Abstract
Volatile organic solvent (inhalant) abuse continues to be a major health concern throughout the world. Of particular concern is the abuse of inhalants by adolescents because of its toxicity and link to illicit drug use. Toluene, which is found in many products such as glues and household cleaners, is among the most commonly abused organic solvents. While studies have assessed outcomes of exposure to inhalants in adult male animals, there is little research on the neurobehavioral effects of inhalants in female or younger animals. In attempt to address these shortcomings, we exposed male and female Long-Evans rats to 20 min of 0, 2,000, 4,000, or 8,000 parts per million (ppm) inhaled toluene for 10 days in rats aged postnatal (PN) day 28-39 (adolescent), PN44-PN55, or >PN70 (adult). Animals were observed individually in 29-l transparent glass cylindrical jars equipped with standard photocells that were used to measure locomotor activity. Toluene significantly increased activity as compared to air exposure in all groups of male and female rats with the magnitude of locomotor stimulation produced by 4000 ppm toluene being significantly greater for female adults than during any age of adolescence. The results demonstrate that exposure to abuse patterns of high concentrations of toluene through inhalation can alter spontaneous locomotor behavior in rats and that the expression of these effects appears to depend upon the postnatal age of testing and sex of the animal.
1. Introduction
The practice of inhaling chemical vapors from products found in society for the purpose of achieving a quick intoxication is known as inhalant abuse. While inhalant abuse continues to be a lesser recognized form of substance abuse, products that are typically abused are easily accessible, easily used, and easily concealed. Abuse typically involves 15 to 20 inhalations of very high solvent concentrations (usually several thousand ppm) which occur over a very short period of time (10-15 minutes) [11,31]. While the exact concentration inhaled varies by compound, estimates are between 5,000 and 15,000 ppm for toluene, one of the most commonly abused solvents [16,65]. Toluene is found in varying percentages in a wide variety of commonly available household products such as gasoline, paint, varnish, glue, shoe polish, fingernail polish, and rubber cement. Recent national data suggests that greater than half of the commonly abused inhalants contain varying amounts of toluene and over 30% of inhalant users under 18 years old have inhaled toluene [46-48]. While earlier Household Surveys showed large increases in the number of new users (> 200% between 1990 and 2002), trends have leveled off and the rates of use have been stable for the last four years. Nevertheless, a substantial number of male and female adolescents still report use of inhalants. In 2005, 877,000 individuals 12 years old or older used inhalants for the first time with 72.3 percent being under the age of 18 years at first use (average age was 16.1 years of age)[46,48]. Hence, inhalant abuse is largely a problem in children and adolescents.
While rodent studies have been very helpful in understanding the neurological and behavioral consequences of inhalant exposure [5], most of these studies have used adult rodents. Recent research, however, suggests that adolescent and adult animals may respond differently to the administration of psychoactive drugs such as inhalants [52,53]. In rats, adolescence has been most commonly defined as the two-week period from postnatal day 28 to postnatal day 42 (PN28-PN42) [52,53]. During this period, rats exhibit behavioral patterns that are characteristic of adolescent mammals of many species, including increased risk taking, increased orientation towards and interaction with peers, and increased preference for novelty [52-54]. In addition, a number of studies have shown that significant neural pruning and re-organization occurs during the period of adolescent development [52,53]. In light of these findings, it is not surprising that differences in the effects of drugs of abuse in adolescent rodents, as compared to adult rodents, have been reported. For example, younger animals have been shown to develop less sensitization than adult animals following repeated exposure to stimulants such as cocaine [14],[29], amphetamine [28], and methylphenidate [9,10]. Similar results have been observed for alcohol [12], [39]. While the effects of toluene have been investigated in younger animals, the majority of these studies have focused on gestational exposure followed by later assessment of toluene’s effects in pre-weanling and older animals [see review [6]]. For example, results from our laboratory and others have shown that repeated, brief, high-concentration binge maternal toluene exposure adversely impacts prenatal development and early postnatal maturation of pups (PN22-PN63), as well as their spontaneous exploration and amphetamine-induced locomotor activity [6], [7], [22,27]. These results suggest that exposure to high concentrations of toluene during early development may be more deleterious than exposure during adulthood. Since inhalants are a class of compounds abused primarily by teenagers and young adults, delineation of any age-dependent differences during this developmental stage is important for understanding abuse of these compounds.
To this end, the purpose of the current study was to evaluate age-dependent and sex differences in responsivity to inhalants such as toluene. Locomotor activity was chosen as the endpoint for this investigation for a couple of reasons. First, the effects of toluene and other abused inhalants on activity in rodents have been well-characterized. For example, inhaled toluene concentrations similar to those encountered in abuse settings produce a profile of effects in adult rodents that progress from motor excitation at low concentrations (i.e., 500-4,000 ppm) to sedation, motor impairment and anesthesia at higher concentrations of 6,000-15,000 ppm [4],[24], [68]. Biphasic dose-response curves for locomotor activity have also been documented for acute concentrations of 1,1,1-trichloroethane (TCE; 500 ppm – 14,000 ppm) [8],[4], [60], [64]. Concentrations of these solvents that initially produce only sedation or anesthesia can result in coma and ultimately death by respiratory depression when exposure is prolonged or continuous [5]. Repeated exposure to solvents such as toluene and TCE produces sensitization to the motor increasing effects of these compounds [5], [24]. Interestingly, repeated toluene or TCE exposure also enhances cocaine’s or diazepam’s motor increasing effects, respectively [3],[64], suggesting a common neurochemical pathway. The second reason for assessment of locomotor activity (vs. other possible dependent measures) was the resulting ability to investigate sensitization, a phenomenon whereby initial drug-induced stimulation of locomotor activity in rodents is enhanced following repeated administration of the abused drug. Close association between dopamine pathways that govern ambulatory motor activity and those involved in reward has led to the hypothesis that locomotor sensitization represents a form of neural adaptation that concomitantly results in an increase in the sensitivity of reward pathways to stimulation by dopamine [44,45]. According to the sensitization theory, this hypersensitivity is progressive and may lead to an increase in subjectively felt “wanting” or “craving” for the drug [44,45], resulting in continued or increased use. Regardless of whether or not this theory is ultimately validated, the behavioral phenomenon of locomotor sensitization to drugs of abuse (particularly psychomotor stimulants) has been investigated in both adult and adolescent rodents. Hence, the use of this measure facilitates comparisons of patterns of behavior produced by repeated exposure to toluene as compared to repeated administration of other drugs of abuse.
2. Methods
2.1. Subjects
Timed pregnant adult female Long-Evans rats (Harlan, Dublin, VA), ordered for arrival on gestation day 14, gave birth in the animal facility at Virginia Commonwealth University a week later to all immature (PN28-PN39 and PN44-PN55) rats included in this study. Dams were individually housed in clear plastic cages (52 × 28 × 22 cm) with sawdust bedding available in each cage for nesting. The AAALAC-certified vivarium had a temperature-controlled (20-22°C) environment with a 12-hour light-dark cycle (lights on at 7 a.m.). The dams were left undisturbed except for providing food, water, and fresh bedding until they gave birth (postnatal day 0, PN0). Pups were sexed and culled to no more than 10 pups per litter. Forty-eight litters were required for completion of the entire study. Pups remained with their dams until weaning at PN21. On PN21, pups were separated from the dam and were randomly selected (one male and one female per litter) for each of the treatment groups described below. Subsequently, they were pair-housed with a same-sex rat from another litter. The rat pups were tested for 10 days between ages PN28 - PN39 or PN44 - PN55. Drug naïve rats in the adult condition were ordered (Harlan) at an age from PN65-PN75 and were also pair-housed with a same sex rat. Adult animals were allowed to acclimate to the vivarium for at least one week before testing; hence, adult rats were at least PN70 when testing began. We recognize that shipping stress may affect the behavior of rats; however, we note that baseline rates of activity (which might also be affected by exposure to stress) were similar across age and sex, although we also realize that this similarity does not necessarily eliminate the possibility that toluene exposure may represent a challenge that may bring out stress effects that are latent under baseline conditions. Group sizes were as follows: 5 male and 5 female adult rats in the air group and in each toluene concentration group; 8 male and 9 female rats aged PN28-PN39 in the air and 4000 ppm groups and 5 adolescent rats of each sex at each of the other two concentrations; 5 female rats aged PN44-PN55 at each toluene concentration plus 5 in the air condition; and 3 male rats aged PN44-PN55 in the air condition, 4 male rats of this age that were exposed to toluene 4000 ppm, and 5 PN44-PN55 male rats at each of the other two toluene concentrations. PN44-PN55 rats were tested in order to determine whether increases in activity after repeated exposure in the younger PN28-PN39 rats was due to sensitization or to a developmental increase in sensitivity. Throughout the experiment, all rats had free access to food and water in their home cages. These studies were carried out in accordance with guidelines published in the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and were approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University.
2.2. Apparatus
Vapor exposures were conducted in 29-l transparent glass cylindrical jars (47 cm H × 35 cm diameter; total floor space = 962 cm2) which were outfitted with Plexiglas tops. Briefly, vapor generation commenced when solvent was injected through a port in the Plexiglas top onto filter paper suspended below the sealed lid and located 32 cm from the bottom of the chamber, as described previously [67]. A fan, mounted on the inside of the lid, was then turned on which volatilized and distributed toluene within the chamber. Nominal chamber concentrations did not vary by more than 10% from measured concentrations as determined by single wavelength monitoring infrared spectrometry (Miran 1A, Foxboro Analytical, North Haven, CT). Two pairs of standard photocells, mounted at right angles to each other outside and 4 cm above the glass bottom inside each static exposure chamber, were used to measure locomotor activity. Locomotor activity was operationally defined as the sum of the interruptions of either photocell beam (counts) during each 20-min exposure to air or inhalants. A computer with Med-PC software and interfacing (Med Associates, Georgia, VT) was used to record locomotor counts. In addition, the possibility of human exposure to inhalants was minimized by housing the vapor exposure chambers under a standard laboratory hood. During all exposure sessions, the hood fan was turned on.
2.3. Procedure
Male and female rats (both pups and adult) were assigned to receive exposure to air or to one of the toluene concentrations (2000, 4000 or 8000 ppm). In order to control for possible litter effects [26], each rat pup of a given age was randomly chosen from a different litter such that none of the rats of the same age and sex were from the same litter. An exception was that one male and one female from the same litter may have received the same toluene concentration (i.e., assignment of males and females to groups occurred independently of each other). Toluene or air exposure and locomotor testing began on PN28, PN44, or > PN70. Rats of each age that were assigned to the air or 4000 ppm were tested first. The choice of this concentration was based upon our extensive prior research with toluene. After the large age differences in the effect of 4000 ppm were observed, assessment of a smaller and larger concentration (2000 and 8000 ppm, respectively) was initiated in order to determine whether this effect was concentration-dependent. Because the large number of animals in the study and the small number of exposure chambers (i.e., 2 chambers) prevented testing all rats during the same two-week period, rats were tested in cohorts. Each cohort contained rats of both sexes; some cohorts contained rats of the same age and others contained rats of different ages. Timely completion of the study required that rats be tested throughout the day during each two-week test cycle. Each individual rat was tested at approximately the same time each day; however, test times for rats within the same group were spread throughout the day (e.g., one rat from a group may have always been tested in the morning whereas another rat was always tested in the afternoon).
During the study, rats were transported to the laboratory at least one hour prior to the start of the experiment. Body weights were measured daily during this pre-session period of acclimation to the experimental environment. At the start of the session, each rat was placed into one of the vapor exposure jars and exposed to air or toluene during a 20-min session. After the session, each rat was returned to its home cage. For the next 4 days, rats were exposed daily to air or their assigned concentration of toluene during a 20-min session. On days 6 and 7 of the experiment (PN33-PN34 and PN49-PN50 for the two groups of immature rats/sex), animals were left undisturbed in their home cages in the vivarium. The purpose of this interruption in daily toluene exposure was to determine the effects of intermittent (vs. continuous) administration, as the former has been shown to enhance development of sensitization to locomotor stimulants [62,63]. Subsequently, rats received 5 more daily exposure and testing sessions (PN35-PN39 and PN51-PN55 for the two groups of immature rats/sex). Throughout the exposure regimen, an individual rat was always tested in the same exposure chamber. Due to the short duration of adolescence in rats, placement into the locomotor chambers did not occur prior to toluene or air exposure (i.e., no habituation period). Nevertheless, baseline locomotor activity remained relatively constant across all days of the study in all groups.
2.4. Chemicals
Toluene (T-324, Fisher Scientific Co., Fairlawn, NJ) was purchased commercially (purity ≥ 99.5%). Vapor concentrations were calculated nominal concentrations.
2.5. Statistical Analysis
Locomotor counts were measured as the number of photocell beam breaks. During toluene or air exposures, mean (±SEM) numbers of locomotor counts were calculated for the entire 20-min session. A four-way split-plot ANOVA (age X sex X concentration X exposure session) was performed on data for all rats, with exposure session as the repeated factor and the other three factors as the between-subjects factors. Power estimates for each of the main effects and the four-way interaction exceeded 80% (0.8). Subsequently, separate two-way split-plot ANOVAs were run for each sex and age (concentration X exposure session). Changes in body weights (weight on day 10 - weight on day 1) were calculated for rats within each age and sex group. Separate one-way ANOVAs were performed to compare mean change in body weights across toluene concentration in each group. When ANOVAs were significant, Tukey post hoc tests (α=0.05) were used to compare individual means.
3. Results
3.1.Weight
As expected, immature rodents of both sexes weighed less than same-sex adult rodents. Further, they exhibited more weight gain over the course of the two week exposure period than did adults. Within each age group, however, toluene did not significantly affect the amount of weight gain for either sex (p > 0.05 for each one-way ANOVA; data not shown), suggesting that toluene did not affect growth rate (i.e., decreased weight, had it occurred, would have been a possible indication of toxicity).
3.2.Locomotor Activity
The initial 4-way ANOVA revealed significant main effects for all four factors: age [F(2,107)=31.1, p<0.01], sex [F(1,107)=10.6, p<0.01], concentration [F(3,107)=42.3, p<0.01], and session number [F(9,944)=84.4, p<0.01]. In addition, most of the 2- and 3-way interactions were significant, including interactions for age X concentration [F(6,107)=15.4, <0.01], sex X session [F(9,944)=3.9, <0.01], concentration X session [F(27,944)=12.8, <0.01], sex X age X session [F(18,944)=1.9, <0.01], sex X session X concentration[F(27,944)=2.6, <0.01], as well as the 4-way age X sex X concentration X exposure session interaction [F(54,944)=2.0, p<0.01]. Given the complex pattern of significant differences, all subsequent analysis of the results was based upon separate 2-way (concentration X session) ANOVAs for each age and sex group.
Figure 1 shows the effects of exposure to toluene and air on locomotor activity in female (top panels) and male (bottom panels) rats across three different ages. In female adolescent rats (PN28-PN39) [Fig. 1, top left panel], post hoc analysis of the significant concentration X session interaction [F(27,208)=3.84, p<0.01] revealed that the locomotor activity of rats exposed to any of the toluene concentrations (2000, 4000 or 8000 ppm) was significantly higher (compared to activity level on day 1 and/or compared to air exposure) on several days during the second week of exposure. Female rats aged PN44-PN55 [Fig. 1, top middle panel] also showed significantly greater activity as compared to air and to day 1 after several days of exposure to 4000 or 8000 ppm toluene but not after exposure to 2000 ppm toluene [F(27,142)=5.71, p<0.01 for the concentration X session interaction]. Nevertheless, the 4-way overall ANOVA results suggest that the magnitude of locomotor stimulation produced by 4000 ppm toluene was significantly greater in PN44-PN55 rats than in PN28-PN39 rats. The pattern of toluene effects observed in adult female rats was similar to those at younger ages (Fig. 1,top right panel), with one notable difference. In adult female rats, 4000 ppm toluene significantly increased activity on the first day of exposure (as compared to the air condition) and activity remained elevated throughout the rest of the two-week exposure period [F(27,144)=2.7, p<0.01 for the concentration X session interaction]. Initial activity was not increased by any other toluene concentration in adults and was not increased by any concentration in younger and older adolescents. Increased activity as compared to day 1 was also observed with exposure to 8000 ppm toluene, but this increase did not occur until day 4 of exposure, indicating the development of sensitization. The increased activity produced by 8000 ppm over time was also significant compared to the air group on days 8 and 9 of exposure. The magnitude of increases in activity seen in adult female rats during exposure to 4000 and 8000 ppm toluene was significantly greater than the magnitude of increases that occurred in younger rats, as suggested by results of the 4-way ANOVA.
Figure 1.
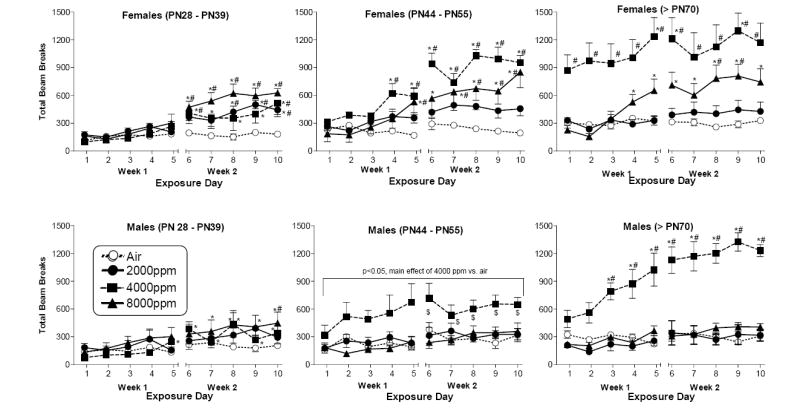
Effects of toluene and air on locomotor counts in female (top panels) and male (bottom panels) rats aged PN28-PN39 (left panels), PN44-PN55 (middle panels), and adult (>PN70; right panels). Each point represents the mean (± SEM) of locomotor counts (measured as photocell beam breaks) during a 20-min exposure session. N = 5 adult rats of each sex at all concentrations; N = 9 female and 8 male adolescent rats for air and 4000 ppm and 5 adolescent rats of each sex for 2000 and 8000 ppm; N = 5 female rats aged PN44-PN55 at all concentrations, 3 male rats aged PN44-PN55 for air, 4 male rats in this age group for 4000 ppm, and 5 male rats in this age group for 2000 and 8000 ppm. * indicates significant ANOVA interaction and Tukey post hoc difference (p<0.05) from day 1. # indicates significant ANOVA interaction and Tukey post hoc difference (p<0.05) from air. $ indicates a significant ANOVA main effect and Tukey post hoc difference (p<0.05) from day 1 (i.e., collapsed across concentrations). In addition, analysis of data for male rats aged PN44-PN55 revealed main effect of concentration, for which Tukey post hoc test results indicated a significant difference (p<0.05) of 4000 ppm from air (collapsed across days), as indicated on the graph.
In male adolescent rats (PN28-PN39) [Fig. 1, bottom left panel], post hoc analysis of the significant concentration X day interaction [F(27,190)=1.96, p<0.01] revealed that exposure to 8000 ppm toluene significantly increased activity as compared to air on the 10th day of exposure. In addition, changes across time occurred at the 4000 and 8000 ppm toluene concentrations. Each of these concentrations significantly increased activity (compared to initial exposure to the concentration) on several days during the latter part of the first week and during the second week of exposure. Analysis of the data for male rats aged PN44-PN55 [Fig. 1, bottom middle panel] showed significant main effects for concentration [F(3,13)=6.03, p<0.01] and day [F(9,116)=4.43, p<0.01], but the concentration X day interaction [F(27,116)=1.0, p>0.05] was not significant. Post hoc analysis of the main effect for concentration revealed that exposure to 4000 ppm toluene (collapsed across day) significantly increased activity as compared exposure to air. In addition, post hoc analysis of the main effect for day showed significant increases in activity (collapsed across toluene concentration) during the second week of exposure. The activity of adult male rats (>PN70) was also enhanced by exposure to 4000 ppm toluene with respect to air exposure and across days [F(27,144)=7.68, p<0.01 for concentration X day interaction] (Fig. 1, bottom right panel). Post hoc analysis of this concentration X day interaction revealed that, from day 3 onward, 4000 ppm toluene significantly increased activity compared to air and compared to its initial effect on day 1. Further, the pattern of significant results in the overall 4-way ANOVA suggests that the magnitude of the locomotor stimulation produced by 4000 ppm toluene in adult male rats was significantly larger than the stimulation observed in either group of younger rats.
4. Discussion
Over the range of concentrations assessed in the present study (2000 – 8000 ppm), toluene produced only increases in activity. The pattern of these activity increases varied widely as a function of concentration, number of exposure sessions, age and sex. Acutely, exposure to 2000 or 8000 ppm toluene failed to alter locomotion in rats of any age or sex. Only the 4000 ppm concentration of toluene increased activity upon initial exposure. Further, it did so only in female adult rats, although a main effect of this concentration (collapsed over sessions) was also observed in males aged PN44-PN55. In general, previous studies in adult male mice [4,13,66] and rats [24,25,68] have shown that toluene’s acute locomotor effects are biphasic: excitatory at lower concentrations (i.e., 500-4,000 ppm) followed by sedation and motor impairment at higher concentrations (i.e., 6000-15,000 ppm) with recovery of function shortly after removal from toluene exposure. While the discrepancies between the results for adult male rats in this study and those of previous reports are somewhat puzzling, a wide variety of factors have been shown to contribute to the pattern of effects produced by a given solvent or drug on motor activity, including age, sex, task parameters (e.g., habituation, duration), species, and strain. In particular, previous studies have demonstrated inter-strain differences in the CNS effects of pesticides and inhaled anesthetics in adult male rodents [32,51], suggesting that strain may have played a role in the discrepancies noted above. At least one previous study, for example, reported that 5000 ppm was the minimal effective concentration required to produce increases in motor activity in adult male Long-Evans rats [25]. Since toluene effects on the motor activity of female rodents or adolescent rodents of either sex have not been well-studied, it is difficult to determine whether or not results would differ under parameters used in other labs.
In contrast to the lack of acute toluene effects on locomotion in most of the groups, clear evidence of sensitization was observed with repeated exposure. During at least one of the later sessions, one or more concentrations of toluene increased activity, compared to air and compared to initial toluene exposure, in rats of all ages and both sexes. While a few previous studies have reported the development of sensitization after repeated exposure of adult male rodents to inhaled [62,63] or systemically administered toluene [40-42], the present study is perhaps the first to examine the effects of toluene in a sensitization paradigm in adolescent rats. Our results here reveal that development of sensitization to the locomotor stimulant effects of toluene in adolescent rats of both sexes was minimal and did not occur until the second week of exposure sessions. Development of sensitization in older rats of both sexes was both quick0er and more pronounced, as it occurred during the first week of exposure and with successive increases in magnitude during the period of transition from adolescence to adulthood. Several factors may have conceivably played a role in mediation of these age differences following acute and repeated exposure to toluene. Since this study was developmental in nature, maturation may have contributed to age differences in drug effects. Indeed, chronic daily exposure to very high concentrations of toluene (10,000 – 40,000 ppm) from PN3 to age 8 weeks resulted in pronounced hypnotic effects and loss of righting reflex in male rats at PN3 [30]. The magnitude of toluene-induced hypnotic effects and loss of righting reflex were dramatically reduced during adolescence followed by a partial return to levels observed during infancy by adulthood at 8 weeks. This effect was not due to tolerance, as initial exposure to toluene at 8 weeks was similar to that observed in the chronically exposed rats, nor was it due to age-related changes in toluene elimination, as brain and liver levels were similar in the 8-week-old rats regardless of length of toluene exposure. Hence, although toluene concentrations used in the present study were 10-fold lower than those in this previous study, it is nevertheless possible that the decreased effects of acute exposure to toluene on locomotion in adolescents resulted from maturational differences in sensitivity to toluene; i.e., adolescents younger than at least PN35 (age at which first increases over air or day 1 occurred) had not yet reached an age where sensitivity to toluene or other solvents had developed. If this were the case, however, we would have expected to see increased activity upon initial exposure in the PN44-PN55, which was not observed. A second alternative is that adolescents require a longer period of exposure before stimulant effects on motor activity appear. In this case, we would expect to see results like those we obtained; i.e., initial exposure did not increase activity in PN44 rats, even though the animals in the PN44-PN55 group were only slightly older at the start of the exposure regimen than the adolescents were at the end. Interestingly, this group also showed a pattern of sensitization development that was mid-way between those of adolescents and adults. Hence, maturation cannot fully account for age differences seen here.
Age differences in toluene pharmacokinetics are another factor that could have affected response following exposure. Levels of isoenzymes of cytochrome P450, a primary hepatic enzyme responsible for toluene metabolism to benzyl alcohol and o- and p-cresol, have been shown to vary across age and sex in drug-naïve rats [34,35,61], although functional consequences of these differences on behavior have not been demonstrated. Since pharmacokinetic parameters were not measured in the present study, their influence cannot be completely eliminated. For example, levels of CYP2E1, the P450 isoenzyme most commonly associated with toluene metabolism, decrease between the ages of 3 and 8 weeks in both male and female and levels of CYP2C11, which is associated with toluene metabolism in male rats only, increase [57], suggesting that younger rats might metabolize toluene more quickly. Several other findings, however, argue against age-related pharmacokinetic differences as a sole explanation for the observed age differences in toluene’s effects on activity. First, whereas 4000 ppm toluene dramatically increased activity in adult and PN44-PN55 rats during the first week of exposure, a 2-fold increase (4000 to 8000 ppm) or decrease (4000 to 2000 ppm) in toluene concentration failed to produce any change in responsivity of the adolescent rats, as compared to air exposure. Some degree of change would have been expected over this concentration range if younger rats were merely metabolizing toluene at a faster or slower rate. Second, prior to puberty male and female rats have approximately equal levels of CYP2E1 [34]; yet, female adolescent rats in this study exhibited greater toluene-induced stimulation of activity than did male adolescent rats, particularly during the second week of exposure. Finally, adolescent animals have also been found to be differentially sensitive to the acute effects of other CNS depressants that do not share the same pharmacokinetic mechanisms as toluene. Indeed, previous studies have shown that the degree to which a specific drug will affect adolescents differently than adults may be affected by multiple factors, including the specific behavior measured [55], the exact age of the animal [59], and the route of administration [43]. For example, adolescent rodents are less sensitive to ethanol-induced motor disruption and loss of righting reflex than are adult rodents [23,33]. Similarly, zolpidem, but not pentobarbital, also produced shorter sleep times in adolescent mice than in adults [33]. Mechanistic studies have suggested that these behavioral differences may co-occur with developmental changes in underlying neural systems [for review [55]], including alterations in GABAA receptor binding [33]. Similar to ethanol, toluene interacts with GABAA receptors [1,2], suggesting that this mechanism may also contribute to the age differences observed in the present study. In addition, younger animals have been shown to develop less locomotor sensitization than adult animals following repeated exposure to other drugs of abuse such as alcohol [12], [39], cocaine [14],[29], amphetamine [28], and methylphenidate [9,10]. Together, these results suggest that initial sensitivity to the acute locomotor effects of toluene (and perhaps those of other inhalants) follows a developmental time-course in which sensitivity increases as rats leave adolescence and enter adulthood.
In addition to age differences in toluene’s effects on activity, our results also demonstrate pronounced sex differences. Although both sexes showed the same general pattern of enhanced sensitivity with age, both acute and repeated effects of toluene on locomotion were more pronounced in females of all ages as compared to age-matched males. Further, these differences were observed without evidence of any sex differences in baseline activity (i.e., activity after air exposure), which are sometimes reported [58]. The fact that these sex differences in toluene’s effect on activity occurred in pre-pubertal adolescents and in both groups of older rats suggests that the differences were not entirely related to hormonal status. These results are also in agreement with other studies demonstrating that females may be more sensitive to the effects of abused drugs [15]. For example, several studies have shown that females are more sensitive than males to alterations in locomotor activity following administration of stimulants such as cocaine [36,49,50], d-amphetamine [20,56] and MDMA [37]. Development of the sensitization to the elevated locomotor activity seen in rats treated repeatedly with stimulants has been demonstrated to be mediated by dopaminergic activation [38]. Interestingly, there is now evidence that dopaminergic systems may also play a role in the acute, and possibly the long term, effects of toluene on locomotor activity as well [40-42]. Indeed, cross-sensitization has been shown from cocaine to toluene [3]. Sex differences have also been reported in preclinical studies with CNS depressants, including ethanol and pentobarbital [17-19,69], although pharmacokinetics likely contributes strongly to sex differences in ethanol sensitivity. In contrast, males were reported to be more sensitive to the locomotor effects of high concentrations of toluene (10,000 – 30,000 ppm) in an early study [21], although comparisons were based upon observation of a few rats without accompanying statistical analysis. The extent to which sex-dependent differences in metabolism or other pharmacokinetic parameters may contribute to the observed sex differences in the behavioral effects of toluene and other CNS depressants has not yet been determined.
In summary, this study presents initial evidence that repeated exposure to abuse patterns of high concentrations of toluene through inhalation can significantly alter spontaneous locomotor behavior in rats and the expression of these sensitivities appears to depend upon both the age and sex of the animal. Compared to adults, adolescent rats exhibit less sensitivity to the initial effects of toluene and show less sensitization with repeated exposure. The reasons for these differences are unclear. The sensitization theory proposes that sensitization represents a neural adaptation within dopaminergic reward pathways; hence, one possibility is that the decreased sensitization to the effects of toluene and other abused substances observed in adolescent animals may reflect developmental differences in the brain’s dopamine system, as previous research has shown that this system undergoes massive pruning and re-organization during adolescence [52,53]. Whether these early exposures result in enduring effects on behavior or brain development, however, remains to be determined. It is possible that repeated toluene exposure may produce long-lasting changes in brain development which may lead to brain systems being hypersensitive to toluene and other drugs of abuse. However, these results also indicate that further studies are necessary to evaluate neurochemical and metabolic activity in animals exposed to toluene to ascertain the degree to which these factors may play a role in the observed age-dependent differences in sensitivity. Finally, these results emphasize the importance of sex-related behavioral differences in response to toluene.
Acknowledgments
Research supported by National Institute on Drug Abuse grants DA-016644 (JLW), DA-019151 (SEB), and training grant DA-07027 (MJW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bale AS, Tu Y, Carpenter-Hyland EP, Chandler LJ, Woodward JJ. Alterations in glutamatergic and gabaergic ion channel activity in hippocampal neurons following exposure to the abused inhalant toluene. Neuroscience. 2005;130:197–206. doi: 10.1016/j.neuroscience.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 2.Beckstead MJ, Weiner JL, Eger EI, 2, Gong DH, Mihic SJ. Glycine and gamma-aminobutyric acid(A) receptor function is enhanced by inhaled drugs of abuse. Molecular pharmacology. 2000;57:1199–205. [PubMed] [Google Scholar]
- 3.Beyer CE, Stafford D, LeSage MG, Glowa JR, Steketee JD. Repeated exposure to inhaled toluene induces behavioral and neurochemical cross-sensitization to cocaine in rats. Psychopharmacology. 2001;154:198–204. doi: 10.1007/s002130000614. [DOI] [PubMed] [Google Scholar]
- 4.Bowen SE, Balster RL. A direct comparison of inhalant effects on locomotor activity and schedule-controlled behavior in mice. Experimental and clinical psychopharmacology. 1998;6:235–47. doi: 10.1037//1064-1297.6.3.235. [DOI] [PubMed] [Google Scholar]
- 5.Bowen SE, Batis JC, Paez-Martinez N, Cruz SL. The last decade of solvent research in animal models of abuse: mechanistic and behavioral studies. Neurotoxicol Teratol. 2006;28:636–47. doi: 10.1016/j.ntt.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Bowen SE, Hannigan JH. Developmental toxicity of prenatal exposure to toluene. The AAPS journal. 2006;8:E419–24. doi: 10.1007/BF02854915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowen SE, Mohammadi MH, Batis JC, Hannigan JH. Gestational toluene exposure effects on spontaneous and amphetamine-induced locomotor behavior in rats. Neurotoxicol Teratol. 2006 doi: 10.1016/j.ntt.2006.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowen SE, Wiley JL, Evans EB, Tokarz ME, Balster RL. Functional observational battery comparing effects of ethanol, 1,1,1-trichloroethane, ether, and flurothyl. Neurotoxicol Teratol. 1996;18:577–85. doi: 10.1016/0892-0362(96)00064-5. [DOI] [PubMed] [Google Scholar]
- 9.Brandon CL, Marinelli M, Baker LK, White FJ. Enhanced reactivity and vulnerability to cocaine following methylphenidate treatment in adolescent rats. Neuropsychopharmacology. 2001;25:651–61. doi: 10.1016/S0893-133X(01)00281-0. [DOI] [PubMed] [Google Scholar]
- 10.Brandon CL, Marinelli M, White FJ. Adolescent exposure to methylphenidate alters the activity of rat midbrain dopamine neurons. Biological psychiatry. 2003;54:1338–44. doi: 10.1016/s0006-3223(03)00787-x. [DOI] [PubMed] [Google Scholar]
- 11.Brouette T, Anton R. Clinical review of inhalants. The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2001;10:79–94. doi: 10.1080/105504901750160529. [DOI] [PubMed] [Google Scholar]
- 12.Brunell SC, Spear LP. Effects of acute ethanol or amphetamine administration on the acoustic startle response and prepulse inhibition in adolescent and adult rats. Psychopharmacology. 2006;186:579–86. doi: 10.1007/s00213-006-0380-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bushnell PJ, Evans HL, Palmes ED. Effects of toluene inhalation on carbon dioxide production and locomotor activity in mice. Fundam Appl Toxicol. 1985;5:971–7. doi: 10.1016/0272-0590(85)90178-2. [DOI] [PubMed] [Google Scholar]
- 14.Campbell JO, Wood RD, Spear LP. Cocaine and morphine-induced place conditioning in adolescent and adult rats. Physiology & behavior. 2000;68:487–93. doi: 10.1016/s0031-9384(99)00225-5. [DOI] [PubMed] [Google Scholar]
- 15.Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends in pharmacological sciences. 2004;25:273–9. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Cavender F. Aromatic Hydrocarbons. In: Clayton FCG, editor. Patty’s Industrial Hygiene and Toxicology. Wiley; New York: 1993. p. 1329. [Google Scholar]
- 17.Cha YM, Li Q, Wilson WA, Swartzwelder HS. Sedative and GABAergic effects of ethanol on male and female rats. Alcohol Clin Exp Res. 2006;30:113–8. doi: 10.1111/j.1530-0277.2006.00005.x. [DOI] [PubMed] [Google Scholar]
- 18.Craft RM, Leitl MD. Potentiation of morphine antinociception by pentobarbital in female vs. male rats. Pain. 2006;121:115–25. doi: 10.1016/j.pain.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Devaud LL, Alele P, Ritu C. Sex differences in the central nervous system actions of ethanol. Crit Rev Neurobiol. 2003;15:41–59. doi: 10.1615/critrevneurobiol.v15.i1.20. [DOI] [PubMed] [Google Scholar]
- 20.Forgie ML, Stewart J. Six differences in the locomotor-activating effects of amphetamine: role of circulating testosterone in adulthood. Physiology & behavior. 1994;55:639–44. doi: 10.1016/0031-9384(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 21.Gause EM, Mendez V, Geller I. Exploratory studies of a rodent model for inhalant abuse. Neurobehav Toxicol Teratol. 1985;7:143–8. [PubMed] [Google Scholar]
- 22.Hass U, Lund SP, Hougaard KS, Simonsen L. Developmental neurotoxicity after toluene inhalation exposure in rats. Neurotoxicol Teratol. 1999;21:349–57. doi: 10.1016/s0892-0362(99)00013-6. [DOI] [PubMed] [Google Scholar]
- 23.Hefner K, Holmes A. An investigation of the behavioral actions of ethanol across adolescence in mice. Psychopharmacology (Berl) 2007;191:311–22. doi: 10.1007/s00213-006-0646-2. [DOI] [PubMed] [Google Scholar]
- 24.Himnan DJ. Tolerance and reverse tolerance to toluene inhalation: effects on open-field behavior. Pharmacology, biochemistry, and behavior. 1984;21:625–31. doi: 10.1016/s0091-3057(84)80048-9. [DOI] [PubMed] [Google Scholar]
- 25.Hinman DJ. Biphasic dose-response relationship for effects of toluene inhalation on locomotor activity. Pharmacology, biochemistry, and behavior. 1987;26:65–9. doi: 10.1016/0091-3057(87)90535-1. [DOI] [PubMed] [Google Scholar]
- 26.Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–8. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- 27.Hougaard KS, Hass U, Lund SP, Simonsen L. Effects of prenatal exposure to toluene on postnatal development and behavior in rats. Neurotoxicol Teratol. 1999;21:241–50. doi: 10.1016/s0892-0362(98)00053-1. [DOI] [PubMed] [Google Scholar]
- 28.Laviola G, Adriani W, Morley-Fletcher S, Terranova ML. Peculiar response of adolescent mice to acute and chronic stress and to amphetamine: evidence of sex differences. Behavioural brain research. 2002;130:117–25. doi: 10.1016/s0166-4328(01)00420-x. [DOI] [PubMed] [Google Scholar]
- 29.Laviola G, Wood RD, Kuhn C, Francis R, Spear LP. Cocaine sensitization in periadolescent and adult rats. The Journal of pharmacology and experimental therapeutics. 1995;275:345–57. [PubMed] [Google Scholar]
- 30.Lorenzana-Jimenez M, Salas M. Behavioral effects of chronic toluene exposure in the developing rat. Neurotoxicol Teratol. 1990;12:353–7. doi: 10.1016/0892-0362(90)90054-g. [DOI] [PubMed] [Google Scholar]
- 31.Marjot R, McLeod AA. Chronic non-neurological toxicity from volatile substance abuse. Human toxicology. 1989;8:301–6. doi: 10.1177/096032718900800408. [DOI] [PubMed] [Google Scholar]
- 32.Moser VC, McDaniel KL, Phillips PM. Rat strain and stock comparisons using a functional observational battery: baseline values and effects of amitraz. Toxicology and applied pharmacology. 1991;108:267–83. doi: 10.1016/0041-008x(91)90117-w. [DOI] [PubMed] [Google Scholar]
- 33.Moy SS, Duncan GE, Knapp DJ, Breese GR. Sensitivity to ethanol across development in rats: comparison to [3H]zolpidem binding. Alcohol Clin Exp Res. 1998;22:1485–92. [PubMed] [Google Scholar]
- 34.Nakajima T, Wang RS. Induction of cytochrome P450 by toluene. The International journal of biochemistry. 1994;26:1333–40. doi: 10.1016/0020-711x(94)90175-9. [DOI] [PubMed] [Google Scholar]
- 35.Nakajima T, Wang RS, Katakura Y, Kishi R, Elovaara E, Park SS, Gelboin HV, Vainio H. Sex-, age- and pregnancy-induced changes in the metabolism of toluene and trichloroethylene in rat liver in relation to the regulation of cytochrome P450IIE1 and P450IIC11 content. The Journal of pharmacology and experimental therapeutics. 1992;261:869–74. [PubMed] [Google Scholar]
- 36.Niyomchai T, Akhavan A, Festa ED, Lin SN, Lamm L, Foltz R, Quinones-Jenab V. Estrogen and progesterone affect cocaine pharmacokinetics in female rats. Brain research bulletin. 2006;68:310–4. doi: 10.1016/j.brainresbull.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Palenicek T, Votava M, Bubenikova V, Horacek J. Increased sensitivity to the acute effects of MDMA (“ecstasy”) in female rats. Physiology & behavior. 2005;86:546–53. doi: 10.1016/j.physbeh.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 38.Pierce RC, Kalivas PW. Repeated cocaine modifies the mechanism by which amphetamine releases dopamine. J Neurosci. 1997;17:3254–61. doi: 10.1523/JNEUROSCI.17-09-03254.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rezvani AH, Levin ED. Adolescent and adult rats respond differently to nicotine and alcohol: motor activity and body temperature. Int J Dev Neurosci. 2004;22:349–54. doi: 10.1016/j.ijdevneu.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Riegel AC, Ali SF, French ED. Toluene-induced locomotor activity is blocked by 6-hydroxydopamine lesions of the nucleus accumbens and the mGluR2/3 agonist LY379268. Neuropsychopharmacology. 2003;28:1440–7. doi: 10.1038/sj.npp.1300193. [DOI] [PubMed] [Google Scholar]
- 41.Riegel AC, Ali SF, Torinese S, French ED. Repeated exposure to the abused inhalant toluene alters levels of neurotransmitters and generates peroxynitrite in nigrostriatal and mesolimbic nuclei in rat. Annals of the New York Academy of Sciences. 2004;1025:543–51. doi: 10.1196/annals.1316.079. [DOI] [PubMed] [Google Scholar]
- 42.Riegel AC, French ED. Abused inhalants and central reward pathways: electrophysiological and behavioral studies in the rat. Annals of the New York Academy of Sciences. 2002;965:281–91. [PubMed] [Google Scholar]
- 43.Ristuccia RC, Spear LP. Sensitivity and tolerance to autonomic effects of ethanol in adolescent and adult rats during repeated vapor inhalation sessions. Alcohol Clin Exp Res. 2005;29:1809–20. doi: 10.1097/01.alc.0000183010.72764.cd. [DOI] [PubMed] [Google Scholar]
- 44.Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- 45.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 46.SAMHSA. No NSDUH07-0315. U.S. Department of Health and Human Services; 2007. The NSDUH Report March 15, 2007 Patterns and Trends in Inhalant Use by Adolescent Males and Females: 2002-2005. [Google Scholar]
- 47.SAMHSA. Results from the 2002 National Survey on Drug Use and Health: National Findings (HTML) In: Rockville, editor. M Department of Health and Human Services. 2003. [Google Scholar]
- 48.SAMHSA. Results from the 2005 National Survey on Drug Use and Health: National Findings (HTML) In: Rockville, editor. M Department of Health and Human Services. 2006. [Google Scholar]
- 49.Sell SL, Dillon AM, Cunningham KA, Thomas ML. Estrous cycle influence on individual differences in the response to novelty and cocaine in female rats. Behavioural brain research. 2005;161:69–74. doi: 10.1016/j.bbr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 50.Sell SL, Scalzitti JM, Thomas ML, Cunningham KA. Influence of ovarian hormones and estrous cycle on the behavioral response to cocaine in female rats. The Journal of pharmacology and experimental therapeutics. 2000;293:879–86. [PubMed] [Google Scholar]
- 51.Sonner JM, Gong D, Li J, Eger EI, 2nd, Laster MJ. Mouse strain modestly influences minimum alveolar anesthetic concentration and convulsivity of inhaled compounds. Anesthesia and analgesia. 1999;89:1030–4. doi: 10.1097/00000539-199910000-00039. [DOI] [PubMed] [Google Scholar]
- 52.Spear L. Modeling adolescent development and alcohol use in animals. Alcohol Res Health. 2000;24:115–23. [PMC free article] [PubMed] [Google Scholar]
- 53.Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and biobehavioral reviews. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 54.Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Developmental psychobiology. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- 55.Spear LP, Varlinskaya EI. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Dev Alcohol. 2005;17:143–59. [PubMed] [Google Scholar]
- 56.Stohr T, Schulte Wermeling D, Weiner I, Feldon J. Rat strain differences in open-field behavior and the locomotor stimulating and rewarding effects of amphetamine. Pharmacology, biochemistry, and behavior. 1998;59:813–8. doi: 10.1016/s0091-3057(97)00542-x. [DOI] [PubMed] [Google Scholar]
- 57.Thomas PE, Bandiera S, Maines SL, Ryan DE, Levin W. Regulation of cytochrome P-450j, a high-affinity N-nitrosodimethylamine demethylase, in rat hepatic microsomes. Biochemistry. 1987;26:2280–9. doi: 10.1021/bi00382a031. [DOI] [PubMed] [Google Scholar]
- 58.Tropp J, Markus EJ. Sex differences in the dynamics of cue utilization and exploratory behavior. Behavioural brain research. 2001;119:143–54. doi: 10.1016/s0166-4328(00)00345-4. [DOI] [PubMed] [Google Scholar]
- 59.Varlinskaya EI, Spear LP. Ontogeny of acute tolerance to ethanol-induced social inhibition in Sprague-Dawley rats. Alcohol Clin Exp Res. 2006;30:1833–44. doi: 10.1111/j.1530-0277.2006.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Warren DA, Bowen SE, Jennings WB, Dallas CE, Balster RL. Biphasic effects of 1,1,1-trichloroethane on the locomotor activity of mice: relationship to blood and brain solvent concentrations. Toxicol Sci. 2000;56:365–73. doi: 10.1093/toxsci/56.2.365. [DOI] [PubMed] [Google Scholar]
- 61.Waxman DJ, Dannan GA, Guengerich FP. Regulation of rat hepatic cytochrome P-450: age-dependent expression, hormonal imprinting, and xenobiotic inducibility of sex-specific isoenzymes. Biochemistry. 1985;24:4409–17. doi: 10.1021/bi00337a023. [DOI] [PubMed] [Google Scholar]
- 62.Wiaderna D, Tomas T. Assessment of long-term effects of exposure to toluene based on the analysis of selected behavioral responses with particular reference to the ability to trigger behavioral hypersensitivity in rats. International journal of occupational medicine and environmental health. 2002;15:239–45. [PubMed] [Google Scholar]
- 63.Wiaderna D, Tomas T. Effects of repeated exposure to toluene or amphetamine on locomotor activity in rats. International journal of occupational medicine and environmental health. 2000;13:317–24. [PubMed] [Google Scholar]
- 64.Wiley JL, Fagalde RE, Buhler KG, LaVecchia KL, Balster RL. Evaluation of 1,1,1-trichloroethane and flurothyl locomotor effects following diazepam treatment in mice. Pharmacology, biochemistry, and behavior. 2002;71:163–9. doi: 10.1016/s0091-3057(01)00645-1. [DOI] [PubMed] [Google Scholar]
- 65.Wilkins-Haug L. Teratogen update: toluene. Teratology. 1997;55:145–51. doi: 10.1002/(SICI)1096-9926(199702)55:2<145::AID-TERA5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 66.Wood RW, Colotla VA. Biphasic changes in mouse motor activity during exposure to toluene. Fundam Appl Toxicol. 1990;14:6–14. doi: 10.1016/0272-0590(90)90226-a. [DOI] [PubMed] [Google Scholar]
- 67.Woolverton WL, Balster RL. Behavioral and lethal effects of combinations of oral ethanol and inhaled 1,1,1-trichloroethane in mice. Toxicology and applied pharmacology. 1981;59:1–7. doi: 10.1016/0041-008x(81)90446-4. [DOI] [PubMed] [Google Scholar]
- 68.Yavich L, Patkina N, Zvartau E. Experimental estimation of addictive potential of a mixture of organic solvents. Eur Neuropsychopharmacol. 1994;4:111–8. doi: 10.1016/0924-977x(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 69.Zambricki EA, Dalecy LG. Rat sex differences in anesthesia. Comp Med. 2004;54:49–53. [PubMed] [Google Scholar]


