Abstract
We assessed the relationship between grip preference and hand use in chimpanzees in 2 experiments. In experiment 1, we evaluated consistency in hand use and grip preference across 4 food types. The chimpanzees showed population-level right-handedness and there are significant positive associations for both hand and grip use across food types. In experiment 2, we assessed validity of hand use in relation to grip preference in 2 colonies of chimpanzees via the same methodology. Differences in hand preferences between colonies were associated with variation in the observed grip preferences. There was no evidence of rearing effects on handedness in either colony. We discuss the overall results in the context of the evolution of handedness in relation to increasing motor demands as manifest in variation on grasping behavior.
Keywords: Chimpanzee, hand preference, grip preference
There has been considerable historical (Ettlinger, 1988; Fagot and Vauclair, 1991; Marchant and McGrew, 1991; Warren, 1980) and contemporary (Corballis, 1992, in press; Hopkins and Cantalupo, in press; McGrew and Marchant, 1997; Palmer, 2002) debate regarding the presence or absence of population-level hand preferences in nonhuman primates and the factors that influence the expression of handedness in nonhumans. The question of whether nonhuman primates express population-level asymmetries is of considerable theoretical interest because of the alleged role of hemispheric specialization in the evolution of complex human behavior including tool-use, bipedalism and language (Bradshaw and Rogers, 1993). In the absence of a clear transition from no handedness to handedness throughout the evolutionary continuum, we can only postulate how handedness and hemispheric specialization evolved. Further understanding of factors that influence the expression of handedness in nonhuman primates is important to evaluate various models of the evolution of hemispheric specialization in primates.
The most commonly used measure of hand preference in nonhuman primates is simple reaching (Lehman, 1993). Despite numerous studies in a variety of nonhuman primate species, there is little evidence that it induces population-level handedness. However, recent studies suggested that posture can influence the expression of directional biases in hand use for simple reaching (Fagot and Vauclair, 1991; MacNeilage et al., 1987; Westergaard et al., 1998). In prosimians, preferential use of the left hand is increased when they adopt an upright versus a tripedal posture (Ward et al., 1993). This is also the case in some New and Old World monkeys and lesser apes (Hook-Costigan and Rogers, 1997; Olson et al., 1990). However, among great apes, there is increased use of the right versus the left hand when they adopt an upright posture (Hopkins, 1993; Hopkins et al., 1993; Olson et al., 1990, cf. De Vleeschouwer et al. 1995). Irrespective of directional differences between species, it seems clear that manipulating posture canalizes hand use and results in more robust manifestations of individual hand preference.
In addition to posture, at least in great apes, there is an association between grip preferences and hand use. Gorillas (Christel, 1994), orangutans (Christel, 1994) chimpanzees (Jones-Engel and Bard, 1995; Hopkins et al., 2002; Tonooka and Matsuzawa, 1995) and bonobos (Christel et al., 1998), all show greater use of the right hand when they use the thumb and index finger to grip versus other types of grasping techniques. The association between hand use and grip preference is significant from a comparative perspective because different species exhibit different types of grips according to the morphology of the hand, which has implications for predictions regarding hand preferences within a species. Grip preference is clearly an important factor in determining hand preferences, yet historically, it has been overlooked or ignored.
In order to further examine the association between grip preference and hand use in chimpanzees, we conducted 2 studies largely aimed at evaluating the consistency and generalizability of this effect. In previous studies on grip preference and hand use, no measures of consistency and reliability were reported within a sample of apes. Thus, it is not clear whether some of the effects were specific to certain contexts or test conditions at the time of testing. Hopkins et al. (2002) reported increased preferential use of the right hand in relation to thumb-index finger grasping for 2 types of foods (raisins and peanuts) in 140 chimpanzees. In the current study, we evaluated the consistency of this pattern of results by testing the same sample of subjects 2 years after the initial data were collected via 2 additional foods that differed in shape and texture. If hand use and grip preferences are stable and reliable, then we hypothesized that significant positive associations in hand use and grip preference would occur across 1) food types and 2) time periods. Additionally, we hypothesized that the chimpanzees would show increased preferential use of the right hand for thumb-index grips for all food types.
The second aim of the study was to evaluate the validity of the reported association between hand and grip preferences. In the previous study, Hopkins et al. (2002) noted population-level right handedness in chimpanzees, particularly for thumb-index grasping responses. The results were somewhat different from findings in another, relatively large (n = 105) sample of captive chimpanzees in which there was no evidence of population-level handedness (Tonooka and Matsuzawa 1995).Whether the differences, with respect to population-level handedness, reflect demographic aspects of the subject populations or differences in methodology is unclear. Therefore, in Experiment 2, we also assessed hand use and grip preferences in a sample of captive chimpanzees at The University of Texas M. D. Anderson Cancer Center (BASTROP) via the same methods as those employed by Hopkins et al. (2002). If the different results from Hopkins et al. (2002) and from Tonooka and Matsuzawa (1995) reflect methodological differences, then population-level handedness should be found in the second (BASTROP) colony of chimpanzees. Moreover, preferential use of the right hand should be greater when subjects employ a thumb-index grip compared to other types of grip preferences.
Combining the Yerkes and Bastrop data generated a reasonable sample size to evaluate the effect of certain subject variables—rearing history, sex, and offspring parity—on handedness: Rearing history is of particular theoretical interest because McGrew and Marchant (1997) suggested that population-level handedness is restricted to captive-born, human-raised chimpanzees, whereas Hopkins & Cantalupo (in press) have questioned their interpretation. Parity is also of theoretical interest because studies in human subjects have indicated that there are higher incidences of left-handedness in first-born and latter born individuals. Recently, Hopkins and Dahl (2000) reported a higher incidence of left-handedness among first and latter born chimpanzees for a task measuring coordinated bimanual actions. The higher incidence of left-handedness in birth order groups resembles a similar curvilinear association between parity and incidences of spontaneous abortions and stillbirths (Hopkins et al. 2000), which suggests that at least some manifestations of left-handedness in chimpanzees may be due to an unstable prenatal environment or to perinatal events, as has been argued for humans.
METHODS, EXPERIMENT 1
Subjects
Subjects were 147 captive chimpanzees (Pan troglodytes) at the Yerkes National Primate Research Center (YNPRC) of Emory University: 85 females and 62 males. Within the female sample, there were 53 mother-reared and 32 human-reared individuals. Within the male sample, there were 23 mother-reared and 39 human-reared individuals. Subjects ranged in age from 3 to 44 yr (Mean = 15.1, s.d. = 5.1).
Materials
We recorded hand use and grip morphology for simple reaching for 4 different foods: peanuts, raisins, stick pretzels, and M&M minis. The raisins were soft and sticky and ca. 13 × 10 × 6 mm. The raw peanuts were shelled and each kernel was divided along the natural fissure of the peanut. The shelled peanut halves were hard and smooth and ca. 15 × 9 × 5 mm. The pretzels were cylindrical and ca. 60 mm long and 4 mm in diameter. The M&M minis had a smooth candy coating and measured 9 mm in diameter and were ca. 3.5 mm thick.
Procedure
We conducted the experiment in the outside portion of the focal subject’s home cage. The outdoor cages of subjects at the YNPRC Main Center are 6 × 3 × 3 m and had with concrete flooring. The outdoor cages of subjects at the YNPRC Field Station were 50 × 50 m and had a natural dirt, grass, and bark surface.
To begin each trial, the experimenter threw a single piece of the target food into the focal subject’s home cage. The subjects were required to locomote to the food and to use a hand to bring the item to the mouth. Hand use and grip type were recorded for each discrete response until a total of 20 (pretzels and M&Ms) or 25 (peanuts and raisins) responses occured. We did not record trials in which the subject used the mouth to pick up the food item. Note that subjects were required to locomote ≥2 steps between each trial to ensure that the experimenter could not influence hand use by the position of the food and that another piece of food was not thrown into the cage until the prior piece had been consumed to ensure that each response was discrete. Accordingly, we secured independence of data points in the assessment of hand preference (Lehman, 1993; Hopkins, 1999; McGrew and Marchant, 1997).
Grip type was either thumb-index, middle-index, or single digit (Figure 1). Thumb-index gripping occurred when a subject abducted the thumb to the lateral side or tip of the index finger to secure the food item. A middle-index grip occurred when a subject grasped the food between the index and middle finger with the hand either prone or supine. Thumb-index and middle-index grip types were by far the most common, but occasionally subjects engaged in single digit responses, i.e., used one finger to press down hard enough on the food that it stuck while being taken to the mouth.
Fig. 1.

Three grip types of chimpanzees. Top: Thumb-index; Middle-index, Bottom: single digit.
To assess the consistency of hand use and grip type over time, a span of ca. 18–24 mo separated the collection of data on peanuts and raisins from the collection of data on M&Ms and pretzels. We recorded reaching data for peanuts and raisins between April and September, 2000. The presentation of either peanuts or raisins was counterbalanced across subjects. We recorded reaching data for the M&Ms and pretzels over the same months during 2002. We did not attempt to counterbalance the presentation of M&Ms or pretzels, however, all 20 responses were completed with one food type before introducing the other food type.
Data Analysis
For each subject, we tabulated the total number of left and right hand responses made for each grip type. We characterized handedness in 3 ways. First, we calculated an overall handedness index (SUM-HI) based on the total number of left- and-right hand responses when summed across the 4 food types. The number of left-hand responses was subtracted from the number of right-hand responses and divided by the total number of responses (R−L/R+L) to yield the overall handedness index. Secondly, for each subject, we calculated a handedness index (HI) for each food type following the same formula used to derive the SUM-HI score. Thirdly, we derived binomial z-scores based on the frequencies in hand use and used the values to classify subjects as left-handed, ambiguously-handed or right-handed. We classified subjects with z-scores either less than −1.95 or >1.95 as left- or right-handed, respectively. We classified subjects with z-scores between −1.95 and 1.95 as ambiguously-handed. When necessary, we performed analyses post hoc, via Tukey’s Honestly Significant Difference (HSD) with alpha at p < .05.
Results
Descriptive Statistics
To evaluate population-level handedness, we performed one-sample t-tests on the HI values for each food separately, as well as the overall SUM-HI score. There is significant population-level right-handedness for peanuts [t(144) = 4.07, p < .001], raisins [t(142) = 2.45, p < .02], pretzels [t(135) = 1.98, p < .05], and M&Ms [t(139) = 3.34, p < .001], and for the SUM-HI score [t(146) = 3.34, p < .001]. Although the mean HI scores varied between foods, a one-way repeated measures analysis of variance revealed no significant effect of food type on handedness.
Via the more traditional classification schema used in nonhuman primate handedness research (z-scores), there were 37 left-, 48 ambiguously- and 62 right-handed chimpanzees, a distribution that differs significantly from chance [χ²(2, N = 147) = 6.41, p < .05]. The number of right-handed chimpanzees is significantly higher than the number of left-handed ones [χ²(1, N = 99) = 6.31, p < .02], but did not differ from the number of ambiguously-handed subjects [χ²(1, N = 110)= 1.78, n.s.]. A chi-square test of independence revealed no interaction between rearing and hand preference and a borderline significant interaction between sex and hand preference [χ²(2, N = 147) = 4.90, p < .08]. For the males, the numbers of left-, ambiguously- and right-handed subjects are 20, 22, and 20 respectively. In contrast, the numbers of left-, ambiguously- and right-handed females are 17, 26, and 42, respectively. Thus, the females are slightly more right-handed than the males are.
Intercorrelations in Hand Use and Grip Morphology
The correlation coefficients for the handedness index values for each food type are in Table I. The correlation coefficients for the proportion of thumb-index responses for each food type are in Table II. For both sets of data, test-retest coefficients are positive and significant across the 4 foods, which suggests that hand use and grip preference are consistent across food types and time periods in the sample.
Table I.
Correlations between handedness index values across food types
p < .01.
Table II.
Correlations between percentage of thumb-index responses across food types
p < .01.
Age, Sex and Rearing Effects on Hand Use
For this analysis, the SUM-HI score and the absolute value of the SUM-HI score (ABS-SUMHI) were the dependent measures of interest. Sex and rearing history (mother-reared and human-reared) served as inter group independent variables. For both the SUM-HI score and the ABS-SUMHI score, there is no significant main effects or interactions. Pearson product moment correlations revealed a positive association between age and the ABS-SUMHI score (r = .207, df = 145, p < .02). Older subjects had stronger hand preferences than younger individuals did.
Grip Morphology and Hand Use
In this analysis, we summed the total number of left and right hand responses for each grip type for each subject. We then compared the values via a mixed-model ANOVA with hand (left, right) and grip type (thumb-index, index-middle and single digit) as repeated measures. Sex and rearing history served as inter group variables. We used the total number of responses, rather than responses for each food type, because the previous correlation analysis revealed that grip type is consistent across foods. Using the total number of responses increased statistical power by reducing the number of independent variables in the model.
The analysis revealed significant interactions between grip type and rearing history [F(2, 254) = 11.53, p < .001] and grip type and hand use [F(2, 254) = 4.00, p < .02]; the mean number of thumb-index, middle-index and single digit responses for subjects in each rearing condition are in Table III. Analysis post hoc revealed that human-reared subjects made more thumb-index responses than mother-reared subjects did. In contrast, mother-reared subjects made significantly more middle-index responses than human-reared subjects did. There is no significant rearing difference for the single digit responses. The chimpanzees made significantly more thumb-index responses with the right versus the left hand. There is no other significant difference between hands as a function of grip type. The consistency in the finding of an interaction between grip morphology and hand use is further highlighted in Table IV where in the mean number of right- and left-hand responses for thumb-index and middle-index responses are shown for each food type. We omitted single digit responses because they were so infrequent. For all 4 foods, the number of right-hand thumb-index responses is significantly higher than for the left hand. There is no significant difference for the middle-index responses for any of the foods.
Table III.
Mean number (and standard errors) of thumb-index and middle-index responses as a function of rearing history
| Mother-reared | Human-reared | |
|---|---|---|
| Thumb-Index | 22.53 (1.86) | 31.26 (1.86) |
| Middle-Index | 20.66 (1.82) | 11.44 (1.81) |
| Single Digit | 1.47 (.56) | 2.50 (.56) |
Note. Values in parentheses are the standard errors.
Table IV.
Mean number of left and right hand responses for thumb-index and middle-index grip types for each food
| Thumb-index |
Middle-index |
|||||
|---|---|---|---|---|---|---|
| Left | Right | t | Left | Right | t | |
| Raisins | 6.31 (.50) | 9.30 (.54) | 4.00 | 3.26 (.38) | 4.00 (.47) | 1.53 |
| Peanuts | 5.94 (.50) | 7.50 (.56) | 2.25 | 4.96 (.46) | 5.85 (.54) | 1.44 |
| Pretzels | 5.38 (.44) | 6.97 (.48) | 2.56 | 3.59 (.39) | 3.71 (.45) | 0.25 |
| M&M | 5.65 (.41) | 7.19 (.48) | 2.53 | 2.55 (.34) | 3.51 (.45) | 1.98 |
| Mean Total | 23.34 (1.67) | 30.37 (1.75) | 3.35 | 14.66 (1.36) | 17.75 (1.66) | 1.86 |
Discussion
The results are relatively straightforward. First, captive chimpanzees showed preferential use of the right hand for thumb-index versus middle-index and single-digit grips. The increased preferential use of the right hand for thumb-index responses is evident for 4 separate foods. Second, hand use and grip type were consistent across foods and time. Thus, individual differences in hand use and grip preferences are reliable and stable in chimpanzees.
The results from Experiment 1 are restricted to the Yerkes colony of chimpanzees and we sought to further examine whether the observed association between grip preference and hand use would generalize to a second colony of chimpanzees. If hand use in relation to grip type is not idiosyncratic to the Yerkes colony, then we expected that a second colony of chimpanzees should show a similar pattern of results.
METHODS, EXPERIMENT 2
Subjects
The first group of subjects comprised 135 chimpanzees at the Department of Veterinary Sciences of The University of Texas M. D. Anderson Cancer Center (BASTROP). There were 74 females and 61 males ranging from 6 to 40 yr (Mean = 20.1 years, s.d. = 11.55). They lived in a variety of social and physical settings including pairs in indoor-outdoor runs, small groups (n = 3 – 7) in Primadomes, and large, multimale–multifemale groups (n = 8 – 16) in outdoor corrals. The corrals are 22 m in diameter and contain grass ground cover, climbing structures and other movable enrichment objects; each had an indoor area (Riddle et al., 1982). The primadomes are 10.7 m in diameter and contained either grass, sand, or aspen chips as ground cover, and climbing structures and enrichment objects that were similar to those in the corrals. The indoor areas of the Primadomes are conventional indoor/outdoor runs totaling 2.4 × 6.1 × 2.4 m. Indoor/outdoor runs have concrete floors, raised resting boards, barred ceilings, some movable enrichment objects, and cinder-block walls. Of the 74 female subjects, 33 were mother-reared, 13 were nursery-reared, 26 were wild-caught, and the rearing histories of the remaining 2 females are unclear. Among the 61 males, 30 were mother-reared, 12 were nursery-reared, 16 were wild-caught, and 3 have unclear rearing histories.
The second group of subjects consisted of 144 chimpanzees housed at the Yerkes National Primate Research Center of Emory University. The data for the colony have been described by Hopkins et al., (2002), but some of the descriptive information from this study will be provided for comparison of the 2 chimpanzee colonies. Within the Yerkes sample, there were 81 females (38 mother-reared, 31 nursery-reared and 12 wild-caught) and 63 males (20 mother-reared, 40 nursery-reared and 3 wild-caught).
For both colonies, mother-reared chimpanzees had been reared by their biological mothers for >30 days. Nursery-reared subjects were brought to and raised in the nursery before they reached 31 days of age. Wild-caught chimpanzees were captured in Africa. Although their exact ages the time of capture is unknown, most were likely young individuals <5 yr old. In order to simplify data analysis, we categorized the small number of chimpanzees with unknown rearing histories as wild-caught because this was their most likely origin.
Procedure
We collected hand preference and grip morphology data via procedures essentially identical to those in Experiment 1. The only exceptions are that we collected data for only one food item (raisins) and ≥40 responses for each subject. On each trial, we threw a raisin into the subject’s home cage ≥3 m from it so that it had to locomote to the raisin, pick it up, and bring it to its mouth. When the chimpanzee acquired the raisin, the experimenter recorded both the hand and the grip type used to pick it up. We recorded only one reaching response each trial to assure independence of data points (McGrew and Marchant, 1997, cf. Hopkins, 1999). Thus, we threw individual raisins into enclosures and did not throw the next raisin until the subject retrieved the first. Subjects were required to locomote ≥3 strides between reaching responses to maintain postural readjustment between trials. We recorded hand use as left or right and grip type as thumb-index, middle-index, and single digit grips (Figure 1).
At Bastrop, testing occurred in the outdoor portion of the runs, Primadomes, and corrals, and no individuals were separated for testing purposes. Similarly, at Yerkes, we tested the chimpanzees in the outdoor corrals or outdoor enclosures and no animal was separated from its group for tests. We collected ≥40 responses from each subject with the range in responses between 40 and 77 for the sample (Mean = 52.65, s.d. = 5.85).
Data Analysis
We characterized handedness in two ways. First, we calculated an overall handedness index (SUM-HI) per the formula in Experiment 1. Second, we derived binomial z-scores based on the frequencies in hand use and used them to classify subjects as left-handed, ambiguously-handed, or right-handed. As in the first experiment, we categorized subjects with z-scores less than −1.95 as left-handed and subjects with z-scores >1.95 as right-handed. Subjects with z-scores between −1.95 and 1.95 are ambiguously-handed. Thirdly, we derived the proportions of thumb-index, middle-index and single digit responses by dividing the total number of responses for each grip type by the total number of responses. When necessary we performed analyses post hoc, via Tukey’s Honestly Significant Difference (HSD) with alpha at p < .05.
Results
Comparison of Handedness and Grip Preference between Colonies
Handedness
In the initial analysis, we evaluated colony differences in overall hand use. Via the SUM-HI value, there is a borderline significant difference between colonies for overall handedness [t(277) = 1.68, p < .10] with the handedness index score higher for YERKES (Mean = .109) versus BASTROP colony (Mean = .023). In terms of hand preference classifications, a chi-square test of independence failed to reveal a significant interaction between colony and hand preference distribution. In the BASTROP colony, there are 32 left-handed, 63 ambiguously-handed, and 40 right-handed subjects. In the YERKES colony, there are 26 left-handed, 62 ambiguously-handed and 56 right-handed subjects. Chi-square goodness-of-fit tests indicated that the number of ambiguously-handed subjects is significantly higher than the number of left-handed ones [χ²(2, N = 183) = 24.631, p < .01] and the number of right-handed subjects is significantly higher than the number of left-handed ones [χ²(2, N = 154) = 9.37, p < .01].
Grip Type
We compared the 2 colonies on grip type via a mixed-model ANOVA. For each subject, we calculated the percentage of thumb-index, middle-index and single digit responses and they served as repeated measures. Colony served as the inter group variable. There is a significant 2-way interaction between grip type and colony [F(2, 554) = 15.52, p < .001]. Analysis post hoc indicates that YERKES chimpanzees made more thumb-index and single digit responses than BASTROP chimpanzees did. In contrast, BASTROP chimpanzee made significantly more middle-index responses than YERKES chimpanzees did.
Grip Type and Hand Use
In the next analysis, we compared handedness as a function of grip type between the two colonies. For each grip type, we derived a handedness index following the formula on p. 264. The two indices served as repeated measures in a mixed-model analysis of variance. Colony served as the between group variable. The analysis revealed a significant main effect for grip type [F(1, 248) = 6.26, p < .01] with themean handedness score for thumb-index responses (Mean = .128) significantly higher than middle-index responses (Mean = .028). No other main effect or interactions are significant.
One problem with the analysis is the ability of a very small number of observations to contribute disproportionately to either handedness index value depending on the individual chimpanzee’s preferred grip type. Therefore, we performed the same analysis again, but selected only subjects from both colonies that exhibited ≥10 responses each for the thumb-index and middle-index grip types. The analysis revealed a significant main effect for grip type [F(1, 153) = 8.74, p < .001] and a borderline significant main effect for colony [F(1, 153) = 3.16, p < .08]. The mean handedness index for thumb-index and for middle-index responses for each colony are in Figure 2. For both colonies, the mean HI score is higher for thumb-index than for middle-index responses; however, YERKES chimpanzees had a higher handedness index score overall for both grip types than BASTROP subjects did.
Fig. 2.
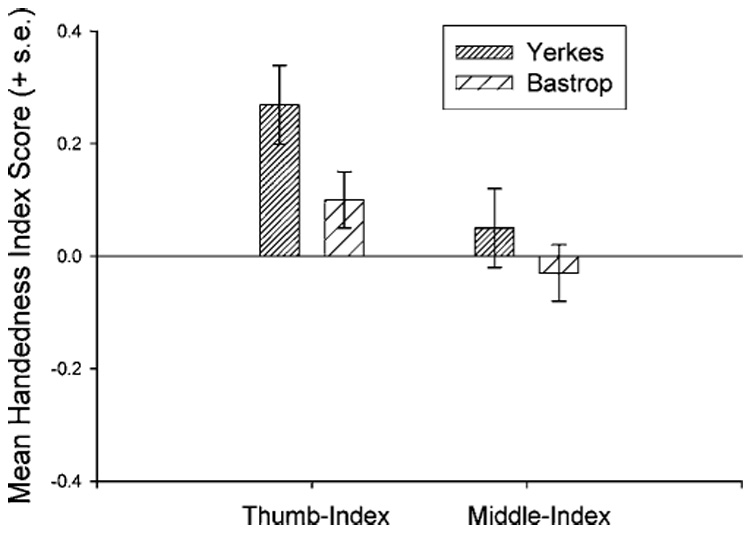
Mean handedness index scores (+s.e) for thumb-index and middle-index grips in YERKES and BASTROP chimpanzees.
Sex and Rearing Differences
For this analysis, the SUM-HI and percentage thumb-index responses served as dependent measures in a 2-factor ANOVA. Sex and rearing history (mother-reared, human-reared and wild caught) served as between group variables. There is no significant main effect or interactions. For the percentage of thumb-index responses, there is a significant 2-way interaction between sex and rearing history [F(2, 273) = 4.43, p < .02]. Analyses post hoc indicate that female nursery-reared subjects have a higher percentage of thumb-index responses than those of males and of females in all other rearing conditions.
Parity Effects
To evaluate whether birth order (or parity) had a significant effect on hand preferences for reaching, we performed an analysis using birth order and colony as grouping variables. To conserve statistical power, we recoded the literal birth orders of the chimpanzees (which ranged from 1 to 18) into 7 discrete categories including first, second, third, fourth, fifth, sixth, and seventh-plus. The seventh-plus group consisted of chimpanzees that had birth orders of 7 or higher. We used a birth order of 7 to define latter born subjects because the average number of offspring born to each female in the combined sample is 4.21, with a standard deviation of 2.67. Thus, subjects with birth orders of 7 or higher were one or more standard deviations from the mean number of births for all females.
We performed a 2-factor ANOVA with colony and birth order serving as the intra group variables. The SUM-HI score served as the dependent variable. There is a significant main effect for birth order [F(6, 190) = 2.34, p < .02]. The mean SUM-HI score as a function of birth order is in Figure 3. Analysis post hoc indicated that first-born chimpanzees have significantly lower SUM-HI scores than those of second-, third-, fourth-, and sixth-born subjects. In addition, seventh-plus-born subjects have significantly lower SUM-HI scores than those of fourth-born chimpanzees. The birth order effects did not significantly interact with the colony variable.
Fig. 3.
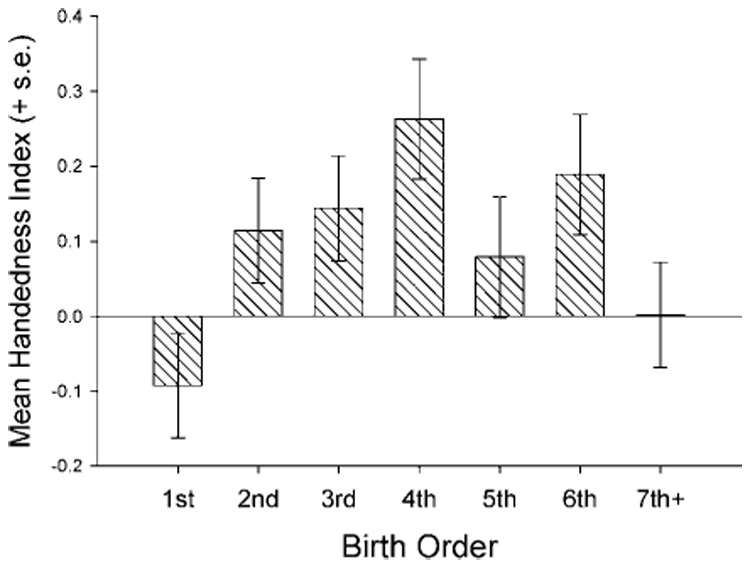
Mean handedness index scores (+s.e.) as a function of offspring parity.
Discussion
There are 4 main results in experiment 2. First, evidence of increased preferential use of the right hand for thumb-index responses was replicated in a second sample of chimpanzees. Second, there are colony differences in the proportion of thumb-index responses. Third, there is evidence of rearing differences in grip preference. Human-reared subjects, particularly females, showed a higher proportion of thumb-index responses than females and males in all other rearing conditions did. Fourth, parity had a significant effect on handedness with increased left-handedness in 1st-born chimpanzees versus ones with parities between 2 and 6.
The evidence of increased preferential use of the right hand for thumb-index responses in BASTROP chimpanzees is consistent with the data from YERKES chimpanzees as well as the chimpanzees studied by Tonooka and Matsuzawa (1995). Thus, the effect appears to be very robust and reliable across different colonies of chimpanzees. The overall handedness data from the YERKES colony differ slightly from the BASTROP and Tonooka and Matsuzawa (1995) chimpanzees, in that they were more right-handed subjects than in the other 2 colonies. The most parsimonious explanation for this finding is that the YERKES chimpanzees, as a whole, produced more thumb-index responses than the BASTROP chimpanzees and the chimpanzees studied by Tonooka and Matsuzawa (1995). Because increased preferential use of the right hand is associated with the use of thumb-index responses, it is not surprising that this sample of chimpanzees showed greater use of the right hand in simple reaching. Why the YERKES chimpanzees show higher rates of thumb-index responses is not clear. One explanation may be in differences in the type of substrate on which the food items were presented. Many of the chimpanzees tested at YERKES live in cages or compounds with cement floors, whereas many of the BASTROP apes live in grassy outdoor compounds. Grasping food off the hard cement floors may have increased the motor demands of the tasks, and therefore induced greater use of the thumb and index finger.
In terms of rearing and sex effects, the only significant effect was in the use of thumb-index responses with human-reared females making them proportionally more than males and females in all other conditions did. The origin of the effect is not clear because there is no reason a priori to assume that rearing would interact with sex to influence grip preference. Perhaps the human-reared apes are imitating the types of grips produced by humans around them, but we have no data to support this interpretation.
There is also evidence of increased left-hand use as a function of parity. First and 7th+ born subjects have lower handedness index scores versus subjects with parities between 2 and 6 (with the exception of 5). The results are consistent with previous findings in chimpanzees for a measure of coordinated bimanual actions (Hopkins and Dahl, 2000). There is evidence that birth order and maternal age or both influence handedness in humans (Searleman et al., 1989), and the pattern of results is similar to that reported here. Of course, the operational definition of latter-born subjects differs between species, with human latter-born individuals being typically defined as subjects with parities of either 3 or 4 and beyond. The value is higher in chimpanzees, at least based on the manner in which we defined it. Birth order per se is not the likely variable that causes increased incidences of left handedness in either chimpanzees or humans but instead maternal age or increased periparturitional stress associated with increasing maternal age or parity may be the underlying mechanisms that alter the development of handedness. As we have argued elsewhere (Hopkins et al., 2000), the evidence of birth order effects on handedness in chimpanzees are interesting because the data cannot be easily explained by nonbiological mechanisms, such as socio-economic status or other factors that have been used to explain findings in human subjects.
The evidence that grip type influences hand preference has potential implications for explaining discrepancies in findings on handedness between captive and wild chimpanzees. Some researchers have suggested that population-level handedness is restricted to captive populations (McGrew and Marchant, 1997; Palmer, 2002) and therefore is an artifact of being born and raised by humans. Others have argued that differences in the types of measures and how handedness is assessed is a more likely explanation for discrepancies between wild and captive ape populations (Hopkins, 1999; Hopkins and Cantalupo, in press). Although simple reaching has been studied extensively in wild and captive chimpanzees, as far as we know, no one has considered the potential influence of grip preference on hand use in wild apes. Grip preferences have been described for feeding in wild gorillas by Byrne et al. (2001) and nut-cracking in wild chimpanzees by Boesch and Boesch (1993), but it has not been explicitly assessed in the context of hand use for reaching or other motor actions. Studies in wild apes that assess the association between hand use and grip preference could provide for important parallels to studies in captive apes.
The extent to which variation in grip type influences other manifestations of handedness in relation to other tasks, such as tool-use or grooming, is also not clear. Specifically, Boesch and Boesch (1993) described the different kinds of grips used by apes when cracking open nuts. In many instances, they adopt a power grasp for nut-cracking. In contrast, there is little information on grip use for other forms of tool-use such as termite fishing but it is likely that the apes would be less prone to adopt a power grip but instead use some types of precision grip (Markze, 1997). Recent functional brain imaging studies in humans have shown that different neural systems and the degree of asymmetry expressed in hand use are different for power contrasted with precision grasping (Ehrsson et al., 2000). Although primarily contralateral hemisphere activation was more pronounced in relation to the preferred hand, power actions elicited larger activational asymmetries between hemispheres than precision grips. (Ehrsson et al. 2000). Accordingly, in chimpanzees, different degrees of asymmetry would be expressed for various kinds of tool-use based on the motor demands of the task. Presumably power actions would elicit stronger asymmetries at the individual and possibly specific level than precision grips would.
In summary, chimpanzees show increased preferential use of the right hand for grasping responses involving the thumb and index finger. This pattern of results is consistent across subjects using different foods and consistent across colonies of chimpanzees using the same food type. Grip type has seldom been considered in relation to hand preference in nonhuman primates and our results strongly suggest it is a critical factor. Whether the pattern in chimpanzees generalizes to other species warrants further investigation. The collective results suggest that selection toward increasing motor skill enhanced the expression of left hemisphere specializations in motor functions, including handedness (Hopkins and Russell, 2002). Which ecological factors selected for the increasing motor demands in chimpanzees is unclear, but tool-use is certainly a possibility. However, other factors may have been important, such as feeding or social functions such as grooming. Additional research on grip preference and hand use in different species should shed important light on the relation between the evolution of handedness in relation to motor functions associated with grasping in primates, including humans.
ACKNOWLEDGMENTS
The research was supported by NIH grants NS-36605, NS-42867, U42-RR-15090 and RR-00165 to the Yerkes National Primate Research Center or The University of Texas M. D. Anderson Cancer Center (UTMDACC). The Yerkes Center and the UTMDACC Department of Veterinary Sciences are fully accredited by the American Association for Accreditation of Laboratory Animal Care. American Psychological Association guidelines for the ethical treatment of animals were adhered to during all aspects of this study. We thank Dr. Marianne Christel for providing helpful comments on the paper. We thank Nicole Buehler, Margaret Remkus, and Amy McCrory for dedicated data collection at UTMDACC. Correspondence concerning this article should be addressed to Dr. William D. Hopkins, Division of Psychobiology, Yerkes National Primate Research Center, Emory University, Atlanta, Georgia, 30322. E-mail: lrcbh@rmy.emory.edu or whopkins@berry.edu
REFERENCES
- Boesch C, Bosech H. Different hand postures for pounding nuts with natural hammers by wild chimpanzees. In: Preuschoft H, Chivers DJ, editors. Hands of primates. New York: Springer-Verlag; 1993. pp. 31–43. [Google Scholar]
- Bradshaw J, Rogers LJ. The Evolution of Lateral Asymmetries, Language, Tool Use and Intellect. San Diego: Academic Press; 1993. [Google Scholar]
- Byrne RW, Corp N, Byrne JM. Manual dexterity in the gorilla: Bimanual and digit role differentiation in a natural task. Anim. Cognit. 2001;4:347–361. doi: 10.1007/s100710100083. [DOI] [PubMed] [Google Scholar]
- Corballis MC. The Lopsided Brain: Evolution of the Generative Mind. New York: Oxford University Press; 1992. [Google Scholar]
- Christel MI. Catarrhine primates grasping small objects: Techniques and hand preferences. In: Anderson JR, Roeder JJ, Thierry B, Herrenschmidt N, editors. Current Primatology vol IV: Behavioral Neuroscience, Physiology and Reproduction. Strasbourg: Universite Louis Pasteur; 1994. pp. 37–49. [Google Scholar]
- Christel MI, Kitzel S, Niemitz C. How Precisely do bonobos (Pan Paniscus) grasp small objects? Int. J. Primatol. 1998;19:165–194. [Google Scholar]
- Ehrsson HH, Fagergren A, Jonsson T, Westling G, Johansson RS, Forssbery H. Cortical activity in precision-versus power-grip tasks: An fMRI study. J. Neurophysiol. 2000;83:528–536. doi: 10.1152/jn.2000.83.1.528. [DOI] [PubMed] [Google Scholar]
- Ettlinger G. Hand preference, ability, and hemispheric specialization: How far are these factors related in the monkey? Cortex. 1988;24:389–398. doi: 10.1016/s0010-9452(88)80002-9. [DOI] [PubMed] [Google Scholar]
- Fagot J, Vauclair J. Manual laterality in nonhuman primates: A distinction between handedness and manual specialization. Psycholo. Bull. 1991;109:76–89. doi: 10.1037/0033-2909.109.1.76. [DOI] [PubMed] [Google Scholar]
- Hook-Costigan MA, Rogers LJ. Hand preferences in New World primates. Int. J. Com. Psychol. 1997;9:173–207. [Google Scholar]
- Hopkins WD. Posture and reaching in chimpanzees (Pan) and orangutans (Pongo) J. Com. Psychol. 1993;17:162–168. doi: 10.1037/0735-7036.107.2.162. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. On the other hand: Statistical issues in the assessment and interpretation of hand preference data in nonhuman primates. Int. J. Primatol. 1999;20:851–866. [Google Scholar]
- Hopkins WD, Dahl JF. Birth order and hand preference in chimpanzees (Pan troglodytes): Implications for pathological models of human handedness. J. Comp. Psychol. 2000;114:302–306. doi: 10.1037/0735-7036.114.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Russell JL. Further evidence of a right hand advantage in motor skill by chimpanzees (Pan troglodytes) Neuropsychologia. 2004;42:990–996. doi: 10.1016/j.neuropsychologia.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Dahl JF, Pilcher D. Birth order and left-handedness revisited: Some recent findings in chimpanzees (Pan troglodytes) and their implications for developmental and evolutionary models of human handedness. Neuropsychologia. 2000;38:1626–1633. doi: 10.1016/s0028-3932(00)00068-3. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Bennett A, Bales S, Lee J, Ward JP. Behavioral laterality in captive bonobos (Pan paniscus) J. Comp. Psychol. 1993;107:403–410. doi: 10.1037/0735-7036.107.4.403. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Cantalupo C. Does variation in sample size explain individual differences in hand preferences of chimpanzees (Pan troglodytes)? An empirical study and reply to Palmer. Am. J. Phys. Anthropol. 2002 doi: 10.1002/ajpa.10170. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Cantalupo C, Wesley MJ, Hostetter A, Pilcher D. Grip morphology and hand use in chimpanzees (Pan troglodytes): Evidence of left hemisphere specialization in motor skill. J. Experim. Psychol. Gen. 2002;131:412–423. doi: 10.1037//0096-3445.131.3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Engel LE, Bard KA. Precision grips in young chimpanzees. Am. J. Primatol. 1996;39:1–15. doi: 10.1002/(SICI)1098-2345(1996)39:1<1::AID-AJP1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Lehman RAW. Manual preference in prosimians, monkeys, and apes. In: Ward JP, Hopkins WD, editors. Primate Laterality: Current Behavioral Evidence of Primate Asymmetries. New York: Springer-Verlag; 1993. pp. 107–124. [Google Scholar]
- MacNeilage PF, Studdert-Kennedy MG, Lindblom B. Primate handedness reconsidered. Behav. Brain Sci. 1987;10:247–303. [Google Scholar]
- Marchant LF, McGrew WC. Laterality of function in apes: A meta-analysis of methods. J. Hum. Evol. 1991;21:425–438. [Google Scholar]
- Marzke MW. Precision grips, hand morphology and tools. Am. J. Phys. Anthropol. 1997;102:91–110. doi: 10.1002/(SICI)1096-8644(199701)102:1<91::AID-AJPA8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- McGrew WC, Marchant LF. On the other hand: Current issues in and meta-analysis of the behavioral laterality of hand function in nonhuman primates. Yearbook Phys. Anthropol. 1997;40:201–232. [Google Scholar]
- Olson DA, Ellis JE, Nadler RD. Hand preferences in captive gorillas, orangu-tans, and gibbons. Am. J. Primatol. 1990;20:83–94. doi: 10.1002/ajp.1350200203. [DOI] [PubMed] [Google Scholar]
- Palmer AR. Chimpanzee right-handedness reconsidered: Evaluating the evidence with funnel plots. Am. J. Phys. Anthropol. 2002;118:191–199. doi: 10.1002/ajpa.10063. [DOI] [PubMed] [Google Scholar]
- Searleman A, Porac C, Coren S. Relationship between birth order, birth stress, and lateral preferences: A critical review. Psychol. Bull. 1989;105:397–408. doi: 10.1037/0033-2909.105.3.397. [DOI] [PubMed] [Google Scholar]
- Tonooka R, Matsuzawa T. Hand preferences in captive chimpanzees (Pan troglodytes) in simple reaching for food. Int. J. Primatol. 1995;16:17–34. [Google Scholar]
- De Vleeschouwer K, Van Elsacker L, Verheyen RF. Effect of posture on hand preferences during experimental food reaching in bonobos (Pan paniscus) J. Comp. Psychol. 1995;109:203–207. doi: 10.1037/0735-7036.109.2.203. [DOI] [PubMed] [Google Scholar]
- Ward JP, Milliken GW, Stafford DL. Patterns of lateralized behavior in prosimians. In: Ward JP, Hopkins WD, editors. Primate Laterality: Current Behavioral Evidence of Primate Asymmetries. New York: Springer-Verlag; 1993. pp. 43–76. [Google Scholar]
- Warren JM. Handedness and laterality in humans and other animals. Physiol. Psychol. 1980;8:351–359. [Google Scholar]
- Westergaard GC, Kuhn HE, Suomi SJ. Bipedal posture and hand preference in humans and other primates. J. Comp. Psychol. 1998;112:56–63. doi: 10.1037/0735-7036.112.1.55. [DOI] [PubMed] [Google Scholar]


