data).
Interestingly, our data indicate that the modulatory impact from tetraspanins can be both negative and positive, perhaps depending on whether or not tetraspanin-associated integrins are engaged in ligand binding, and on a nature of the ligand. Indeed, dephosphorylation of FAK observed upon cell detachment suggests that disengaged and ligand bound integrins trigger opposite biochemical signals. Thus, it is conceivable that when integrins are unoccupied, additional clustering with anti-TM4SF mAbs may enhance negative signals emanating from the tetraspanin-associated integrins. Conversely, additional clustering occurring in cells plated on ECM ligands (e.g., when integrins are engaged) can potentiate positive signaling outcome. The results describing an opposite, ligand-dependent effect of CD9 on adhesion-mediated tyrosine phosphorylation of FAK in HT1080 cells, are particularly interesting. Again, it is possible that in cells plated on collagen, conformations assumed by α6β1 and α3β1, two tetraspanin-binding integrins in HT1080 cells, favor signaling pathways that counteract ligand-dependent phosphorylation of FAK mediated by the α2β1 integrin, a principal collagen receptor in HT1080 cells. On the other hand, when cells are plated on laminin, CD9 enhances positive (e.g., leading to phosphorylation of FAK) signaling via ligand-bound α6β1 integrin. Although exact mechanisms remains to be uncovered, the observed modulatory effect of the tetraspanins on adhesion-dependent signaling may involve the enzymes that are associated with integrin–TM4SF protein complexes (e.g., protein kinases or phosphoinositidylinositol 4-kinase (Skubitz et al. 1996; Berditchevski et al. 1997b). For example, locally produced phosphoinositides may have a pronounced effect on a molecular organization and, consequently, signaling capacity of the adhesion complexes by directly influencing actin cross-linking activities of filamin, α-actinin, and MARCKS, cytoskeletal proteins that are themselves either directly bound or localized in a close proximity to integrins (Furuhashi et al. 1992; Fukami et al. 1992). Further, recent data indicate that the intracellular composition of the adhesion complexes may be dependent upon a conformation of integrins involved (Miyamoto et al. 1995; Pfaff et al. 1998). Thus, it is conceivable that a cyto-architecture of the integrin–tetraspanin protein complexes may be affected by the nature of integrin–ECM ligand interactions. Consequently, by alternating protein targets for the complex-associated enzymes, distinct ECM ligands may differentially regulate the final signaling outcome. Alternatively, signaling properties of the integrin–tetraspanin protein complexes may be differentially affected through a cross-talk with a distinct integrin partner.
Various signaling pathways link actin cytoskeleton reorganization with phosphorylation of FAK, including trans-phosphorylation caused by integrin clustering, phosphorylation by activated tyrosine kinases of src family, or through the involvement of cytosolic protein tyrosine phosphatases (Miyamoto et al. 1995; Parsons and Parsons 1997; Shen et al. 1998; Tamura et al. 1998). Thus, it will be important to establish which of these signaling cascades is regulated by TM4SF proteins. In this regard, the fact that ectopic expression of CD9 in HT1080 cells could alter the kinetic of FAK dephosphorylation points to a possible connection between tetraspanins and activation of protein tyrosine phosphatase(s).
Whether influenced by TM4SF proteins directly (through the actin cytoskeleton rearrangement) or indirectly (via activation of other signaling pathways), the level of tyrosine phosphorylation of FAK is a critical factor that determines a molecular architecture of adhesion complexes, their signaling capacities, and, ultimately, their role in cell migration (Guan 1997). Thus, our data demonstrating involvement of tetraspanins in this process provide an important insight into the mechanisms that may link the function of TM4SF proteins with cell motility.
Acknowledgments
We thank all our colleagues who provided us with the reagents used in this study. We also thank L. Tompkins for invaluable help in preparation of the sections for immunoelectron microscopy and Dr. T. Sugiura, M. Griffiths, and E. Gilbert for critical review of the manuscript.
Work was supported by the Cancer Research Campaign grant SP2369/0101 (to F. Berditchevski).
Footnotes
1.used in this paper: FAK, focal adhesion kinase; TM4SF, transmembrane 4 superfamily
References
- Barnstable C.J., Bodmer W.F., Brown G., Galfré G., Milstein C., Williams A.F., Zeigler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens—new tools for genetic analysis. Cell. 1978;14:9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Berditchevski F., Bazzoni G., Hemler M.E. Specific association of CD63 with the VLA-3 and VLA-6 integrins. J. Biol. Chem. 1995;270:17784–17790. doi: 10.1074/jbc.270.30.17784. [DOI] [PubMed] [Google Scholar]
- Berditchevski F., Chang S., Bodorova J., Hemler M.E. Generation of monoclonal antibodies to integrin-associated proteins. Evidence that α3β1 complexes with EMMPRIN/basigin/OX47/M6 J. Biol. Chem. 272 1997. 29174 29180a [DOI] [PubMed] [Google Scholar]
- Berditchevski F., Tolias K.F., Wong K., Carpenter C.L., Hemler M.E. Novel link between integrins, TM4SF proteins (CD63, CD81) and phosphatidylinositol 4-kinase J. Biol. Chem. 272 1997. 2595 2598b [DOI] [PubMed] [Google Scholar]
- Berditchevski F., Zutter M.M., Hemler M.E. Characterization of novel complexes on the cell surface between integrins and proteins with 4 transmembranes (TM4 proteins) Mol. Biol. Cell. 1996;7:193–207. doi: 10.1091/mbc.7.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershadsky A., Chausovsky A., Becker E., Lyubimova A., Geiger B. Involvement of microtubules in the control of adhesion-dependent signal transduction. Curr. Biol. 1996;6:1279–1289. doi: 10.1016/s0960-9822(02)70714-8. [DOI] [PubMed] [Google Scholar]
- Claas C., Seiter S., Claas A., Savelyeva L., Schwab M., Zöller M. Association between the rat homologue of CO-029, a metastasis-associated tetraspanin molecule and consumption coagulability. J. Cell Biol. 1998;141:267–280. doi: 10.1083/jcb.141.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePasquale J., Izzard C. Accumulation of talin in nodes at the edge of the lamellipodium and separate incorporation into adhesion plaques at focal contacts in fibroblasts. J. Cell Biol. 1991;113:1351–1359. doi: 10.1083/jcb.113.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domanico S.Z., Pelletier A.J., Havran W.L., Quaranta V. Integrin α6Aβ1 induces CD81-dependent cell motility without engaging the extracellular matrix migration substrate. Mol. Biol. Cell. 1997;8:2253–2265. doi: 10.1091/mbc.8.11.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J.-T., Lamb P.W., Rinker-Schaeffer C.W., Vukanovic J., Ichikawa T., Isaacs J.T., Barret J.C. KAI 1, a metastasis suppressor gene for prostate cancer on human chromosome 11p11.2. Science. 1995;268:884–886. doi: 10.1126/science.7754374. [DOI] [PubMed] [Google Scholar]
- Fukami K., Furuhashi K., Inagaki M., Endo T., Hatano S., Takenawa T. Requirement of phosphatidylinositol 4,5-bisphosphate for alpha-actinin function. Nature. 1992;359:150–152. doi: 10.1038/359150a0. [DOI] [PubMed] [Google Scholar]
- Furuhashi K., Inagaki M., Hatano S., Fukami K., Takenawa T. Inositol phospholipid-induced suppression of F-actin-gelating activity of smooth muscle filamin. Biochem. Biophys. Res. Commun. 1992;184:1261–1265. doi: 10.1016/s0006-291x(05)80018-x. [DOI] [PubMed] [Google Scholar]
- Guan J.-L. Role of focal adhesion kinase in integrin signaling. Int. J. Biochem. Cell Biol. 1997;29:1085–1096. doi: 10.1016/s1357-2725(97)00051-4. [DOI] [PubMed] [Google Scholar]
- Hadjiargyrou M., Kaprielian Z., Kato N., Patterson P.H. Association of the tetraspan protein CD9 with integrins on the surface of S-16 Schwann cells. J. Neurochem. 1996;67:2505–2513. doi: 10.1046/j.1471-4159.1996.67062505.x. [DOI] [PubMed] [Google Scholar]
- Hamamoto K., Ohga S., Nomura S., Yasunaga K. Cellular distribution of CD63 antigen in platelets and in three megakaryocytic cell lines. Histochem. J. 1994;26:367–375. doi: 10.1007/BF00157770. [DOI] [PubMed] [Google Scholar]
- Hemler M.E., Mannion B.A., Berditchevski F. Association of TM4SF proteins with integrinsrelevance to cancer. Biochim. Biophys. Acta. 1996;1287:67–71. doi: 10.1016/0304-419x(96)00007-8. [DOI] [PubMed] [Google Scholar]
- Hemler M.E., Ware C.F., Strominger J.L. Characterization of a novel differentiation antigen complex recognized by a monoclonal antibody (A-1A5)unique activation-specific molecular forms on stimulated T cells. J. Immunol. 1983;131:334–340. [PubMed] [Google Scholar]
- Higashiyama S., Iwamoto R., Goishi K., Raab G., Taniguchi N., Klagsbrun M., Mekada E. The membrane protein CD9/DRAP27 potentiates the juxtacrine growth factor activity of the membrane-anchored heparin-binding EGF-like growth factor. J. Cell Biol. 1995;128:929–938. doi: 10.1083/jcb.128.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeyama S., Koyama M., Yamaoko M., Sasada R., Miyake M. Suppression of cell motility and metastasis by transfection with human motility-related protein (MRP-1/CD9) DNA. J. Exp. Med. 1993;177:1231–1237. doi: 10.1084/jem.177.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai T., Kakizaki M., Nishimura M., Yoshie O. Molecular analysis of the association of CD4 with two members of the transmembrane 4 superfamily, CD81 and CD82. J. Immunol. 1995;155:1229–1239. [PubMed] [Google Scholar]
- Jones P.H., Bishop L.A., Watt F.M. Functional significance of CD9 association with beta 1 integrins in human epidermal keratinocytes. Cell. Adhes. Commun. 1996;4:297–305. doi: 10.3109/15419069609010773. [DOI] [PubMed] [Google Scholar]
- Lagaudriere-Gesbert C., Le Naour F., Lebel-Binay S., Billard M., Lemichez E., Boquet P., Boucheix C., Conjeaud H., Rubinstein E. Functional analysis of four tetraspans, CD9, CD53, CD81, and CD82, suggests a common role in costimulation, cell adhesion, and migrationonly CD9 upregulates HB-EGF activity. Cell Immunol. 1997;182:105–112. doi: 10.1006/cimm.1997.1223. [DOI] [PubMed] [Google Scholar]
- Lemke H., Hammerling G.J., Hohmann C., Rajewsky K. Hybrid cell lines secreting monoclonal antibody specific for major histocompatibility antigens of the mouse. Nature. 1978;271:249–251. doi: 10.1038/271249a0. [DOI] [PubMed] [Google Scholar]
- Maecker H.T., Todd S.C., Levy S. The tetraspanin superfamilymolecular facilitators. FASEB J. 1997;11:428–442. [PubMed] [Google Scholar]
- Mannion B.A., Berditchevski F., Kraeft S.-K., Chen L.B., Hemler M.E. TM4SF proteins CD81 (TAPA-1), CD82, CD63 and CD53 specifically associate with α4β1 integrin. J. Immunol. 1996;157:2039–2047. [PubMed] [Google Scholar]
- Matsumoto A.K., Martin D.R., Carter R.H., Klickstein L.B., Ahearn J.M., Fearon D.T. Functional dissection of the CD21/cd19/TAPA-1/Leu-13 complex of B lymphocytes. J. Exp. Med. 1993;178:1407–1417. doi: 10.1084/jem.178.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake M., Koyama M., Seno M., Ikeyama S. Identification of the motility-related protein (MRP-1), recognized by monoclonal antibody M31-15, which inhibits cell motility. J. Exp. Med. 1991;174:1347–1354. doi: 10.1084/jem.174.6.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S., Teramoto H., Coso O.A., Gutkind J.S., Burbelo P.D., Akiyama S.K., Yamada K.M. Integrin functionmolecular hierarchies of cytoskeletal and signaling molecules. J. Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Iwamoto R., Mekada E. Membrane-anchored heparin-binding EGF-like growth factor (HB-EGF) and diptheria toxin receptor-associated protein (DRAP27)/CD9 form a complex with integrin α3β1 at cell-cell contact sites. J. Cell Biol. 1995;129:1691–1705. doi: 10.1083/jcb.129.6.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nermut M.V., Eason P., Hirst R., Kellie S. Cell substratum adhesion in RSV-transformed rat fibroblasts. Exp. Cell Res. 1991;193:382–397. doi: 10.1016/0014-4827(91)90111-7. [DOI] [PubMed] [Google Scholar]
- Nobes C.D., Hall A. Rho, Rac, and Cdc42 GTPase regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Parsons J.T., Parsons S.J. Src family protein tyrosine kinasescooperating with growth factor and adhesion signaling pathways. Curr. Opin. Cell. Biol. 1997;9:187–192. doi: 10.1016/s0955-0674(97)80062-2. [DOI] [PubMed] [Google Scholar]
- Peters P.J., Neefjes J.J., Oorschot V., Ploegh H.L., Geuze H.J. Segregation of MHC class II molecules from MHC class I molecules in the Golgi complex for transport to lysosomal compartments. Nature. 1991;349:669–676. doi: 10.1038/349669a0. [DOI] [PubMed] [Google Scholar]
- Pfaff M., Liu S., Erle D.J., Ginsberg M. Integrin beta cytoplasmic domains differentially bind to cytoskeletal proteins. J. Biol. Chem. 1998;273:6104–6109. doi: 10.1074/jbc.273.11.6104. [DOI] [PubMed] [Google Scholar]
- Rabinovitz I., Mercurio A.M. The integrin a6b4 functions in carcinoma cell migration on laminin-1 by mediating formation and stabilization of actin-containing motility structures. J. Cell Biol. 1997;139:1873–1884. doi: 10.1083/jcb.139.7.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford K.J., Thorne R.F., Hersey P. CD63 associates with transmembrane 4 superfamily members, CD9 and CD81, and with beta1 integrins in human melanoma. Biochem. Biophys. Res. Commun. 1996;222:13–28. doi: 10.1006/bbrc.1996.0690. [DOI] [PubMed] [Google Scholar]
- Radford K.J., Thorne R.F., Hersey P. Regulation of tumor cell motility and migration by CD63 in a human melanoma cell line. J. Immunol. 1997;158:3353–3358. [PubMed] [Google Scholar]
- Rubinstein E., Le Naour F., Billard M., Prenant M., Boucheix C. CD9 antigen is an accessory subunit of the VLA integrin complexes. Eur. J. Immunol. 1994;24:3005–3013. doi: 10.1002/eji.1830241213. [DOI] [PubMed] [Google Scholar]
- Schwartz M.A., Schaller M.D., Ginsberg M.H. Integrinsemerging paradigms of signal transduction. Annu. Rev. Cell Dev. Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- Shaw A.R.E., Domanska A., Mak A., Gilchrist A., Dobler K., Visser L., Poppema S., Fliegel L., Letarte M., Willett B.J. Ectopic expression of human and feline CD9 in a human B cell line confers β 1 integrin-dependent motility on fibronectin and laminin substrates and enhanced tyrosine phosphorylation. J. Biol. Chem. 1995;270:24092–24099. doi: 10.1074/jbc.270.41.24092. [DOI] [PubMed] [Google Scholar]
- Shen Y., Schneider G., Cloutier J.-F., Veillete A., Schaller M.D. Direct association of protein-tyrosine phosphatase PTP-PEST with paxillin. J. Biol. Chem. 1998;273:6474–6481. doi: 10.1074/jbc.273.11.6474. [DOI] [PubMed] [Google Scholar]
- Skubitz K.M., Campbell K.D., Iida J., Skubitz A.P.N. CD63 associates with tyrosine kinase activity and CD11/CD18, and transmits an activation signal in neutrophils. J. Immunol. 1996;157:3617–3626. [PubMed] [Google Scholar]
- Slupsky J.R., Seehafer J.G., Tang S.-C., Masellis-Smith A., Shaw A.R.E. Evidence that monoclonal antibodies against CD9 antigen induce specific association between CD9 and the platelet glycoprotein IIb-IIIa complex. J. Biol. Chem. 1989;264:12289–12293. [PubMed] [Google Scholar]
- Tachibana I., Bodorova J., Berditchevski F., Zutter M.M., Hemler M.E. NAG-2, a novel transmembrane-4 superfamily (TM4SF) protein that complexes with integrins and other TM4SF proteins. J. Biol. Chem. 1997;272:29181–29189. doi: 10.1074/jbc.272.46.29181. [DOI] [PubMed] [Google Scholar]
- Tamura M., Gu J., Matsumoto K., Aota S., Parsons R., Yamada K.M. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science. 1998;280:1614–1617. doi: 10.1126/science.280.5369.1614. [DOI] [PubMed] [Google Scholar]
- Tawil N., Wilson P., Carbonetto S. Integrins in point contacts mediate cell spreadingfactors that regulate integrin accumulation in point contacts vs. focal contacts. J. Cell Biol. 1993;120:261–271. doi: 10.1083/jcb.120.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman J.B., Pasqualini R., Takada Y., Hemler M.E. The function and distinctive regulation of the integrin VLA-3 in cell adhesion, spreading and homotypic cell aggregation. J. Biol. Chem. 1993;268:8651–8657. [PubMed] [Google Scholar]
- Wiederkehr A., Staple J., Caroni P. The motility-associated proteins GAP-43, MARCKS, and CAP-23 share unique targeting and surface activity-inducing properties. Exp. Cell Res. 1997;236:103–116. doi: 10.1006/excr.1997.3709. [DOI] [PubMed] [Google Scholar]
- Yáñez-Mó M., Alfranca A., Cabañas C., Marazuela M., Tejedor R., Ursa A., Ashman L.K., De Landázuri M.O., Sanchez-Madrid F. Regulation of endothelial cell motility by complexes of tetraspan molecules CD81/TAPA-1 and CD151/PETA-3 with α3β1 integrin localized at endothelial lateral junctions. J. Cell Biol. 1998;141:791–804. doi: 10.1083/jcb.141.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]