Abstract
Spontaneous social coordination has been extensively described in natural settings but so far no controlled methodological approaches have been employed that systematically advance investigations into the possible self-organized nature of bond formation and dissolution between humans. We hypothesized that, under certain contexts, spontaneous synchrony -a well-described phenomenon in biological and physical settings- could emerge spontaneously between humans as a result of information exchange. Here, a new way to quantify interpersonal interactions in real time is proposed. In a simple experimental paradigm, pairs of participants facing each other were required to actively produce actions, while provided (or not) with the vision of similar actions being performed by someone else. New indices of interpersonal coordination, inspired by the theoretical framework of coordination dynamics (based on relative phase and frequency overlap between movements of individuals forming a pair) were developed and used. Results revealed that spontaneous phase synchrony (i.e., unintentional in-phase coordinated behavior) between two people emerges as soon as they exchange visual information, even if they are not explicitly instructed to coordinate with each other. Using the same tools, we also quantified the degree to which the behavior of each individual remained influenced by the social encounter even after information exchange had been removed, apparently a kind of social memory.
Keywords: Social memory, Interpersonal, Spontaneous Synchronization, Coupling, Emergence, Vision, Entrainment
INTRODUCTION
Social interactions represent a substantial portion of many daily activities in human populations. A common and well described consequence of this interpersonal activity is that an individual’s behavior, whether intentionally or not, is modified through interactions with others (Insel & Fernald, 2004). Thus, from the very first months of life, individuals live vicariously through one another adopting spontaneously, if only temporarily, a similar posture or tempo during a conversation with a peer, or imitating their favorite singer (e.g. Bernieri, Reznick, & Rosenthal, 1988; Condon & Sandler, 1974; McGarva & Warner, 2003; Meltzoff & Decety, 2003; Peery, 1980). Alterations of the individual and collective behaviors range from imitation and mimicry to spontaneous synchronization, and have been observed in groups varying in size from dyads to thousands of individuals (e.g. Barsalou, Niedenthal, Barbey, & Ruppert, 2003; Motter, Nishikawa, & Lai, 2003; Strogatz, 2003).
Synchronization is a form of spontaneous pattern formation that operates according to general principles of self-organization described by nonlinear dynamics (Haken, 1983; Nicolis & Prigogine, 1977). Although different processes can underlie synchronization (see Pikovsky, Rosemblum, & Kurths, 2001 & Strogatz, 2003 for reviews), spontaneous phase synchrony has been observed among very different entities in a broad range of physical, biological and social systems ranging from Josephson junctions (Tsygankov & Wiesenfeld, 2002) to fireflies (Winfree, 1967), sinoatrial pacemakers (Michaels, Matyas, & Jalife, 1987), columns in the visual cortex (Gray, Konig, Engel, & Singer, 1989) and firing neurons (Nunez, Panetsos, & Avendano, 2000). Following on Huygens’s analysis of two clocks synchronizing on a wall (Bennett, Schatz, Rockwood, & Weisenfield, 2002; Hugenii, 1673), many studies have since framed the problem of mutual synchronization in terms of a network of oscillators whose individual behavior is altered by nearest neighbor interactions (Bottani, 1996; Kuramoto, 1984; Pikovsky et al., 2001; Strogatz, 2003; Winfree, 1967, 1980).
The coordination dynamics of human brain and behavior has proven no exception to the principles of self-organized synchronization (Kelso, 1995; Fuchs, Kelso, & Haken, 1992; Kelso, Bressler, Buchanan, de Guzman, Ding, Fuchs, & Holroyd, 1992; Kelso, Fuchs, Lancaster, Holroyd, Cheyne, & Weinberg, 1998). For instance, experiments reveal that humans exchange information (uni- or multi-sensory in nature) to spontaneously coordinate and switch behavioral patterns (e.g. Kelso, 1984; Lagarde & Kelso, 2006). A common social illustration is the clapping of an audience where sometimes applause occurs in unison, with many individuals clapping as a single synchronized ensemble (Néda, Ravasz, Vicsek, & Barbarasi, 2000a, b). Mechanisms governing the phenomenon are highly context-dependent, even within the same audience and depend on whether people applaud in unison with or without music. From an experimental perspective, clapping in synchrony with the beat of the music is equivalent to intentional sensorimotor coordination with an external event, such as a metronome (Kelso, 1995). Several studies have employed the sensorimotor coordination paradigm to investigate interpersonal coordination dynamics for the case when an individual intentionally synchronizes his/her movements with another by means of visual information exchange (e.g. de Rugy, Salesse, Oullier, & Temprado, 2006; Oullier, de Guzman, Jantzen, & Kelso, 2003; Schmidt, Carello, & Turvey, 1990; Temprado, Swinnen, Carson, Tourment, & Laurent, 2003). In such studies however, it is not yet clear whether spontaneous social entrainment actually occurs, i.e., as a two-way interaction where people mutually influence each other, or whether one individual simply acts as a pacing stimulus or “driver” for the other (Kelso, DelColle, & Schöner, 1990). A different scenario, however, is characteristic of the end of a live performance when each individual applauds according to his/her preferred pace and in the absence of driving stimuli from the stage. Nonetheless, the audience will quickly and spontaneously entrain to a common rhythm such that everyone claps in unison. Note that at this moment, an individual’s clapping behavior is influenced solely by exchange of auditory (and possibly visual) information (Néda et al., 2000a,b)11. Individual entities communicating via a medium of information exchange constitutes a minimum requirement for self-organized coordination to emerge (Kelso, 1995; Winfree, 2002).
In spite of an abundant literature addressing social coordination, many questions remain regarding the nature of the behavioral and neural processes mediating the formation and dissolution of bonds between individuals and how such processes may be quantified (Balaban, 2004; Konner, 2004). Three major problems exist when trying to understand spontaneous synchronization in social settings.
The first is the challenge of complexity, both in terms of the large number of units to analyze (e.g. thousands of pairs of clapping hands, cf. Néda et al, 2000b) and/or the nature of the behavior itself (e.g. mother-infant synchronization, cf. Condon & Sandler, 1974). Such compositional and behavioral complexity has hindered experimental attempts to record and quantify both the individual and social dynamics. Even the reduction in dimensional complexity afforded in coordinated behavior can only go so far in elucidating the relationship between group behavior and the individual units of which it is composed.
Second, even when the source and nature of the coupling has been identified, it is difficult to manipulate experimentally relevant variables such as the coupling intensity (e.g. Néda et al., 2000b). Almost by definition, spontaneous behavior is not externally goal directed or explicitly controlled. Most of the results reporting unintentional synchronization in humans are based on observation and categorization methods that rely primarily on the experimenter’s appreciation of a given exemplar behavior rather than a quantitative measure of coupling and individual behavior (e.g. Barsalou et al., 2003; Condon & Sandler, 1974; but see Richardson, Marsh, & Schmidt, 2005).
A third problem comes from the possibility that any change in a person’s behavior induced by interacting with another may persist even after the encounter is over. We term this remnant of a prior social interaction social memory. Social memory implies at the very least, that the intrinsic parameters of the individual components have been altered by virtue of the social interaction. Social memory is thought to play an important role in human actions, and, to a larger extent, on the way we live (Insel & Fernald, 2004). A deeper understanding of social memory may ensue if one is able to quantify the strength and persistence of prior social influences on an individual’s behavior.
In the present study, we focus on a most basic unit of social interaction: a pair of individuals interacting via visual information exchange. Focusing on the dyad constitutes a crucial first step, allowing for experimental control of information exchange and a precise quantification of the nature and strength of the social interaction. Additionally, our paradigm circumvents some of the limitations of existing work on social coordination and provides a more ecologically valid methodology. Previous work (e.g. Schmidt & O’Brien, 1997) hinted at the emergence of spontaneous motor coordination between individuals but the authors explicitly instructed each member of the dyad to try and maintain their own rhythm (i.e. resist the interpersonal influence). Here, we turn the issue around and identify instead the coordinative patterns that emerge only as a function of visual information exchange. Pairs of participants execute rhythmic movements while in full view of each other’s and their own ongoing actions without any other additional task to perform (see Sebanz, Bekkering & Knoblich, 2006 for a review on joint actions).
We test the hypothesis that even without instructions to do so, spontaneous synchronization between partners will occur as soon as they are coupled visually while performing the rhythmic task. Further, we explore the possibility that once the visual coupling is removed, individual movements, although no longer synchronized, might remain influenced by the social encounter after it is over, thereby implicating memory as a distinguishing feature of human self-organizing systems. Just as kinematic studies have elucidated the neural basis of motor control (see contributions, e.g. in Latash & Lestienne, 2006) the present work sets the stage and provides new methods for neurophysiological investigations of social interaction. So far the latter have tended to assess the behavioral actions of pairs of individuals one at a time or through imitation after some delay. In many everyday social settings, as in the present paradigm, both members of a pair adjust in an ongoing fashion to the other’s behavior in real time. Thus, the present paradigm has genuine potential to expose the neural mechanisms of real time social coordination. A step in this direction has already begun with its replication while simultaneously recording brain activity of each member of the dyad (Tognoli, Lagarde, de Guzman & Kelso, 2007).
MATERIALS AND METHODS
Overview
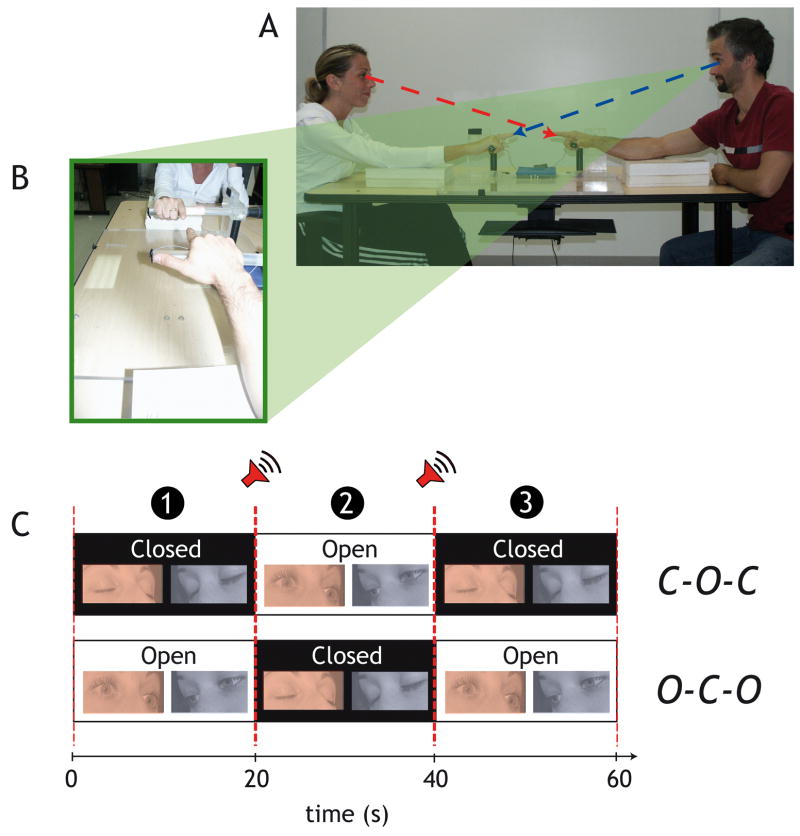
Pairs of participants, sitting in front of each other (see Figure 1A) executed rhythmic finger movements, each at their own preferred pace and amplitude and without the benefit of externally imposed pacing stimuli2. A trial was partitioned equally into three time-contiguous phases during which both participants either exchanged visual information or did not. The interaction was controlled by allowing (or not) visual contact between participants, coupling being mediated by the exchange of visual information regarding the other’s actions. When visual interaction was allowed, participants observed both their own motion and the motion of the other (Figure 1B).
Figure 1. Experimental set-up and design.
(A) Participants sat in front of each other and were instructed to look at each other’s finger when they executed the task with their eyes open. (B) Note that the experimental set-up allows participants to see the movements of their own finger as well as the movements of the other person sitting in front of them. (C) Detail of the experimental procedure in the O-C-O (Open-Closed-Open) and the C-O-C (Closed-Open-Closed) conditions.
Participants
Six pairs of participants (8 males and 4 females, pairs were either mixed or same gender) between the ages of 22 and 55 volunteered for the experiment. All participants (graduate students at Florida Atlantic University) provided informed consent and were naive to the purpose of the study. The experiment received full approval from the IRB of Florida Atlantic University. Our hypothesis states that visual coupling may induce participants to spontaneously synchronize their movements in space and time. The observations of spontaneous adjustments in oscillation frequency necessary to achieve interpersonal synchrony required forming pairs in which participants demonstrated different initial preferred movement frequencies. To do so, the preferred frequency of each participant (movement with eyes open and fixated on a stationary object) was recorded several days prior to the experiment. Pairs were then formed using individuals who differed in intrinsic frequency.
Setting, instructions and task
Two participants sat opposite each other and grasped a plastic dowel in a pronated (palms down) position with a 30 cm separation between their hands (Figure 1). Participants were instructed to move their right index finger up and down continuously at their preferred amplitude and frequency “as if they were going to do it all day”. It was emphasized to the subjects that the trials were to be performed without interrupting ongoing movement. No external metronome was used to pace the movements to prevent possible coordination with respect to the auditory signal rather than with the other member of the dyad (cf. Schmidt et al., 1990). No specific instructions were given as to how participants should move relative to each other. In addition, participants were told not to resist if they felt/realized that their coordination with respect to the other changed over a trial. Within each trial, participants alternated eyes open and eyes closed segments. Participants were further instructed to look at each other’s finger (and thereby their own, Figure 1B) during eyes open segments. To minimize distractions from the surroundings, large black panels were placed behind each participant.
Experimental conditions
Each condition lasted 1 minute and was divided into three 20s segments. Segments were defined by the presence or absence of visual contact which was controlled based on a set of instructions to participants to open or close their eyes. The beginning of each segment was signaled by an auditory beep. The order of presentation of visual information exchange (or the absence thereof) was alternated across trials resulting in two experimental conditions (see Figure 1C):
- Closed-Open-Closed C-O-C: Both participants eyes closed (0 to 20s) -- Both participants eyes open (20 to 40s) -- Both participants eyes closed (40 to 60s);
- Open-Closed-Open O-C-O: Both participants eyes open (0 to 20s) -- Both participants eyes closed (20 to 40s) -- Both participants eyes open (40 to 60s).
Compliance with the instruction to open or close the eyes was monitored by an experimenter hidden from the participants’ view. Both experimental conditions were executed 10 times by each pair of participants. The order of both the conditions and the trials was randomized.
Data acquisition
Finger movements were recorded on an OPTOTRAK 3010 (Northern Digital Inc., Waterloo, Ontario) 3D acquisition system using one infrared emitter (IRED) attached to the tip of the right index finger and three reference IREDS fixed to the supporting apparatus. The reference IREDs defined a vertical plane onto which the finger movements were projected. The projected angle formed by two vectors (the directed line from a reference point to the finger and another directed line from the same reference point to another reference point) was used as the measure of finger movements. Data were sampled at 120 Hz.
Data analysis
In coordination dynamics, the behavior of a given system can be captured by the value of low-dimensional collective variable known as the order parameter. In the vicinity of critical points, emergent behavior is governed by the dynamics of these collective variables (e.g., Haken, 1983; Kelso, 1995). In experimental cases the order parameters are not known in advance but have to be discovered. For the situation of biological coordination an appropriate order parameter describing the system dynamics is the relative phase φ between the elements to (be) coordinate(d) (Haken, Kelso & Bunz, 1985; Kelso, 1984).
The first quantity computed was the peak to peak relative phase (Kelso, 1984; Zanone & Kelso, 1992) between the index finger flexion-extension movements of participants A and B. The relative phase measure (φ) allows for a dimensional reduction of the system as it captures the macroscopic spatio-temporal behavioral pattern. Hence, four degrees of freedom (position and velocity of each component) are compressed onto a single value that summarizes the organization of the (un)coupled system formed by the dyad. Quantitative evaluation of spontaneous synchrony was also provided by the FFT power spectrum overlap (PSO) between the movements of both fingers. PSO measures the percentage of movement frequencies common to both partners in a pair. Defined as the area of intersection between each participant’s um overlap (PSO) between the movements of both fingers. PSO measures the percentage of movement frequencies common to both partners in a pair. Defined as the area of intersection between each participant’s um overlap (PSO) between the movements of both fingers. PSO measures the percentage of movement frequencies common to both partners in a pair. Defined as the area of intersection between each participant’s normalized spectral plots, the PSO is an indicator of the strength of the frequency entrainment between the two participants (Oullier, Bardy, Stoffregen, & Bootsma, 2002). Finally, a third measure, the peak frequency, defined as the frequency at the maximum of the (movement) FFT power spectrum, was computed for each participant in each segment of a trial.
The previously described analysis and the associated linear statistics were performed with Matlab (The Mathworks Inc, Natick, MA). Circular statistics (Batschelet, 1981) applied to the relative phase data were computed with Oriana (Kovach Computing Services, Pentraeth, Wales) and included Kuiper’s test to compare distributions of φ-values with the uniform distribution and Watson’s U2 to compare one distribution to another.
RESULTS
Interpersonal coordination pattern
Trajectory and relative phase
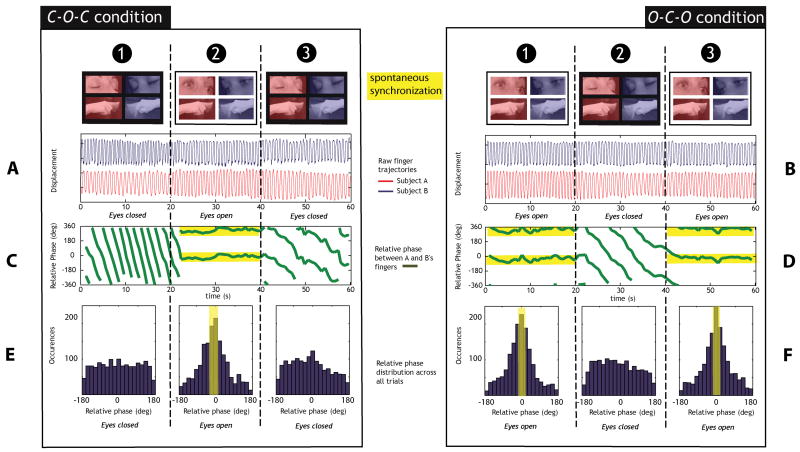
Evolution of the relative phase (between the movements of each individual of a pair) through the three segments of experimental trial indicates if and possibly when spontaneous synchronization emerges. Figure 2A shows the three segments of a Closed-Open-Closed (C-O-C) trial from a representative pair. The left, middle, and right columns (labeled 1, 2 and 3) plot the movement trajectories with the participants’ eyes closed, open, and closed again, respectively. When the eyes were closed (segment 1), each participant produced movements independently at his/her own frequency (Figure 2A, segment 1). Due to intrinsic frequency differences, the relative phase (φ) between the participants’ finger motions exhibited phase wrapping (Figure 2C, segment 1). Phase wrapping occurs when the components oscillate independently at different frequencies. In the first segment of a C-O-C trial, phase wrapping reveals the absence of synchronization as it indicates that individual behaviors are not coordinated.
Figure 2. Relative phase between the participants’ movements.
(A–D) Displacement of the index finger of both participants during representative trials in the (A) closed-open-closed C-O-C and (B) open-closed-open O-C-O conditions. (C–D) Peak-to-peak relative phase φ between the movements of the index finger of the participants during C-O-C (C) and O-C-O (D). (E–F) Distribution of all the relative phase φ-values in 20° bins across all pairs of participants (n=6) and all trials (10 per pair) during C-O-C (E) and O-C-O (F). The yellow overlays outline spontaneous synchronization.
Following a simple auditory cue to open their eyes and look at each other’s finger motion (while being in full view of their own movements), participants spontaneously adopted in-phase coordination illustrated by the peak extension and flexion of movements of participants occurring (more or less) at the same time (Figure 2A, segment 2). This is also indicated by φ stabilizing around 0° constituting a clear measure of their movements being coordinated in an in-phase fashion (Figure 2C, segment 2, yellow overlay). On a signal to close the eyes again, the frequencies diverged and φ fell back into phase wrapping (Figure 2C, segment 3) with movements of each participants no longer being in-phase (Figure 2A, segment 3). Similarly, spontaneous synchronized (in-phase) patterns also emerged during segments of the Open-Closed-Open (O-C-O) condition when participants had their eyes open (Figure 2D, segments 1 and 3).
These spontaneous behaviors during the eyes-open segments were very consistent in both C-O-C and O-C-O conditions as confirmed by the distributions of relative phase-values across all the trials (C-O-C: Figure 2E; O-C-O: Figure 2F). The distributions clearly exhibit a peak value of relative phase around 0°, revealing the spontaneous adoption of a 1:1 synchronized coordination pattern whenever eyes are open and participants are provided with vision of each other’s movements (Figure 2, yellow overlays). Table 1 provides a statistical quantification of the distributions of φ-values across all subjects in every segment of each experimental condition. In eyes closed segments, φ-values are more uniformly distributed compared to eyes open segments, regardless of the experimental condition (C-O-C or O-C-O). Statistical analyses also reveal a substantial decrease in the stability of the interpersonal coordination pattern spontaneously adopted (illustrated by the circular variance of the relative phase) for segments where eyes are open compared to closed. In addition, in the C-O-C condition, a significant difference of relative phase distributions is found when segments 1 (eyes closed) and 2 (eyes open; Watson’s U2 = 6.297, p< .001) and segments 2 (eyes open) and 3 (eyes closed; Watson’s U2 = 3.891, p< .001) are compared. Similarly, in the O-C-O condition, comparison of distributions in segments 1 (eyes open) and 2 (eyes closed; Watson’s U2 = 6.787, p< .001) and segments 2 (eyes closed) and 3 (eyes open; Watson’s U2 = 6.265, p< .001) are highly significant.
Table 1.
Circular statistics of relative phase for each segment of each experimental condition.
| C-O-C condition | O-C-O condition | |||||
|---|---|---|---|---|---|---|
| Segment 1 Closed | Segment 2 Open | Segment 3 Closed | Segment 1 Open | Segment 2 Closed | Segment 2 Open | |
| Circular Variance | 0.95 | 0.56 | 0.85 | 0.50 | 0.88 | 0.53 |
| Circular Standard Deviation | 142.18° | 73.53° | 110.94° | 67.88° | 117.49° | 70.55° |
| Kuiper’s Test (Uniform, V) | 2.074 | 12.108 | 4.314 | 13.765 | 3.739 | 12.706 |
| Kuiper’s Test (p) | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 |
Frequency overlap
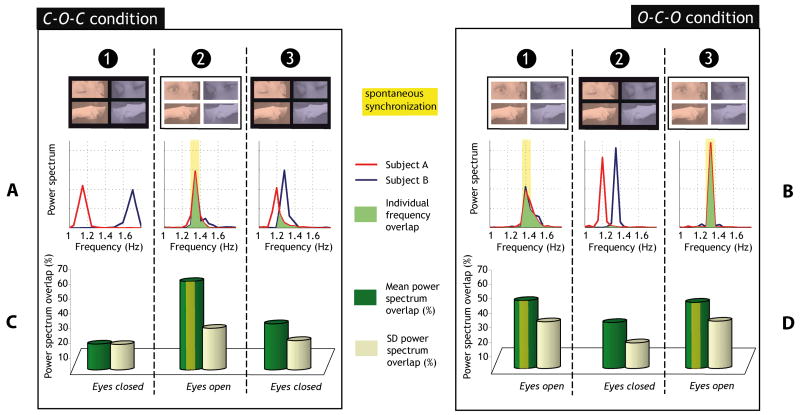
We used the power spectrum overlap (PSO) to gauge the relative strength of movement coordination frequency between the two participants during the eyes-open and eyes-closed segments. The PSO was significantly higher when there was visual exchange in both C-O-C (segment 2) and O-C-O (segments 1 and 3) conditions (Figure 3; see also Table 2 for detailed statistical comparisons). The power spectrum overlap was significantly greater during eyes open segments compared to eyes closed segments of the same condition. No differences were found when comparing between eyes open segments of the O-C-O condition (Table 2).
Figure 3. Frequency overlap between the participants’ movements.
(A–B) Representative trials for the C-O-C (A, same trial as Figures 1A and C) and the O-C-O conditions (B, same trial as Figures 1B and D). Each individual plot represents a 20s segment. Power spectra of the movements of each participant are plotted as well as the frequency overlap. (C–D) Mean and standard deviation of the power spectrum overlap, PSO, across all pairs of participants (n=6) and all trials (10 per pair) for the Closed-Open-Closed C-O-C (C) and the Open-Closed-Open O-C-O (D) conditions. The yellow overlays outline spontaneous synchronization.
Table 2.
Statistical comparisons (Wilcoxon tests) of the percentage of frequency overlap (PSO) between segments of experimental conditions.
| Segments compared | Z | p | Significance level |
|---|---|---|---|
| OCO_1_Open vs OCO_2_Closed | 2.35 | 0.018 | * |
| OCO_1_Open vs OCO_3_Open | 0.34 | 0.731 | ns |
| OCO_2_Closed vs OCO_3_Open | 2.76 | 0.005 | ** |
| COC_1_Closed vs COC_2_Open | 6.29 | 0.001 | ** |
| COC_1_Closed vs COC_3_Closed | 4.30 | 0.001 | ** |
| COC_2_Open vs COC_3_Closed | 5.61 | 0.001 | ** |
| OCO_2_Closed vs COC_1_Closed | 4.19 | 0.001 | ** |
| OCO_2_Closed vs COC_3_Closed | 0.09 | 0.926 | ns |
p < .05
p < .05
ns non significant
OCO: Open-Closed-Open condition
COC: Closed-Open-Closed condition
1, 2 or 3: Segment number within the condition
Open or Closed: Visual information exchange or not
Overall, relative phase and frequency overlap measures lead to the same conclusion: with visual information exchange, participants tend to mutually couple at a common phase and frequency, whereas in the absence of vision of each other’s hand movements, the movement trajectories diverge and behave independently. Importantly, no participant reported having intentionally tracked the finger movements of the other during the experiment. These results enable us to conclude that the coordination was an emergent behavior spontaneously brought about by information exchange. We note again that our results may be distinguished from previous dyadic studies in which one participant was explicitly instructed to track (or drive) the other (e.g. de Rugy et al., 2006; Oullier et al., 2003; Schmidt et al., 1990; Temprado et al., 2003) or to resist the mutual influence each member of the dyad exerted on the other (Schmidt & O’Brien, 1997).
Social memory
Our results might be considered a remarkable example of mutual entrainment between oscillators coupled through a medium of information exchange (Winfree, 2002). Such a view predicts that once the coupling is removed, each oscillator should return to its own intrinsic frequency and any influence of the interaction should disappear. The situation between two people, however, is not so generic. A closer look at the frequency distributions in the C-O-C condition reveals that participants do not revert to their initial ‘preferred’ frequency and may carry a memory of the previous rhythm (from hereon referred to as social memory), when visual exchange is removed.
To quantify this social memory effect, we analyzed the movement frequencies for the C-O-C condition in two ways.
Power spectrum overlap
First, using the power spectrum overlap, we measured the similarity of movement frequency produced by the members of the dyad before and after visual contact (i.e. between segments 1 and 3 of the C-O-C condition). The logic was that if the fingers were acting as classically coupled oscillators they should revert to their respective intrinsic behaviors after severing visual contact. Empirically, therefore, the resulting PSO should be identical for the two eyes-closed time segments of the C-O-C condition. In contrast, the movement frequencies of the members of the dyad showed significantly greater overlap after spontaneous coupling (PSO = 31.3% ± 19.6) than before (PSO = 17.6% ± 15.2). A statistical comparison between the PSO from the two eyes closed segments (1 and 3) of the C-O-C condition revealed significant differences in spite of the absence of visual exchange in both cases (Figures 3A; Table 2). Instead of returning to their preferred frequency following the removal of visual information, participants continued to be influenced by the previous coupled state.
This observation is corroborated by what happened during the second segment of the O-C-O condition (Figures 2D & 3D): the two eyes closed segments that followed eyes open ones (O-C-O segment 2 and C-O-C segment 3) revealed no significant difference in frequency overlap but significantly differed with the segment in which eyes closed did not follow visual exchange (C-O-C segment 1; see Table 2)3. Overall, the frequency overlap (PSO) provides a good quantitative measure of the ‘remnant of attraction’.
Peak movement frequency
Second, we tracked the peak movement frequencies as a participant traverses the three-time segments of the 60s trial. Direct comparison of the two eyes-closed segments of a C-O-C condition revealed a significant difference between pre- (segment 1) and post-coupling frequencies (segment 3), t(119) = 11.23, p< .001. After viewing each other’s finger movements, participants did not relax back to their initial frequency but adopted a new one as a result of their interaction. Effects of visually induced social coupling are also clear in the sequence of relative phase plots, where the moderating effect of the coupled phase-locking state on the previous phase wrapping behavior is expressed by a reduction of the slope of φ (compare segments 1 and 3 of Figure 2C) and the concentration values of the relative phase and its circular variance in each segment (cf. Table 1).
To investigate how long this remnant endures, we ran a simple ancillary experiment that systematically increased the length of the third segment of the C-O-C trial. Whether the third segment lasted 20, 30 or 60s, similar results were observed: participants did not relax back to their initial movement frequency as long as finger oscillations were executed. Moreover, participants consistently started the new trial moving at their previously determined preferred frequency. Hence, the social memory effect observed in the C-O-C trials appeared to disappear once the participants stopped moving or when a new trial began.
Dependence on initial conditions
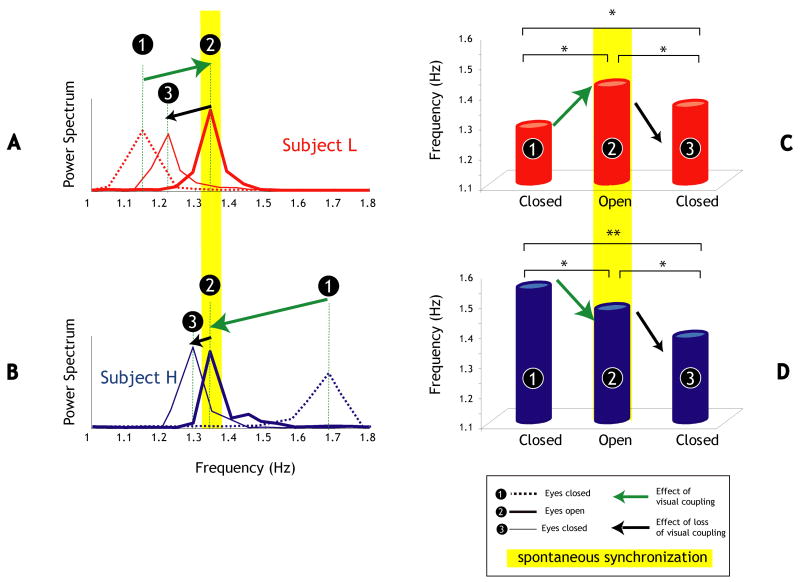
Based on initial frequency screening, participants could readily be identified as having the higher (H) or lower preferred frequency (L) of the pair. Sorting participants with respect to this criterion revealed an unexpected directionality effect that provided greater insight into how initial conditions, in this case initial preferred frequency (L or H), in part determines how the individual movement frequencies evolve throughout the trial. In 86.6% of trials, the participant with the lowest initial frequency of movement (L) increased his/her frequency when switching from eyes closed to eyes open (from segment 1 to segment 2; Figure 4C) whereas the one with the higher initial frequency (H) decreased in 75% of the cases, χ12 = 109.10, p < 0.01 (Figure 4D). When closing their eyes again (from segment 2 to 3), participants L slowed down toward their initial ‘intrinsic’ frequency (83.3% of the cases) and participants H also slowed down away from their own intrinsic frequency when vision was removed (75%; χ12 = 34.10, p < 0.01).
Figure 4. Directionality effect in peak frequency changes in the C-O-C condition.
Power spectrum of the movement of the participant with (A) the lowest (L) initial preferred frequency (red) and (B) the highest (H) initial preferred frequency (blue) for each of the three time segments. For both participants, the effects of opening and closing the eyes is illustrated by green and black arrows respectively. (C–D) Grand average of the peak frequencies for each kind of participant (L & H) in each time segment. T-test significance: * p < .05, ** p < .01.
During the C-O-C condition, there was therefore a different directionality effect in peak frequency change depending on whether a given member of the dyad initially had a higher (or lower) preferred frequency. Shifts in frequency observed across segments 1 and 3 of a C-O-C trial, resulted from participant L increasing frequency (78.3% of the cases) and participant H decreasing frequency (93.3%), as confirmed by a χ2 test (χ12 = 137.08, p < 0.01. These results were confirmed by computation of the average frequency for each participant (L and H) in each segment of the C-O-C condition (Figure 4C and D). Importantly, participants starting the trial with the higher initial movement frequency (H) were more affected by the interaction, as the difference between their initial and final frequencies was significantly higher (H; 0.21 Hz ± 0.12) than for participants who started with lower initial frequency (L; 0.11 Hz ± 0.09; see Figure 4).
DISCUSSION
The present research adopts the theoretical and experimental framework of coordination dynamics to investigate elementary forms of social interaction. A notable feature of this framework is its ability to uncover generic mechanisms common to different kinds of systems at different levels of observation. For instance, the same basic patterns of coordinated behavior and pattern dynamics (multistability, critical fluctuations accompanied by a temporary loss of stability, phase transitions, hysteresis and critical slowing down) have been observed within an individual as in studies of bimanual and single limb movement coordination, studies of sensorimotor coordination between an individual and the environment or between individuals (Jantzen & Kelso, 2007; Kelso, 1995; and Swinnen, 2002 for reviews). Here we investigate how the natural (uninstructed) social influence of one person on another evolves in real time and report two key findings: the first is that humans immediately and spontaneously coordinate their actions with each other when provided vision of the movements of the other’s hand together with their own. The second is that an individual’s intrinsic behavior is altered by the social interaction: that is, the effect of the previous social encounter persists when vision of the other’s movements is no longer available. Dynamical measures such as relative phase and power spectral overlap proved to be adequate quantifications of the spontaneous coupling between individuals, the transition to loss of entrainment and the persistence or ‘social memory’ of the encounter.
What features of the visual information exchange might have facilitated such spontaneous social coordination? Human movements can be affected unintentionally by the vision of an object oscillating in their environment as illustrated by experiments using a moving-room paradigm (e.g. Lee & Lishman, 1975; Oullier et al., 2002; Stoffregen, 1985). In addition, experimental data show that the mere observation of the movements of another person interferes with one’s execution of a similar action (Kilner, Paulignan & Blakemore, 2003). Interestingly, such interference is less noticeable when the movement being observed is generated by human-figured robots (see also Castiello, 2003). Recent work in our laboratory has examined the degree of coordination that occurs when a single individual performs the present task in front of a computer generated hand that moves along a sinusoidal or a pre-recorded realistic trajectory (de Guzman, Tognoli, Lagarde, Jantzen, & Kelso, 2005). Spontaneous synchronization was most likely when participants moved while viewing the computer-generated hand driven by a realistic trajectory. However, unlike the present results, synchronization was not observed in 100% of the trials and, when present, was supported by a significantly lower frequency overlap (de Guzman et al., 2005). One may invoke a one-way coupling to explain these latter findings, since —unlike the present work— the motion of the computer generated hand could not be influenced by the movement of the participant.
Taken together, the foregoing results suggest that biological relevance, and biological motion in particular, play a key role in the formation of social coupling. One explanation of our findings may be found at the neurophysiological level. For instance, some areas of the brain are known to be associated with the perception (but not the execution) of biological motion including the posterior superior temporal sulcus or STS (Allison, Puce, & McCarthy, 2000; Grèzes, Armony, Rowe, & Passingham, 2003; Grèzes, Fonlupt, Bertenthal, Delon-Martin, Segebarth, & Decety, 2001; Iacoboni, Molnar-Szakacs, Gallese, Buccino, Mazziotta, & Rizzolatti, 2005). STS is also known to be a source of afferent input for the so-called human “mirror system” (Rizzolatti & Craighero, 2004). Originally identified in monkeys, mirror neurons are discharging both when one performs a given action and sees the same action performed by someone else (Gallese, Fadiga, Fogassi, & Rizolatti, 1996). They have been located primarily in the ventral premotor cortex and the rostral region of the inferior parietal lobule (Rizzolatti & Craighero, 2004). The human mirror system constitutes a neural mechanism allowing matching between visual perception and the execution of a given action (Rizolatti, Fogassi, & Gallese, 2001) and may also provide a basis for understanding the intentions of others (Iacoboni et al., 2005). Since participants in our experiment were both producing and observing rhythmic coordination, it seems possible that the human mirror neuron system at least partially underlies the spontaneous coordination observed. This hypothesis is supported by the conclusions of a study that replicated our C-O-C condition while brain activity of each member of the dyad was recorded using a dual-EEG system (Tognoli et al., 2007). These authors identified a cortical rhythm that distinguished synchronized and unsynchronized interpersonal coordination whose topography is consistent with neuro-anatomical sources within the human mirror system. In addition, other neural systems are likely to be required for our task to be performed. Among them is the cerebellum which has been reported to play a key role in perceiving (Leube, Knoblich, Erb, Grodd, Bartels, & Kirchera, 2003) and timing one’s movements (Ivry & Spencer, 2004; Jantzen, Oullier, Marshall, Steinberg & Kelso, 2007).
A serendipitous finding in our study was the consistent and persistent influence of social interaction on subsequent rhythmic behavior despite the lack of information exchange between the pair. Such a finding suggests that the connectivity and dynamics of the network engaged in the generation of spontaneous rhythmical movement is modified by social interaction, and that this new organization is retained after the completion of the social visual exchange. Recent evidence in support of this hypothesis suggests that two people engaging in a common task share a representation of each other’s movement dynamics, including trajectory amplitude and frequency (Bosbach, Cole, Prinz, & Knoblich, 2005; Decety & Sommerville, 2003; Sebanz, Knoblich, & Prinz, 2005; see also Saxe, Jamal, & Powell, 2006). Dual-EEG measurement of people involved in a joint task revealed that a stimulus referring to someone else’s action elicited a similar electrophysiological response located in frontal sites as a stimulus referring to one’s own action (Sebanz, Knoblich, Prinz & Wascher, 2006). Sebanz and colleagues (2006) therefore provided evidence that individuals acting in a social context might form shared neurophysiological action representations. To some extent, such a (shared) representation may persist in working memory when vision is removed, i.e. when information exchange is no longer possible (Goldman, Levine, Major, Tank, & Seung, 2003; Seung & Chapman, 2003; Seung, Lee, Reis, & Tank, 2000). This notion is bolstered by evidence showing that observation of another person performing rhythmic movements generates a kinematically specific memory trace of the observed motions in primary motor cortex (Stefan, Cohen, Duque, Mazzocchio, Celnik, Sawaki, Ungerleider, & Classen, 2005). Moreover, representations at the neural level have been shown to be highly flexible and context-dependent (Jantzen, Steinberg, & Kelso, 2004; 2005), influenced both by environmental (Wheeler, Peterson & Buckner, 2000) and task demands (Oullier, Jantzen, Steinberg, & Kelso, 2005).
Clearly, the extent and duration of the carryover or remnant effects found here may reflect many factors, including the strength of the bond that is formed between people, place in the social hierarchy, the willingness of each participant to cooperate, gender differences, personality characteristics and the significance each participant attaches to the social encounter (Insel & Fernald, 2004). Our result showing that the initial conditions (who starts with the higher or lower preferred movement frequency) determines behavior after the social encounter is over hints that such issues may be precisely quantified in well-defined experimental situations such as those afforded by the present paradigm.
In conclusion, one may well ask why this kind of spontaneous interpersonal coordination occurs in the first place? Compelling examples stretching from human evolution through religious ritual and sports to political, war and economical strategy suggest that keeping together in time is one of the most powerful ways to create and sustain communities and communication (McNeill, 1995). Moreover, not moving in synchrony may be too costly for the dyad (see, e.g., Körding Fukunaga, Howard, Ingram, & Wolpert, 2004). Coordination dynamics serves as a natural framework for studying social and biological coordination in real time (Kelso & Engstrøm, 2006). Although observational methods have elucidated various forms of social behaviour (Condon & Sandler, 1974; Meltzoff & Decety, 2003), the present study offers a novel perspective and new metrics to explore systematically a fundamental form of human bonding (or lack thereof), and the self-organizing processes that underlie its persistence and change. In this respect it complements recent developments in several fields such as social cognitive neuroscience (e.g. Singer, Frith, & Wolpert, 2003; Sommerville & Decety, 2006). In addition, behavioural economics and game theory (e.g. Camerer, 1999, 2003; Sally, 2003), socio-economics (e.g. Vinkovic & Kirman, 2006) and neuroeconomics (e.g. Camerer, Lowenstein, & Prelec, 2005; Oullier & Kelso, 2006; Zak, 2004) could also benefit from this paradigm as decision-making is often studied in a disembodied fashion, i.e. with little consideration for the role played by the “bodily dimension of attraction~repulsion” in the way people decide to behave with respect to others.
Acknowledgments
Work supported by the National Institute of Mental Health (NIMH Grants MH42900 and MH01386 to J.A.S.K.). The preparation of this manuscript was supported by the Programme Initiative from the Fondation de l’Académie des Sciences (O.O.), the Enactive Interfaces European Network (IST contract #002114 to J.L.) and the National Institute of Neurological Disorders and Stroke Grant (NS48229-01A1 to J.A.S.K.). The authors would like to thank Craig Richter (Florida Atlantic University), Thomas Stoffregen (University of Minnesota) and Erwann Michel-Kerjan (The Wharton School of the University of Pennsylvania) for helpful discussions on early versions of this manuscript.
ABBREVIATIONS
- φ
Relative phase between the participants’ movements
- C-O-C
Closed-Open-Closed condition
- O-C-O
Open-Closed-Open condition
- PSO
Power Spectrum Overlap
- FFT
Fast Fourier Transform
Footnotes
A beautiful example of the same audience clapping in synchrony at two separate moments of a live musical show is seen in the world-famous New Year’s Concert given every year by the Vienna Philharmonic Orchestra in Austria while and after the Radetzky March by Johann Strauss Jr. is played. This is an unusual piece of classical music in which the conductor leads not only the orchestra but also the audience. Upon a visual cue from the maestro, the audience claps in synchrony with the music. This collective clapping is intentionally synchronized both with auditory and visual signals coming from stage. Although, at the end of the performance, pacing stimuli are no longer provided by the orchestra and conductor, the audience still applauds in unison. From an external point of view both modes of synchronized clapping might look similar, however they are governed by two different mechanisms: intentional and spontaneous synchronization, respectively.
This experimental feature is crucial since the presence of a metronome creates the possibility that the pair will synchronize primarily with the metronome and not necessarily spontaneously with each other (see Schmidt et al., 1990).
Although interesting, the latter result should be considered with caution since it reports a comparison bet ween two eyes closed segments from two different conditions.
References
- Allison T, Puce A, McCarthy G. Social perception from visual cues: Role of the STS region. Trends in Cognitive Science. 2000;4:267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Balaban E. Neurobiology - Why voles stick together. Nature. 2004;429:711–712. doi: 10.1038/429711a. [DOI] [PubMed] [Google Scholar]
- Barsalou LW, Niedenthal PM, Barbey AK, Ruppert JA. Social embodiment. Psychology of Learning and Motivations - Advances in Research and Theory. 2003;43:43–92. [Google Scholar]
- Batschelet E. Circular statistics in biology. New York: Academic Press; 1981. [Google Scholar]
- Bennett M, Schatz MF, Rockwood H, Wiesenfeld K. Huygens’s clocks. Proceedings of the Royal Society of London Series A - Mathematical Physical and Engineering Sciences. 2002;458:563–579. [Google Scholar]
- Bernieri FJ, Reznick JS, Rosenthal R. Synchrony, pseudosynchrony, and dissynchrony - Measuring the entrainment process in mother infant interactions. Journal of Personality and Social Psychology. 1988;54:243–253. [Google Scholar]
- Bosbach S, Cole J, Prinz W, Knoblich G. Inferring another’s expectation from action: The role of peripheral sensation. Nature Neuroscience. 2005;8:1295–1297. doi: 10.1038/nn1535. [DOI] [PubMed] [Google Scholar]
- Bottani S. Synchronization of integrate and fire oscillators with global coupling. Physical Review E. 1996;54:2334–2350. doi: 10.1103/physreve.54.2334. [DOI] [PubMed] [Google Scholar]
- Camerer CF. Behavioral economics: Reunifying psychology and economics. Proceedings of the National Academy of Science of the United States of America. 1999;96:10575–10577. doi: 10.1073/pnas.96.19.10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerer CF. Behavioral game theory: Experiments in strategic interaction. Princeton: Princeton University Press; 2003. [Google Scholar]
- Camerer CF, Loewenstein G, Prelec D. Neuroeconomics: How neuroscience can inform economics. Journal of Economic Literature. 2005;XLIII:9–64. [Google Scholar]
- Castiello U. Understanding other people’s actions: Intention and attention. Journal of Experimental Psychology: Human Perception and Performance. 2003;29:416–430. doi: 10.1037/0096-1523.29.2.416. [DOI] [PubMed] [Google Scholar]
- Condon WS, Sander LW. Neonate movement is synchronized with adult speech - Interactional participation and language acquisition. Science. 1974;183:99–101. doi: 10.1126/science.183.4120.99. [DOI] [PubMed] [Google Scholar]
- de Guzman GC, Tognoli E, Lagarde J, Jantzen KJ, Kelso JAS. Effects of biological relevance of the stimulus in mediating spontaneous visual social coordination. Society for Neuroscience: Abstract Viewer/Itinirary Planner, Program No. 867.21 2005 [Google Scholar]
- de Rugy A, Salesse R, Oullier O, Temprado JJ. A neuro-mechanical model for interpersonal coordination. Biological Cybernetics. 2006;94:427–443. doi: 10.1007/s00422-006-0059-7. [DOI] [PubMed] [Google Scholar]
- Decety J, Sommerville JA. Shared representations between self and other: A social cognitive neuroscience view. Trends in Cognitive Science. 2003;7:527–533. doi: 10.1016/j.tics.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Fuchs A, Kelso JAS, Haken H. Phase transitions in the human brain: Spatial mode dynamics. International Journal of Bifurcation and Chaos. 1992;2:917–939. [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119:593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Goldman MS, Levine JH, Major G, Tank DW, Seung HS. Robust persistent neural activity in a model integrator with multiple hysteretic dendrites per neuron. Cerebral Cortex. 2003;13:1185–1195. doi: 10.1093/cercor/bhg095. [DOI] [PubMed] [Google Scholar]
- Gray CM, Konig P, Engel AK, Singer W. Oscillatory responses in cat visual-vortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989;338:334–337. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- Grèzes J, Fonlupt P, Bertenthal B, Delon-Martin C, Segebarth C, Decety J. Does perception of biological motion rely on specific brain regions? Neuroimage. 2001;13:775–785. doi: 10.1006/nimg.2000.0740. [DOI] [PubMed] [Google Scholar]
- Grèzes J, Armony JL, Rowe J, Passingham RE. Activations related to “mirror” and “canonical” neurones in the human brain: An fMRI study. Neuroimage. 2003;18:928–937. doi: 10.1016/s1053-8119(03)00042-9. [DOI] [PubMed] [Google Scholar]
- Haken H. Advanced synergetics : Instability hierarchies of self-organizing systems and devices. Berlin: Springer-Verlag; 1983. [Google Scholar]
- Haken H, Kelso JAS, Bunz H. A theoretical-model of phase-transitions in human hand movements. Biological Cybernetics. 1985;51:347–356. doi: 10.1007/BF00336922. [DOI] [PubMed] [Google Scholar]
- Hugenii C. Horologium oscillatorium. Paris: Apud F. Muguet; 1673. [Google Scholar]
- Iacoboni M, Molnar-Szakacs I, Gallese V, Buccino G, Mazziotta JC, Rizzolatti G. Grasping the intentions of others with one’s own mirror neuron system. Public Library of Science Biology. 2005;3:529–535. doi: 10.1371/journal.pbio.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Fernald RD. How the brain processes social information: Searching for the social brain. Annual Review of Neuroscience. 2004;27:697–722. doi: 10.1146/annurev.neuro.27.070203.144148. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Spencer RMC. The neural representation of time. Current Opinion in Neurobiology. 2004;14:225–232. doi: 10.1016/j.conb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Jantzen KJ, Steinberg FL, Kelso JAS. Brain networks underlying human timing behavior are influenced by prior context. Proceedings of the National Academy of Science of the United States of America. 2004;101:6815–6820. doi: 10.1073/pnas.0401300101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen KJ, Steinberg FL, Kelso JAS. Functional MRI reveals the existence of modality and coordination-dependent timing networks. Neuroimage. 2005;25:1031–1042. doi: 10.1016/j.neuroimage.2004.12.029. [DOI] [PubMed] [Google Scholar]
- Jantzen KJ, Oullier O, Marshall M, Steinberg FL, Kelso JA. A parametric fMRI investigation of context effects in sensorimotor timing and coordination. Neuropsychologia. 2007;45:673–684. doi: 10.1016/j.neuropsychologia.2006.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen KJ, Kelso JAS. Neural coordination dynamics of human sensorimotor behavior: A Review. In: Jirsa VK, McIntosh AR, editors. Handbook on brain connectivity. Berlin: Springer-Verlag; 2007. pp. 421–462. [Google Scholar]
- Kelso JAS. Phase-transitions and critical behavior in human bimanual coordination. American Journal of Physiology. 1984;246:1000–1004. doi: 10.1152/ajpregu.1984.246.6.R1000. [DOI] [PubMed] [Google Scholar]
- Kelso JAS, DelColle J, Schöner G. Action-perception as a pattern formation process. In: Jeannerod M, editor. Attention and Performance XIII. Hillsdale, NJ: Erlbaum; 1990. pp. 139–169. [Google Scholar]
- Kelso JAS, Bressler SL, Buchanan S, Deguzman GC, Ding M, Fuchs A, et al. A phase-transition in human brain and behavior. Physics Letters A. 1992;169:134–144. [Google Scholar]
- Kelso JAS. Dynamic Patterns: The self-organization of brain and behavior. Cambridge: MIT Press; 1995. [Google Scholar]
- Kelso JAS, Fuchs A, Lancaster R, Holroyd T, Cheyne D, Weinberg H. Dynamic cortical activity in the human brain reveals motor equivalence. Nature. 1998;392:814–818. doi: 10.1038/33922. [DOI] [PubMed] [Google Scholar]
- Kelso JAS, Engstrøm DA. The complementary nature. Cambridge: MIT Press; 2006. [Google Scholar]
- Kilner JM, Paulignan Y, Blakemore SJ. An interference effect of observed biological movement on action. Current Biology. 2003;13:522–525. doi: 10.1016/s0960-9822(03)00165-9. [DOI] [PubMed] [Google Scholar]
- Konner M. The ties that bind - Attachment: the nature of the bonds between humans are becoming accessible to scientific investigation. Nature. 2004;429:705. doi: 10.1038/429705a. [DOI] [PubMed] [Google Scholar]
- Körding KP, Fukunaga I, Howard IS, Ingram JN, Wolpert DM. A neuroeconomics approach to inferring utility functions in sensorimotor control. Public Library of Science Biology. 2004;2:1652–1656. doi: 10.1371/journal.pbio.0020330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramoto Y. Chemical oscillations, waves, and turbulences. Berlin: Springer-Verlag; 1984. [Google Scholar]
- Lagarde J, Kelso JAS. Binding of movement, sound and touch: multimodal coordination dynamics. Experimental Brain Research. 2006;173:673–688. doi: 10.1007/s00221-006-0410-1. [DOI] [PubMed] [Google Scholar]
- Latash ML, Lestienne F, editors. Motor control and learning. Berlin: Springer-Verlag; 2006. [Google Scholar]
- Lee DN, Lishman JR. Visual proprioceptive control of stance. Journal of Human Movement Studies. 1975;1:87–95. [Google Scholar]
- Leube DT, Knoblich G, Erb M, Grodd W, Bartels M, Kircher TTJ. The neural correlates of perceiving one’s own movements. Neuroimage. 2003;20:2084–2090. doi: 10.1016/j.neuroimage.2003.07.033. [DOI] [PubMed] [Google Scholar]
- McGarva AR, Warner RM. Attraction and social coordination: Mutual entrainment of vocal activity rhythms. Journal of Psycholinguistic Research. 2003;32:335–354. doi: 10.1023/a:1023547703110. [DOI] [PubMed] [Google Scholar]
- McNeill WH. Keeping together in time. Cambridge: Harvard University Press; 1995. [Google Scholar]
- Meltzoff AN, Decety J. What imitation tells us about social cognition: a rapprochement between developmental psychology and cognitive neuroscience. Philosophical transactions of the Royal Society of London. Series B - Biological sciences. 2003;358:491–500. doi: 10.1098/rstb.2002.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels DC, Matyas EP, Jalife J. Mechanisms of sinoatrial pacemaker synchronization - A new hypothesis. Circulation Research. 1987;61:704–714. doi: 10.1161/01.res.61.5.704. [DOI] [PubMed] [Google Scholar]
- Motter AE, Nishikawa T, Lai YC. Large-scale structural organization of social networks. Physical Reviews E. 2003;68:036105. doi: 10.1103/PhysRevE.68.036105. [DOI] [PubMed] [Google Scholar]
- Néda Z, Ravasz E, Brechet Y, Vicsek T, Barabasi AL. The sound of many hands clapping - Tumultuous applause can transform itself into waves of synchronized clapping. Nature. 2000;403:849–850. doi: 10.1038/35002660. [DOI] [PubMed] [Google Scholar]
- Néda Z, Ravasz E, Vicsek T, Brechet Y, Barabasi AL. Physics of the rhythmic applause. Physical Review E. 2000;61:6987–6992. doi: 10.1103/physreve.61.6987. [DOI] [PubMed] [Google Scholar]
- Nicolis G, Prigogine I. Self-organization in non-equilibrium systems. New York: Wiley; 1977. [Google Scholar]
- Nunez A, Panetsos F, Avendano C. Rhythmic neuronal interactions and synchronization in the rat dorsal column nuclei. Neuroscience. 2000;100:599–609. doi: 10.1016/s0306-4522(00)00305-5. [DOI] [PubMed] [Google Scholar]
- Oullier O, Bardy BG, Stoffregen TA, Bootsma RJ. Postural coordination in looking and tracking tasks. Human Movement Science. 2002;21:147–167. doi: 10.1016/s0167-9457(02)00093-3. [DOI] [PubMed] [Google Scholar]
- Oullier O, de Guzman GC, Jantzen KJ, Kelso JAS. On context dependence of behavioral variability in inter-personal coordination. International Journal of Computer Science in Sports. 2003;2:126–128. [Google Scholar]
- Oullier O, Jantzen KJ, Steinberg FL, Kelso JAS. Neural substrates of real and imagined sensorimotor coordination. Cerebral Cortex. 2005;15:975–985. doi: 10.1093/cercor/bhh198. [DOI] [PubMed] [Google Scholar]
- Oullier O, Kelso JAS. Neuroeconomics and the metastable brain. Trends in Cognitive Sciences. 2006;10:353–354. doi: 10.1016/j.tics.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Peery JC. Neonate-Adult Head Movement - No and Yes Revisited. Developmental Psychology. 1980;16:245–250. [Google Scholar]
- Pikovsky A, Rosenblum M, Kurths J. Synchronization: A universal concept in nonlinear science. Cambridge: Cambridge University Press; 2001. [Google Scholar]
- Richardson MJ, Marsh KL, Schmidt RC. Effects of visual and verbal interaction on unintentional interpersonal coordination. Journal of Experimental Psychology-Human Perception and Performance. 2005;31:62–79. doi: 10.1037/0096-1523.31.1.62. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nature Reviews Neuroscience. 2001;2:661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annual Review of Neuroscience. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Sally D. Dressing the mind properly for the game. Philosophical transactions of the Royal Society of London. Series B - Biological Sciences. 2003;358:583–592. doi: 10.1098/rstb.2002.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, Jamal N, Powell L. My body or yours? The effect of visual perspective on cortical body representations. Cerebral Cortex. 2006;16:178–182. doi: 10.1093/cercor/bhi095. [DOI] [PubMed] [Google Scholar]
- Schmidt RC, Carello C, Turvey MT. Phase transitions and critical fluctuations in the visual coordination of rhythmic movements between people. Journal of Experimental Psychology: Human Perception and Performance. 1990;16:227–247. doi: 10.1037//0096-1523.16.2.227. [DOI] [PubMed] [Google Scholar]
- Schmidt RC, O’Brien B. Evaluating the dynamics of unintended interpersonal coordination. Ecological Psychology. 1997;9:189–206. [Google Scholar]
- Sebanz N, Knoblich G, Prinz WG. How two share a task: Corepresenting stimulus-response mappings. Journal of Experimental Psychology-Human Perception and Performance. 2005;31:1234–1246. doi: 10.1037/0096-1523.31.6.1234. [DOI] [PubMed] [Google Scholar]
- Sebanz N, Knoblich G, Prinz W. How two share a task: Corepresenting stimulus response mappings. Journal of Experimental Psychology: Human Perception and Performance. 2005;31:1234–46. doi: 10.1037/0096-1523.31.6.1234. [DOI] [PubMed] [Google Scholar]
- Sebanz N, Knoblich G, Prinz W, Wascher E. Twin peaks: An ERP study of action planning and control in coacting individuals. Journal of Cognitive Neuroscience. 2006;18:859–870. doi: 10.1162/jocn.2006.18.5.859. [DOI] [PubMed] [Google Scholar]
- Sebanz N, Bekkering H, Knoblich G. Joint action: bodies and minds moving together. Trends in Cognitive Sciences. 2006;10:70–76. doi: 10.1016/j.tics.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Seung HK, Chapman RS. The effect of story presentation rates on story retelling by individuals with Down syndrome. Applied Psycholinguistics. 2003;24:603–620. [Google Scholar]
- Seung HS, Lee DD, Reis BY, Tank DW. The autapse: A simple illustration of short-term analog memory storage by tuned synaptic feedback. Journal of Computational Neuroscience. 2000;9:171–185. doi: 10.1023/a:1008971908649. [DOI] [PubMed] [Google Scholar]
- Singer T, Frith C, Wolpert D. Decoding, imitating and influencing the actions of others: The mechanisms of social interaction. Philosophical transactions of the Royal Society of London. Series B - Biological sciences. 2003;358:429–602. [Google Scholar]
- Sommerville JA, Decety J. Weaving the fabric of social interaction: Articulating developmental psychology and cognitive neuroscience in the domain of motor cognition. Psychonomic Bulletin & Review. 2006;13:179–200. doi: 10.3758/bf03193831. [DOI] [PubMed] [Google Scholar]
- Stefan K, Cohen LG, Duque J, Mazzocchio R, Celnik P, Sawaki L, et al. Formation of a motor memory by action observation. Journal of Neuroscience. 2005;25:9339–9346. doi: 10.1523/JNEUROSCI.2282-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffregen TA. Flow structure versus retinal location in the optical control of stance. Journal of Experimental Psychology: Human Perception and Performance. 1985;11:554–565. doi: 10.1037//0096-1523.11.5.554. [DOI] [PubMed] [Google Scholar]
- Strogatz SH. Sync: The emerging science of spontaneous order. New York: Hyperion Press; 2003. [Google Scholar]
- Swinnen SP. Intermanual coordination: From behavioural principles to neural-network interactions. Nature Reviews Neuroscience. 2002;3:350–361. doi: 10.1038/nrn807. [DOI] [PubMed] [Google Scholar]
- Temprado JJ, Swinnen SP, Carson RG, Tourment A, Laurent M. Interaction of directional, neuromuscular and egocentric constraints on the stability of preferred bimanual coordination patterns. Human Movement Science. 2003;22:339–363. doi: 10.1016/s0167-9457(03)00049-6. [DOI] [PubMed] [Google Scholar]
- Tognoli E, Lagarde J, de Guzman GC, Kelso JA. The phi complex as a neuromarker of human social coordination. Proceedings of the National Academy of Science of the United States of America. 2007;104:8190–8195. doi: 10.1073/pnas.0611453104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsygankov D, Wiesenfeld K. Spontaneous synchronization in a Josephson transmission line. Physical Reviews E. 2002;66:036215. doi: 10.1103/PhysRevE.66.036215. [DOI] [PubMed] [Google Scholar]
- Vinkovic D, Kirman A. A physical analogue of the Schelling model. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:19261–19265. doi: 10.1073/pnas.0609371103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ME, Petersen SE, Buckner RL. Memory’s echo: Vivid remembering reactivates sensory-specific cortex. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11125–11129. doi: 10.1073/pnas.97.20.11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfree AT. Biological rhythms and behavior of populations of coupled oscillators. Journal of Theoretical Biology. 1967;16:15–42. doi: 10.1016/0022-5193(67)90051-3. [DOI] [PubMed] [Google Scholar]
- Winfree AT. The geometry of biological time. New York: Springer-Verlag; 1980. [Google Scholar]
- Winfree AT. On emerging coherence. Science. 2002;298:2336–2337. doi: 10.1126/science.1072560. [DOI] [PubMed] [Google Scholar]
- Zak PJ. Neuroeconomics. Philosophical transactions of the Royal Society of London, Series B - Biological Sciences. 2004;359:1737–1748. doi: 10.1098/rstb.2004.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanone PG, Kelso JAS. Evolution of behavioral attractors with learning - Nonequilibrium phase-transitions. Journal of Experimental Psychology: Human Perception and Performance. 1992;18:403–421. doi: 10.1037//0096-1523.18.2.403. [DOI] [PubMed] [Google Scholar]