Abstract
The vascular endothelial cell cadherin complex (VE-cadherin, α-, β-, and γ-catenin, and p120/p100) localizes to adherens junctions surrounding vascular endothelial cells and may play a critical role in the transendothelial migration of circulating blood leukocytes. Previously, we have reported that neutrophil adhesion to human umbilical vein endothelial cell (HUVEC) monolayers, under static conditions, results in a dramatic loss of the VE-cadherin complex. Subsequent studies by us and others (Moll, T., E. Dejana, and D. Vestweber. 1998. J. Cell Biol. 140:403–407) suggested that this phenomenon might reflect degradation by neutrophil proteases released during specimen preparation. We postulated that some form of disruption of the VE-cadherin complex might, nonetheless, be a physiological process during leukocyte transmigration. In the present study, the findings demonstrate a specific, localized effect of migrating leukocytes on the VE-cadherin complex in cytokine-activated HUVEC monolayers. Monocytes and in vitro differentiated U937 cells induce focal loss in the staining of VE-cadherin, α-catenin, β-catenin, and plakoglobin during transendothelial migration under physiological flow conditions. These events are inhibited by antibodies that prevent transendothelial migration and are reversed following transmigration. Together, these data suggest that an endothelial-dependent step of transient and focal disruption of the VE-cadherin complex occurs during leukocyte transmigration.
Keywords: adherens junctions, catenins, adhesion molecules, cell-to-cell interactions, recruitment
Introduction
Vascular endothelial-specific cadherin (VE-cadherin, cadherin-5) is a transmembrane protein that associates via its cytoplasmic tail with a number of cytosolic proteins (Geiger 1991; Dejana et al. 1995, Dejana et al. 1998; Dejana 1996; Lampugnani et al. 1995), including α-catenin, β-catenin, plakoglobin (γ-catenin), and p120/p100 (Dejana et al. 1995, Dejana et al. 1998; Staddon et al. 1995; Allport et al. 1997). These proteins, in turn, link VE-cadherin to the cytoskeleton. VE-cadherin appears to be critical for vascular structure assembly, as assessed in mouse embryoid bodies lacking VE-cadherin (Vittet et al. 1997). Other in vivo and in vitro studies have implicated the adherens junctions and, primarily, VE-cadherin, in the maintenance of endothelial integrity and control of leukocyte passage (Dejana et al. 1995, Dejana et al. 1998; Del Maschio et al. 1996; Gotsch et al. 1997; Matsuyoshi et al. 1997). Antibodies directed against VE-cadherin promoted an increase in neutrophil recruitment in a murine model of thioglycollate-induced acute peritonitis (Gotsch et al. 1997).
The adhesion of PMN (polymorphonuclear leukocytes) to the apical surface of endothelium previously has been shown to induce signals both in the leukocyte and the endothelial cell. Huang et al. 1993 described coupling of transmigration events to a transient increase in intracellular Ca2+ concentration ([Ca2+]i) within endothelium, and migration was inhibited by >90% after pharmacological chelation of [Ca 2+]i. Others have demonstrated that lymphocytes and monocytes induce similar increases in [Ca2+]i upon adhesion to vascular endothelium (Pfau et al. 1995; Ziegelstein et al. 1994). In addition, Yoshida et al. 1996 have reported that adhesion of leukocytes to activated endothelium induces transmembrane linkage of E-selectin to the cytoskeleton and subsequent rapid dephosphorylation of serine residues in its cytoplasmic domain (Yoshida et al. 1998). Further study (Hixenbaugh et al. 1997; Saito et al. 1998) has demonstrated that neutrophil transendothelial migration is associated with phosphorylation of endothelial cell myosin light chain kinase. These findings support the notion that leukocyte–endothelial adhesive interactions are a required prerequisite of transendothelial migration and may provide a model mechanism of signal transduction that facilitates leukocyte transendothelial migration.
In vivo and in vitro studies have indicated that neutrophil and monocyte transendothelial migration occurs generally between endothelial cell-to-cell lateral borders. Our working hypothesis is that stably adherent leukocytes that are spread on the apical surface induce endothelial-dependent changes in the lateral junctions that precede leukocyte passage and facilitate transmigration. Recent in vitro studies (Del Maschio et al. 1996; Allport et al. 1997) have demonstrated dissociation and degradation of the VE-cadherin complex during transendothelial migration of PMN under static conditions. Subsequently, Moll et al. 1998 used a lysis protocol that involved boiling in 1% SDS, followed by immunoblotting to detect changes in β-catenin and plakoglobin during neutrophil adhesion. Under the latter conditions, coincubation of endothelium and PMN did not alter the gross levels of these catenins (as reported earlier, Del Maschio et al. 1996; Allport et al. 1997), indicating that a nonphysiological degradation of the catenins by neutrophil proteases was occurring during postlysis analysis. An important caveat, however, is that these experiments were not performed under conditions of physiologically relevant flow, and there remains a lack of sensitivity to detect or visualize transient and/or focal changes in the VE-cadherin complex during leukocyte migration.
To avoid nonphysiological proteolysis and to better model the adhesion events seen in vivo, we adopted a method of analysis using a parallel plate flow chamber and then recovered the endothelial cell monolayers for immediate fixation and immunofluorescence staining. The current study also employed freshly isolated human monocytes or PBMC (peripheral blood mononuclear cells: monocytes, lymphocytes), which have significantly lower protease content as compared with human PMN. Further, the presence of lymphocytes in this model provided an internal control because lymphocytes do not transmigrate the HUVEC monolayer. A second strategy employed the U937 monocytic cell line stably transfected with L-selectin (U937L; Luscinskas et al. 1994), which adhere, but do not transmigrate 4 h TNF-α activated HUVEC, and differentiated U937L (U937L-Dif; Chuluyan and Issekutz 1993), which transmigrate within the same time frame (minutes) as human monocytes (Luscinskas et al. 1996).
Using this approach, we observed that monocytes or U937L-Dif, but not lymphocytes or U937L, induce focal disruption of the VE-cadherin complex only at sites of transmigration. This disruption can be inhibited by function blocking antibodies that prevent monocyte or U937L-Dif transmigration, but not by control antibodies. Further, the disruption of the VE-cadherin complex is reversible, in that once all monocyte migration has occurred, the VE-cadherin complex staining is restored. Taken together, these results demonstrate a direct and reversible effect of monocyte transendothelial migration on the VE-cadherin complex.
Materials and Methods
Materials
1 M Hepes solution, DPBS (Dulbecco's PBS), DPBS with Ca2+ and Mg2+ (DPBS+), M199, DME, and RPMI-1640 containing 25 mM Hepes were purchased from BioWhittaker Bioproducts. Recombinant hTNF-α (produced in Escherichia coli) was purchased from Genzyme and contained <10 pg/ml of endotoxin, as determined by the manufacturer. Human serum albumin (HSA; albuminate 25%, sterile and nonpyrogenic solution) was obtained from Baxter Healthcare Corp. All other chemicals were of the highest grade available from Baker Chemical.
Antibodies
The following murine mAbs have been reported previously (purified IgG): anti-CD18 (TS1/18, ATCC); anti-CD49d (anti–α4-integrin, HP2.1, IgG1) was purchased from Immunotech Inc.; anti–PECAM-1 (1.1, IgG2a; Liao et al. 1995); anti-MHC class I (W6/32, IgG2a; Luscinskas et al. 1995). Rabbit polyclonal IgG anti–PECAM-1 (Ab 177; Muller et al. 1993) and rabbit preimmune IgG were used in flow studies. Murine mAbs for immunofluorescence studies were used as purified IgG. Anti–VE-cadherin (clone TEA1/31, IgG1) was purchased from Immunotech Inc.; anti–β-catenin (IgG1) was purchased from Research Diagnostics Inc.; antiplakoglobin (clone PG5.1, IgG2b) was purchased from BioDesign International; anti–α-catenin (clone αCAT-7A4, IgG1) was purchased from Zymed Laboratories. Additionally, mouse mAb to VE-cadherin, (hec-1, IgG2a; Ali et al. 1997) was labeled directly with Oregon green 514 (Molecular Probes Inc.) and used for dual fluorescence Ag staining. Goat anti–mouse IgG Texas red conjugate and goat anti–mouse IgG CY3 conjugate were purchased from Caltag Laboratories. Anti-CD14–FITC conjugate (clone 3C10) has been reported previously (Muller et al. 1993) and anti-CD3–FITC was purchased from Sigma Chemical Co.
Cell Culture
Endothelial Cell Cultures.
HUVEC were isolated from 2–5 umbilical cord veins, pooled, and established as primary cultures in M199 containing 20% FCS (Hyclone; Luscinskas et al. 1989). HUVEC were serially passaged (1:3 split ratio) and maintained in M199 containing 10% FCS, endothelial cell growth factor, porcine intestinal heparin, and antibiotics. Experiments were performed at subculture 1 or 2 using 2-d postconfluent cells.
Leukocyte Cell Lines.
U937L (Luscinskas et al. 1994) and U937L-Dif cells were cultured in RPMI-1640 containing 10% FCS, 1 mM glutamine, and antibiotics, and serially passaged (1:20 split ratio) as required. U937L were differentiated by treatment of cell suspensions (0.3 × 106 cells/ml) with 1 mM dibutyryl cAMP in cell culture media for 72 h (Chuluyan and Issekutz 1993).
Isolation of Leukocytes
Human Peripheral Blood Mononuclear Cells.
PBMC were isolated from whole blood (normal volunteers) by centrifugation on Ficoll-Hypaque density gradients at 15°C (LSM, Organon Teknika). Contaminating RBCs were removed by hypotonic lysis. The percentage of monocytes and lymphocytes was generally 28% and 65%, respectively, as assessed by Wright-Giemsa, and contaminating cells were RBCs with <1% PMN.
Human CD4+ Lymphocytes.
PBMC, isolated as above (in RPMI-1640 containing 5% FCS) were incubated at 4°C for 2 h in the presence of anti-CD4–coupled beads (Dynal Inc.). CD4+ cells bound to the magnetic beads were removed by magnetic isolation and detached from the magnetic beads (DETACHaBEAD CD4, Dynal Inc.). The population was >99% CD4+ lymphocytes as determined by indirect immunofluorescence and flow cytometry.
Human Monocytes.
Monocytes were isolated from freshly drawn leukopacks resulting from platelet pheresis. PBMC, isolated as above, were subjected to counterflow centrifugal elutriation as described (Luscinskas et al. 1994). Monocyte fractions were >94% purity as determined by FACScan (CD14+, CD3−) and Wright-Giemsa, and >99% viable (Trypan blue).
Human Neutrophils.
PMN were isolated as described previously (Luscinskas et al. 1989). PMN were >95% pure as determined by Wright-Giemsa.
Flow Studies
The parallel plate flow chamber used for leukocyte adhesion assays has been described in detail (Shen et al. 1992; Luscinskas et al. 1994). Activated HUVEC monolayers (25 ng/ml TNF-α, 4 h) on 25-mm diam fibronectin-coated glass coverslips were placed in the flow chamber and PBMC (106 cells/ml) or monocytes (0.5 × 106/ml) were drawn across the monolayers for 5 min at 0.52 ml/min (estimated wall shear stress of 1.0 dynes/cm2). In some experiments, this was followed by 12 min of perfusion with buffer alone to allow adherent monocytes to complete the process of transmigration. Leukocyte–endothelial cell interactions were recorded live time by videomicroscopy and adhesion and transmigration determined from 4–8 high power (60× phase-contrast objective) fields. Transmigrated leukocytes were determined as being beneath the endothelial monolayer, i.e., in a different plane of focus, distinct from both adherent leukocytes and the endothelium. Coverslips were fixed immediately in ice-cold methanol at −20°C for 5 min or 2% paraformaldehyde at room temperature for 10 min, washed three times in DPBS+, and stained for VE-cadherin complex as described below. Control monolayers were activated with TNF-α for 4 h and perfused with buffer alone for 3–5 min in the flow chamber and processed in parallel.
Indirect Immunofluorescence Analysis of HUVEC Junctional Proteins
Fixed HUVEC monolayers were blocked with TBS, pH 7.4, containing 0.1 mg/ml salmon sperm DNA, 1% (vol/vol) horse serum, and 1% (vol/vol) goat serum (block) for 20 min at 37°C. Monolayers were then incubated for 45 min at 37°C with specific mAb (10–20 μg/ml in block), rinsed three times in DPBS+, and incubated with goat anti–mouse IgG Texas red/CY3 (1/100 dilution in block) for 45 min at 37°C. Coverslips were rinsed three times in DPBS+ and incubated with anti-CD3–FITC (1/50 dilution) or anti-CD14–FITC (5 μg/ml) for a further 30 min at 37°C, rinsed twice in DPBS+, once in dH2O, and mounted with Vectashield (Vector Labs). Stained HUVEC were visualized as follows: 1, an upright microscope (MicroFot FXA; Nikon, Inc.) equipped for fluorescence and a 20× phase objective. Images were captured using a cooled charged-coupled device (CCD) video camera (SenSys, Photometrics) as described previously (Allport et al. 1997). Exposures were matched in each case (typically 0.3–0.6 s). 2, confocal image analysis, images were captured using a laser scanning confocal microscope (BioRad MRC-1024/2P multiphoton interfaced with Zeiss Axiovert S100 microscope) equipped with a 63× water immersion objective. Serial 0.5-μm sections were taken routinely in the z direction.
Live-Time In Vitro Flow and Immunofluorescence Analysis
Activated HUVEC monolayers were preincubated on ice with 5 μg/ml anti–PECAM-1 antibody, P1.1, for 30 min, followed by 30 min with anti-mouse IgG Texas red. The monolayers were washed, placed in the flow chamber, and perfused with PBMC (5 × 105/ml) at 0.26 ml/min (estimated wall shear stress = 0.5 dynes/cm2) for 15 min. Leukocyte–endothelial interactions were recorded (40× objective) on videotape. At intervals during monocyte transmigration, live-time immunofluorescence images and corresponding phase-contrast micrographs were taken using a computer-controlled cooled CCD camera and Nikon TE-300 inverted microscope equipped for phase-contrast and fluorescence. The immunofluorescence and phase micrographs were subsequently overlaid in register using the Oncor Image software (see Fig. 2).
Figure 2.
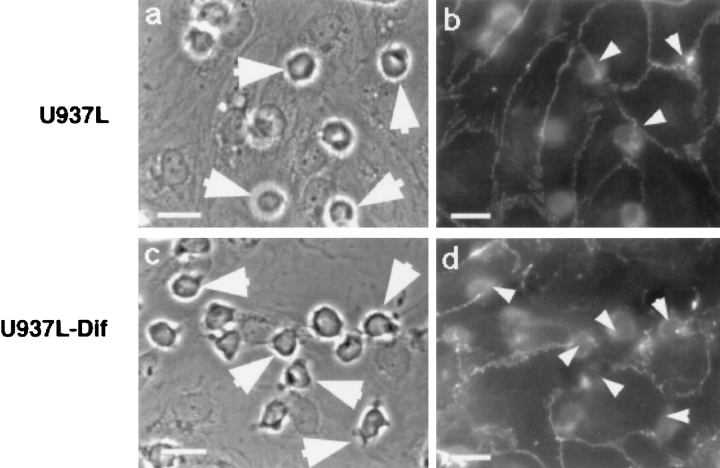
Adhesion and transmigration of U937L-Dif cells induces focal disruption of VE-cadherin staining under flow. U937L or U937L-Dif cells (106/ml) were perfused across 4 h TNF-α activated HUVEC monolayers for 10 min at 1.8 dynes/cm2. Coverslips were stained for VE-cadherin as described. In the presence of U937L cells (a and b) there was no detectable disruption of VE-cadherin staining (b), even where cells were aligned directly over a lateral junction (arrowheads). In contrast, in the presence of U937L-Dif cells (c and d), there was loss of VE-cadherin staining (d, arrowheads) in areas of leukocyte adhesion/transmigration. Bars, 12 μm.
Analysis of Changes in VE-Cadherin Complex Staining
Image Capture by CCD Camera.
Fluorescence (Texas red and FITC channels) and phase images of the same field were captured by photomicroscopy and CCD camera. The phase image was used to locate and quantify the number of adherent leukocytes. A two-color immunofluorescence staining protocol was used to identify changes in junctional staining (red) and simultaneously the number and location of adherent lymphocytes were determined (green FITC-labeled anti-CD3 mAb). The identity and location of monocytes (anti-CD3–FITC negative leukocytes) was then deduced. The phase-contrast, red, and green images for each field were pseudocolored (Oncor Image software) and then overlaid in register for direct analysis of the location of monocytes/lymphocytes in relation to disruption of VE-cadherin complex staining. A leukocyte was considered to be associated with an observed staining disruption, if it was located within one leukocyte diameter. Data were normalized to the total number of monocytes or lymphocytes per field because the range of adherent/transmigrated leukocytes per field varied due to monocyte string formation (Luscinskas et al. 1996; Lim et al. 1998).
Image Capture by Confocal Microscopy.
Serial z-sections of each field were captured sequentially using the same specifications and separate excitation wavelengths to prevent bleed-through between emission channels. This method allowed accurate detection of gaps in the VE-cadherin complex staining with no contribution from the FITC channel. CY3 labeled junctional proteins were excited at 568 nm and FITC-labeled monocytes were excited at 488 nm. The individual Z-series were subsequently rendered as a single two-color Z-series demonstrating the location of each leukocyte (x, y, and z) in relation to junctional staining using Confocal Assistant software (BioRad). Further manipulation of confocal Z-series was performed using NIH Image 1.62 software. This allowed us to isolate an area of interest from each Z-series and replot the data in an x-z direction. The new y-series was then collapsed to generate a single composite image.
Statistics
Adhesion and transmigration data were collected by ANOVA, and Student's two-sample t test was used to calculate statistical significance (Excel, Microsoft Corp). P values of <0.05 were considered significant.
Results
Monocytes Transmigrate at the Lateral Borders of Endothelial Cells Under Flow
Previous reports have indicated that leukocytes might migrate endothelium via either transcellular or paracellular routes. To study the adherens junctions during monocyte transendothelial migration, it was first necessary to establish that monocytes migrate via paracellular routes in our model. Using live-time immunofluorescence microscopy and the parallel plate flow chamber, we perfused PBMC across activated HUVEC monolayers that had been prestained with a nonblocking anti–PECAM-1 (P1.1, Liao et al. 1995) and secondary Texas red-labeled goat anti–mouse IgG, to visualize the staining pattern of PECAM-1 at the lateral junctions. There was no difference in the number of monocyte interactions or their rate of transmigration in the presence of the P1.1 and secondary antibodies when compared with media alone (data not shown). Phase-contrast and immunofluorescence images for each field were captured at intervals and later overlaid in register to produce photomicrographs (representative shown in Fig. 1, a–c). PECAM-1 staining in live HUVEC are depicted in red (Fig. 1 a), the corresponding phase image of adherent (Fig. 1 b, asterisk) and transmigrating (Fig. 1 b, arrows) monocytes is shown in Fig. 1 b, and the immunofluorescence and phase images were overlaid to generate Fig. 1 c. Quantitative measure showed that the majority of cells migrated between endothelial cells (80%, n = 3, three coverslips per experiment, total 22 fields). Hence, this model is appropriate to study the VE-cadherin complex components of the EC lateral junctions during transmigration of leukocytes.
Figure 1.
Live-time assessment of monocytes transmigration across activated HUVEC monolayers at lateral junctions. Activated HUVEC monolayers, preincubated with 5 μg/ml anti–PECAM-1 antibody, P1.1, and anti-mouse IgG Texas red were washed and then perfused with PBMC (5 × 105/ml) for 15 min. Live-time immunofluorescence images and corresponding phase-contrast micrographs were taken at intervals and subsequently overlaid in register. PECAM-1 staining is shown in red (a) and the corresponding phase image is shown in b. Actively migrating monocytes are indicated by arrows and a newly arrested monocyte is indicated by the asterisk. An overlay of the two images is indicated in c. Bars, 10 μm.
Adhesion and Transmigration of U937L-Dif cells, but Not U937L Cells, Under Flow Induces Loss of VE-Cadherin Staining
Significant numbers of U937L cells adhered to 4 h TNF-α activated HUVEC (400 ± 167 cells/mm2, n = 3), but few transmigrated the endothelial monolayer (≤4%). Similar numbers of U937L-Dif cells stably adhered to activated endothelium (290 ± 126 cells/mm2, n = 3) and by five minutes, approximately half of the adherent cells had transmigrated (138 ± 49 cells/mm2). This level of migration and the time course are similar to that observed for isolated blood monocytes as previously reported by us for this system (Luscinskas et al. 1996).
Coverslips from the experiments described were recovered and stained for VE-cadherin. Random fields were visualized under phase-contrast and fluorescence optics and the analysis of monolayers showed that U937L cells adhere to the endothelial surface, but remain round and appear unactivated (Fig. 2 a, arrows). Despite this level of U937L adhesion, essentially no disruption of the VE-cadherin staining was observed (Fig. 2 b), even when the U937L cells were located immediately over an endothelial tricellular corner (Fig. 2, arrowheads). By comparison, adherent U937L-Dif cells appear flattened and irregular (activated), and rapidly transmigrate the HUVEC monolayer (Fig. 2 c, arrows). U937L-Dif transmigration is accompanied by focal loss of VE-cadherin staining close to adherent/transmigrated U937L-Dif cells (Fig. 2 d, arrowheads). The U937L-Dif–dependent loss of VE-cadherin could be prevented by inhibiting migration using antibodies to PECAM-1, (rabbit polyclonal antibody, Ab 177). U937L-Dif cell transmigration was inhibited by 61% (138 ± 49 migrated cells/mm2, n = 3 in the presence of preimmune IgG, and 36 ± 20 cells/mm2 in the presence of Ab 177, n = 3, P < 0.05), whereas there was no effect on adhesion, as seen previously under static conditions (Muller et al. 1993; Liao et al. 1995, Liao et al. 1997). The presence of Ab 177 reduced U937L-Dif–dependent dissociation of VE-cadherin to a level equivalent to the baseline level seen with U937L cells (a decrease from 24.1 ± 5.7% to 5.4 ± 4.4%, n = 3, P < 0.05). One would not expect 100% of adherent/transmigrated U937L-Dif cells to be associated with VE-cadherin loss due to the fact that transmigration is not a synchronized event, but occurs continuously throughout the short perfusion period, and only ∼50% of the U937L-Dif cells transmigrate. At any given moment, therefore, only a percentage of adherent leukocytes are actively migrating and inducing changes in the VE-cadherin complex.
Taken together, these results indicate that in this model nonphysiological U937L cell-dependent degradation of the VE-cadherin complex does not occur and demonstrates that this is a valid method of investigating leukocyte-dependent changes in VE-cadherin complex. In addition, these data support the hypothesis that only leukocytes transmigrating the HUVEC are associated with focal changes and indicates that PECAM-1 engagement by leukocytes may play a role in capacitating the leukocyte at the apical lateral junction to induce changes in VE-cadherin or in signaling to the endothelium to disassemble the VE-cadherin complex.
Transendothelial Migration of Monocytes Induces Focal Changes in VE-Cadherin Complex
We next examined the effects of either isolated human peripheral blood monocytes or buffer alone on VE-cadherin staining, under flow. In the absence of leukocytes, perfusion of the monolayers with flow buffer does not alter the staining pattern of the VE-cadherin complex as assessed by laser scanning confocal microscopy (Fig. 3 a). The monolayer remains intact and staining for VE-cadherin, β-catenin, PECAM-1 (Fig. 3 a), or α-catenin (data not shown) is continuous at lateral junctions. In some areas there appear to be gaps in the staining when a single confocal Z-section is examined (identified by arrowheads), but the staining pattern of each protein is actually continuous when neighboring z-sections are included in the analysis. These data indicate that while the VE-cadherin complex forms a continuous staining band around the periphery of each cell, the band is not located within the same plane. These data are consistent with the predominant role of VE-cadherin complex in the maintenance of in vivo and in vitro barrier function (Breviario et al. 1995; Dejana 1996; Matsuyoshi et al. 1997; Dejana et al. 1998).
Figure 3.



Transendothelial migration of monocytes under flow in vitro induces focal changes in the VE-cadherin complex. Monocytes (0.5 × 106/ml) or buffer alone were perfused across activated HUVEC monolayers for 5 min at 1.0 dynes/cm2. The monolayers were recovered from the chamber and were immediately fixed in 2% paraformaldehyde (RT, 10 min) and stained for adherens junction proteins as described in Materials and Methods. Serial Z-sections were taken on the laser scanning confocal microscope where the CY3 and FITC channels were acquired sequentially using excitation wavelengths of 568 and 488 nm, respectively, to prevent bleed-through, and subsequently merged (a and b). Each series of panels (1–4) depicts sections from a Z-series from the apical (1) to the basolateral (4) surface. The Z-series was also projected in the x-z direction and shown at the bottom of each corresponding Z-series (Z). Selected images from b were further processed using NIH Image as described in Materials and Methods to generate c. a, Adherens junctional protein staining (VE-cadherin, left; β-catenin, middle; PECAM-1, right) is shown in the absence of monocytes. There were no detectable gaps in the VE-cadherin staining. Any apparent gaps in the staining pattern (arrowheads) were eliminated when neighboring sections were examined. b, In the presence of monocytes, gaps in the VE-cadherin staining pattern were seen where a monocyte was actively transmigrating the monolayer (arrow). There was no loss of staining where a monocyte was merely adherent to the apical surface of the monolayer (asterisk). Similarly, actively transmigrating monocytes induced loss of β-catenin staining (middle panels, arrows). In contrast, no detectable loss of PECAM-1 staining was observed in the presence of actively migrating monocytes. c, Selected regions (A, B, and C, bound by white lines) were projected in the x-z direction and collapsed to generate single images of VE-cadherin staining, β-catenin staining, and PECAM-1 staining in the presence of monocytes. Bars, 10 μm.
In contrast, gaps in the VE-cadherin complex staining were identified at locations of actively transmigrating monocytes. Using two-color immunofluorescence, VE-cadherin staining (red) was absent in sequential confocal z-sections where the monocytes (green) were actively transmigrating the endothelium (Fig. 3 b, arrows). The loss of staining was seen only in the immediate location of the migrating monocyte. The lack of yellow color indicated no colocalization of the monocyte (green) with VE-cadherin (red) staining and that there was simply a close apposition between the monocyte and the endothelial cell. This is further demonstrated when a selected region within panels 1–4 of Fig. 3 b is replotted in the x-z direction (Fig. 3 c, white lines, A). In addition, there was no loss of VE-cadherin staining if the monocyte was exclusively above (yet-to-transmigrate, identified by *) or below (completed transmigration) the endothelium (Fig. 3 b). This analysis suggested that disruption of the VE-cadherin complex occurs only during active transmigration, and that the complex quickly reseals following leukocyte passage. Subsequent analysis demonstrated a direct correlation between the timing of monocyte transmigration and the loss of VE-cadherin staining (see Fig. 5). Furthermore, β-catenin staining also was disrupted only in the presence of actively migrating monocytes (Fig. 3 b, arrows, and c, B), thus indicating that both components were absent from the lateral junctions in the region of transendothelial migration. For both VE-cadherin staining and β-catenin staining, 50% of adherent monocytes (total 133 monocytes, 18 fields (63×), four separate experiments) were actively transmigrating and, of the migrating cells, 90% were associated with loss of adherens junction staining.
Figure 5.
Monocyte-dependent loss of VE-cadherin is reversible over time. PBMC or isolated CD4+ lymphocytes were perfused across activated HUVEC as indicated. Leukocyte–endothelial interactions were recorded by live-time videomicroscopy and analyzed from at least six fields at 3, 7, and 12 min of perfusion. Total interacting cells were 436 ± 44, 770 ± 336, and 1,155 ± 353 cells/mm2, respectively. Fixed monolayers were stained for VE-cadherin and the number of monocytes or lymphocytes associated with loss of VE-cadherin were determined as described. The range of total leukocytes per field was 23–68 cells. Data are expressed as mean ± SD, n = 3 (three coverslips analyzed per experiment, three fields per coverslip). *Indicates significance when compared with the 3-min time-point. Transmigration data are shown as filled symbols (•, PBMC; ▪, CD4+ lymphocytes), VE-cadherin staining data are shown as open symbols (○, monocytes; □, CD4+ lymphocytes).
As a control, we examined PECAM-1 staining during monocyte transmigration under the same conditions. We found no discernible loss of PECAM-1 staining at the lateral junctions (Fig. 3 b). Of the fraction of monocytes (54%) that were actively transmigrating the endothelium, only 3% were associated with a gap in the PECAM-1 staining. There are a few caveats in this analysis, however, because both the endothelium and the monocytes express PECAM-1. Hence, staining of monolayers for PECAM-1 highlights not only the lateral junctions, but also the monocytes (Fig. 3b and Fig. c), resulting in a colocalization of PECAM-1 and CD14 (Fig. 3b and Fig. c, seen as yellow staining). In addition, as permeabilization of the monolayers with NP-40 was required for these analyses, intracellular PECAM-1 is detected also, resulting in a high background of staining in both the HUVEC and monocytes. Taken together, these findings indicate that only components of the adherens junction complex are lost during monocyte transmigration, perhaps due to their requirement as sealing elements, but recovered once the monocyte has completed transendothelial migration.
On the basis of the confocal data, demonstrating that the same data would be obtained using a single image or a Z-series, (VE-cadherin staining was absent from all z-sections in the location of actively transmigrating monocytes, Fig. 3 b), we performed further analysis of stained monolayers using the fluorescence microscope and CCD camera. In addition, we made use of PBMC instead of purified monocytes, which allowed us to observe the effect of lymphocyte adhesion on the VE-cadherin complex also. Lymphocytes do not transmigrate HUVEC monolayers in our hands under these conditions and served as an internal control.
Activated HUVEC monolayers were perfused with PBMC (106/ml, 5 min, 1.0 dynes/cm2) and then stained for either VE-cadherin, β-catenin, α-catenin, plakoglobin, or PECAM-1, or VE-cadherin and β-catenin together, as described in Materials and Methods. Lymphocytes were identified as CD3+ staining cells, and monocytes were inferred as CD3− cells. Immunofluorescence staining was visualized and recorded using the fluorescence microscope and CCD camera. Under these conditions, >40% interacting monocytes (adhesion vs. transmigration cannot be distinguished at the magnification used in this assay) were associated with loss of each VE-cadherin complex component (Table ). This was similar to the data obtained using confocal microscopy (see above). Double staining of the same monolayers for β-catenin and VE-cadherin indicated that transmigration of monocytes induced simultaneous loss of VE-cadherin and β-catenin at the site of adhesion/transmigration. For VE-cadherin or β-catenin alone, 40% of leukocytes were associated with loss of staining (similar to the percent of monocytes actively migrating above; Table , n = 4), and all leukocyte-dependent loss of VE-cadherin was also associated with loss of β-catenin. We conclude that each adherens junction component is affected simultaneously during monocyte transmigration.
Table 1.
Transmigration of Monocytes Is Associated with Loss of VE-Cadherin, β-Catenin, α-Catenin, and Plakoglobin in the Adjacent Endothelial Monolayer
| Junctional protein examined | Interacting monocytes associated with staining loss |
|---|---|
| % | |
| VE-Cadherin | 40 ± 16 |
| β-Catenin | 43 ± 3 |
| α-Catenin | 38 ± 12 |
| Plakoglobin | 54 ± 1 |
| PECAM-1 | 8 ± 3 |
Data are expressed as the percentage of monocytes per field that are associated with loss of either VE-cadherin, α-catenin, β-catenin, plakoglobin, or PECAM-1 staining (see Materials and Methods for criteria of scoring). Data are from four fields (20× objective) per coverslip and from at least two coverslips per experiment. The range in cell numbers was 11–53 cells per field. Data are mean ± SD.
Loss of VE-Cadherin Does Not Correlate with Clustering of Monocyte Adhesion/Transmigration
If disruption of VE-cadherin in our flow assay system was dependent on diffusion of released proteases from adherent mononuclear leukocytes, then disruption of VE-cadherin would be predicted to occur in areas of high density adhesion (clusters of monocytes), rather than in areas of low density (single cells). To address this issue, experiments with PBMC (from above) were analyzed further to determine the profile of leukocyte binding with respect to disruption of VE-cadherin complex (Table ). Interestingly, adherent monocytes were more often seen to alter VE-cadherin when bound as single cells or in pairs of cells (83 ± 14%, n = 4) as compared with clusters of adherent cells (15 ± 13%, n = 4, P < 0.001). These data further suggest that loss of VE-cadherin, β-catenin, or plakoglobin was not due simply to release or diffusion of granule proteases from fixed leukocytes. Here, we point out that from the confocal microscopy data (Fig. 3), there is little evidence to suggest monocyte-dependent proteolysis of VE-cadherin.
Table 2.
Number of Monocyte Interactions Associated with Loss of Endothelial Cell VE-Cadherin, β-Catenin, and Plakoglobin
| Monocytes associated with loss of: | |||
|---|---|---|---|
| Monocytes in group | VE-Cadherin | β-Catenin | Plakoglobin |
| n | % | % | % |
| >5 | 1 ± 2 | 2 ± 3 | 0 ± 0 |
| 3–5 | 15 ± 13 | 31 ± 3 | 37 ± 5 |
| 1 or 2 | 83 ± 14 | 69 ± 6 | 64 ± 5 |
Flow adhesion assays using PBMC were performed as detailed in Materials and Methods. HUVEC monolayers were recovered and stained for components of the VE-cadherin complex. Monocytes associated with loss of VE-cadherin, β-catenin, or plakoglobin were scored as to whether they were in groups of: >5; 3–5; or 1 or 2 monocytes. The results are expressed as the percentage of monocytes in each group associated with loss of junctional staining (mean ± SD).
Inhibition of Transendothelial Migration Reduces Loss of VE-Cadherin Staining
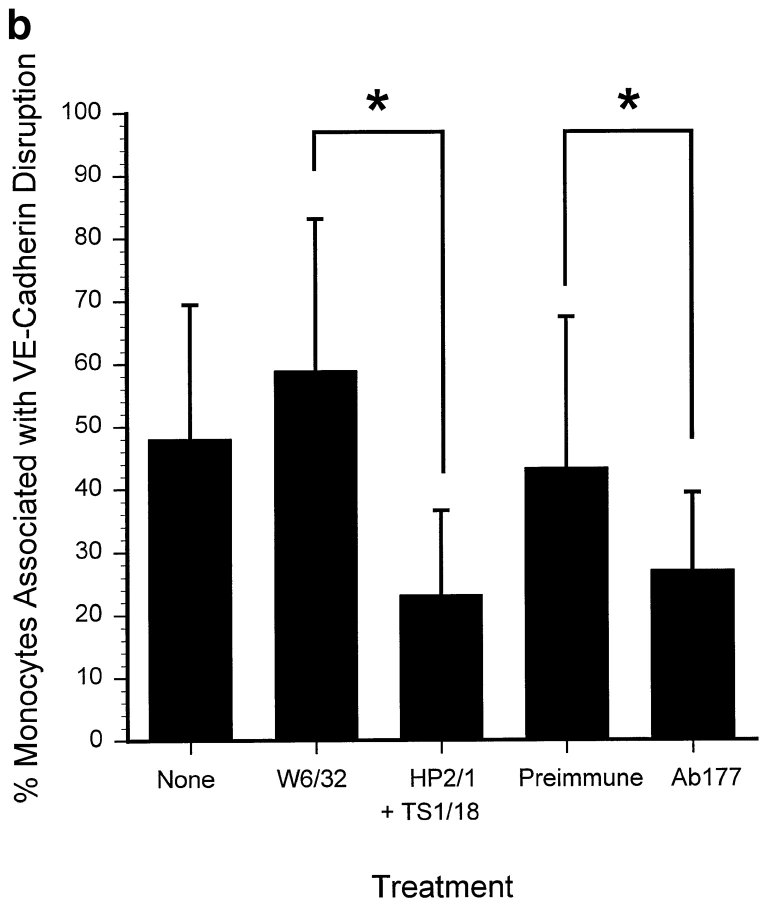
To determine further whether adhesion of monocytes alone is sufficient to induce disruption of the VE-cadherin complex, or if monocyte transmigration is required, we performed flow experiments in the presence of function blocking antibodies that prevent transmigration. These included anti–PECAM-1 (Ab 177) that does not impair adhesion, but prevents transmigration in this system (Muller et al. 1993; Liao et al. 1995, Liao et al. 1997), and a combination of anti–α4-integrin (HP2.1) and anti–β2-integrin (TS1/18) mAbs, which prevent a large proportion of monocyte firm adhesion and subsequent transendothelial migration under flow (Chuluyan and Issekutz 1993; Meerschaert and Furie 1994; Luscinskas et al. 1996). Fig. 4 a demonstrates that the presence of either Ab 177 or the combination of HP2.1 and TS1/18 results in a significant inhibition of transendothelial migration. It was not possible to determine from the videotape the proportion of monocytes present in the field of PBMC; therefore, all these data refer to total cell populations. Specifically, the combination of HP2.1 and TS1/18 mAb reduce the total leukocyte migration in presence of control mAb level from 50.7 ± 8.6% (W6/32 control, n = 3) to 19.7 ± 7.5% (combination mAb, n = 3, P < 0.01), an inhibition of 61.1%. Similarly, Ab 177 reduces transmigration by 54.7% (46.7 ± 9.2% transmigration with preimmune IgG vs. 21.1 ± 6.6% with Ab 177; n = 3, P < 0.001).
Figure 4.
Inhibition of monocyte transmigration prevents monocyte-dependent changes in VE-cadherin staining. PBMC (106/ml) were incubated in the presence of either binding control (W6/32 mAb, 10 μg/ml) or the combination of α4-integrin (mAb HP2.1) and β2-integrin (TS1/18 mAb, each at 10 μg/ml) for 15 min at room temperature. In additional experiments, PBMC and the HUVEC monolayer were incubated in the presence of either Ab 177 or preimmune IgG (20 μg/ml) for 30 min before use in flow assays. a, The total interacting cells (rolling, adherent, and transmigrated) were comparable in each case, 602 ± 157 cells/mm2 in the presence of HP2.1 and TS1/18; 573 ± 53 cells/mm2 in the presence of Ab177; and 575.8 ± 170 cells/mm2 in the presence of preimmune IgG. Data are the mean ± SD, n = 4 (four coverslips analyzed per experiment). b, The number of monocytes associated with changes in VE-cadherin were quantified as described in Materials and Methods. Three fields were taken from each coverslip (two coverslips analyzed per experiment). Data are expressed as the mean ± SD, n = 4. The range of total leukocytes per field was 13–65 cells, with most fields falling between 25 and 47 cells.
We then examined the effect of these antibodies on leukocyte-induced dissociation of VE-cadherin. In the presence of both HP2.1 and TS1/18, monocyte-dependent dissociation of VE-cadherin was reduced significantly from 58.9 ± 24.2% of monocytes (Fig. 4 b, W6/32) to 23.2 ± 13.4% (Fig. 4 b, n = 3, P < 0.01). Similarly, anti–PECAM-1 reduced monocyte-dependent disruption of VE-cadherin by 37% (Fig. 4 b, n = 3, P < 0.02), indicating that inhibition of transmigration reduced leukocyte-dependent dissociation of the VE-cadherin complex, as demonstrated with U937L-Dif cells (see above).
Monocyte-induced Dissociation of VE-Cadherin Is Reversible
From our confocal microscopy data, we noted that restoration of VE-cadherin staining occurred following completion of transmigration. In addition, recent in vitro studies (Huang et al. 1988; Haselton et al. 1996; Burns et al. 1997; Lennon et al. 1998) suggest that transendothelial migration of leukocytes does not lead to a rapid increase in HUVEC monolayer permeability. We hypothesized, therefore, that leukocyte-induced dissociation of the VE-cadherin complex must be focal and rapidly reversible after transmigration. This question was addressed by varying the protocol for the experiments as follows. PBMC were perfused across activated HUVEC monolayers for three minutes and then the monolayers either were fixed immediately for staining analyses, or perfused for a further four or nine minutes with buffer alone (no leukocytes) before fixation. We observed by live-time phase-contrast microscopy with a 60× objective that no new adhesive interactions occurred after five minutes and that monocyte transmigration had reached a plateau by seven minutes (Fig. 5). As a control, purified CD4+ T cells were used in parallel experiments to simulate the conditions used for PBMC.
After three minutes of perfusion, 46.6 ± 21.1% of monocytes (Fig. 5, ○, n = 3), and 6.6% of lymphocytes (Fig. 5, □, n = 3) were associated with disruption of VE-cadherin staining, which is similar to the actual percentage of leukocytes transmigrating as assessed by live-time videomicroscopy. By seven minutes, no further migration occurred, and loss of VE-cadherin staining had declined significantly to 16.9 ± 7.0% (Fig. 5, ○, P < 0.01). After 12 min of perfusion, monocyte-dependent loss of VE-cadherin had declined further to 11.7 ± 8.9% (P < 0.001), suggesting that the VE-cadherin complex was restored after completion of monocyte transmigration. In contrast, no significant change was observed in the low level of purified CD4+ T cells (Fig. 5, □, 6.9%) associated with loss of VE-cadherin staining. Some loss of VE-cadherin staining was associated with adherent lymphocytes in the PBMC experiments (16.5 ± 11.0%, n = 3), however, lymphocytes were usually observed in areas of high monocyte binding also. As isolated monocytes induce loss of VE-cadherin staining (Fig. 3), and because no change in VE-cadherin was observed for isolated CD4+ T cells alone, the loss in VE-cadherin staining at sites of adherent lymphocytes (PBMC) is most likely attributed to monocyte-dependent events.
Discussion
The multistep model of leukocyte attachment, rolling, and arrest has been well defined, however, the underlying mechanisms that mediate the last step of the cascade, transmigration through the endothelial clefts, has not been clearly established. To this end, we have explored the role of the VE-cadherin complex during monocyte transendothelial migration under flow in vitro across HUVEC monolayers. To circumvent postlysis degradation events (Moll et al. 1998; Allport, J.R., unpublished observations), we developed a model using the U937 cell line, monocytes, or PBMC under flow conditions, followed by immunofluorescence analysis of VE-cadherin complex staining. This system establishes two important conditions: first, it brings leukocytes in contact with the endothelium in a physiologically relevant context that would not occur in a static adhesion assay, and second, allows continuous live time monitoring of leukocyte adhesion, arrest, and ultimately transmigration, and the status of the endothelial cell monolayers. Further advantages are that the endothelial cell monolayers can be recovered for in depth analyses using immunofluorescence staining and that both U937L cells and mononuclear leukocytes have significantly less proteolytic capabilities as compared with neutrophils, especially in regard to neutrophil elastase, a serine protease which can cleave endothelial VE-cadherin (Carden et al. 1998). Using this approach, we demonstrate that leukocyte adhesion/transmigration still induces focal changes in VE-cadherin complex that correlate with the location of actively transmigrating leukocytes.
Leukocyte Transmigration Correlates with Changes in VE-Cadherin Complex In Vitro
In this flow model, human peripheral blood monocytes and differentiated U937L cells (U937L-Dif cells) transmigrate TNF-α activated HUVEC at similar rates and to a similar extent (48–50%; Chuluyan and Issekutz 1993; Luscinskas et al. 1996), whereas purified CD4+ T cells or U937L cells adhere to the endothelium apical surface, but do not transmigrate (<5% of total transmigrate). Both monocytes (Fig. 3) and U937L-Dif cells (Fig. 2) induce transmigration-dependent changes in the VE-cadherin staining, whereas adherent CD4+ T cells (Fig. 5) or U937L cells do not result in any changes in VE-cadherin complex. In additional experiments not presented, over a time course up to 60 min of incubation under static conditions, CD4+ T cells did not transmigrate activated endothelium and did not induce loss of VE-cadherin staining. The monocyte-dependent loss of VE-cadherin is attenuated by anti–PECAM-1 antibodies that reduce transmigration without affecting the level of firm adhesion (Fig. 4; Muller et al. 1993). Moreover, both U937L-Dif cell-dependent changes in VE-cadherin staining and transmigration (but not adhesion) are reduced significantly by anti–PECAM-1 antibodies. These data suggest that a transmigration-initiated event triggers rapid and reversible alterations in the endothelial VE-cadherin complex.
Leukocyte-induced Alterations in VE-Cadherin Under Flow Are Focal and Reversible
Notably, the monocyte- and U937L-Dif-induced changes in VE-cadherin staining occurred focally and mostly at the site of adhesion/transmigration and did not result in global loss of VE-cadherin staining in endothelial cell junctions that lack adherent/migrated leukocytes. In fact, the percentage of transmigrating cells associated with loss of the VE-cadherin complex may represent an underestimate for three reasons. First, the lack of synchronized transmigration; second, only a proportion of the leukocyte population undergoes transendothelial migration; and third, endothelial junctions directly above any leukocytes that had completed transmigration at the time of fixation would have reestablished their lateral junctions and, hence, no disruption would be observed. Most changes occurred in areas of single or two cell adhesion and not in areas of monocyte clusters, suggesting VE-cadherin loss was not due simply to release of increasing levels of proteases (Table ). Optical sectioning by confocal microscopy revealed that the monocytes maintained a clear cut border with the VE-cadherin staining as they transmigrated, with no colocalization of VE-cadherin with the CD14 staining, indicating tight association between the monocyte and endothelial cell junction proteins. An important observation is that the loss of VE-cadherin complex staining was reversible over the time course of migration (Fig. 3 and Fig. 5). Monocyte transmigration induced a transient change in VE-cadherin staining that correlated precisely with the time course of monocyte migration. These data indicate an endothelial cell regulatory mechanism during leukocyte trafficking, as opposed to simple degradation by leukocyte proteases.
Mechanisms that Regulate VE-Cadherin Complex and Leukocyte Transmigration
What is the mechanism by which the VE-cadherin complex is destabilized, disassembled, or retracted to allow passage of leukocytes across the endothelial monolayer? Previous data and the current study indicate a number of possible mechanisms. Our data demonstrates that anti–PECAM-1 antibodies reduce proportionally both migration of monocytes and U937L-Dif cells. Similarly, blockade of both β1 and β2 integrins reduced migration in proportion to loss of VE-cadherin. One possibility is that the anti–PECAM-1 IgG simply prevents the leukocyte from getting close enough to the endothelial cell–cell lateral borders to trigger alterations in VE-cadherin; a purely mechanical interference. Alternatively, signaling events, either negative or positive (Newman 1999), required to trigger alteration of VE-cadherin and allow passage of the leukocyte, may be induced through homophilic interactions between PECAM-1 molecules (Liao et al. 1995; Berman and Muller 1995; Liao et al. 1997). Consequently, if the leukocyte is prevented from such interactions by anti–PECAM-1 antibody, then these signaling events are interrupted. Thus, even leukocytes adherent to the apical surface in the presence of blocking anti–PECAM-1 antibody are prevented from making productive interactions with endothelial PECAM-1. Nonetheless, PECAM-1 is not the only molecule capable of maintaining endothelial apposition during transmigration. PECAM-1–independent mechanisms of transmigration indeed exist. While this work was under review, a PECAM-1 null mouse was reported (Duncan et al. 1999). As these mice have essentially normal leukocyte transmigration, future studies are planned to examine the effect of monocyte transmigration on VE-cadherin complex in PECAM-1 null endothelium.
Another explanation for focal loss of VE-cadherin at transmigration sites is that the entire complex is pushed aside directly by the migrating leukocyte, analogous to a trapdoor mechanism that simply recloses after leukocyte migration. Migrating leukocytes remain in close apposition to endothelial cell junction clefts within estimated distances of ≤100 Å (Huang et al. 1988). This narrow distance could be maintained throughout migration by the pairing of appropriate adhesion molecules on both cells (i.e., PECAM-1–PECAM-1 and/or integrin–IgG interactions). Because VE-cadherin does not appear to have a leukocyte counter receptor, the VE-cadherin complex distribution is altered, whereas PECAM-1 remains localized (Fig. 3). Hence, a trapdoor model envisions that endothelial junction clefts are amenable to being pushed aside by the extruded leading lamella of the crawling leukocyte. A further assumption is that the energy required for bypassing/splitting the VE-cadherin complex comes entirely from the leading lamella of migrating leukocytes.
Several recent studies reviewed in the Introduction have found that engagement of endothelial cell adhesion molecules triggered dramatic changes in the endothelium. More recently, occupancy of surface endothelial leukocyte adhesion molecules by blocking mAb or leukocytes led to increases in both [Ca2+]i and actin stress fibers (Lorenzon et al. 1998). Here, we show transmigrating leukocytes cause a focal and reversible disruption/dispersion of the VE-cadherin complex. Based on these data, we speculate that transmigration, analogous to the multistep adhesion cascade model, is a sequential multistep process that involves active participation of both leukocytes and the endothelium. The nature of the exact intracellular signals and their integration with the physical movements of both cell types will require further studies.
Burns et al. 1997 have reported that leukocytes migrate preferentially at areas where multiple endothelial cells come together (tricellular corners), reporting that both tight junctions and VE-cadherin staining were discontinuous at these regions in their culture system, providing a potential gateway for leukocytes to traverse the endothelium. This mechanism may be a component of monocyte transendothelial migration in our model, but does not seem to predominate for a number of reasons. First, in our culture system, discontinuities in the expression of tight junction components exist along the lateral borders of endothelial cells (data not shown) not only at the points where three or more cells interface. Hence, it is most likely that leukocytes are able to migrate around tight junctions in this model. Second, the confocal micrographs of immunofluorescent stained VE-cadherin in intact HUVEC monolayers did not demonstrate the number of gaps we see in the presence of monocytes or PBMC (Fig. 3). Additionally, any gaps that were detected appeared to occur only in regions where the monolayer was pulled away from the substrate or neighboring cells. Third, although tricellular corners may support a level of transmigration out of proportion to their surface area in the monolayer (Burns et al. 1997), our flow assay system showed no predisposition for monocytes to migrate at multicellular interfaces. Last, and most important, the appearance of gaps in the VE-cadherin staining pattern correlated temporally with the migration of monocytes, in that only during active migration were significant numbers of gaps associated with adherent leukocytes (Fig. 3 and Fig. 5).
Others have suggested (Carden et al. 1998) that migrating leukocytes digest VE-cadherin at the site of adhesion/transmigration and internalize the protein, removing it from the endothelial cell membrane. This would require reexpression of new VE-cadherin at the endothelial cell surface that would most likely come from intracellular stores, as protein synthesis appears not to be required (data not shown). The trivial explanation that loss of VE-cadherin staining is due to inaccessibility of the mAb epitope or loss of the epitope at sites of leukocyte adhesion/transmigration is unlikely for two reasons. First, the VE-cadherin complex appears to move away from the site of adhesion/transmigration as an intact complex because loss of VE-cadherin staining is accompanied by loss of β-catenin and plakoglobin staining (Fig. 3 and Table ). Both are intracellular proteins that would not directly interact with the migrating leukocyte and hence, their epitopes should not be impinged upon. Second, VE-cadherin staining remains at HUVEC junctions with overlying adherent U937L cells, indicating that the epitope for VE-cadherin antibody binding remains accessible (Fig. 2). Since immunostaining of the entire complex is lost, then regained, in concert, it is possible that transmigration triggers dissociation of these large aggregates into smaller groups of molecules, which disperse into neighboring plasmalemma, rendering their staining much less intense. These groups may retain attachments via catenins to actin filaments that would facilitate their reclustering when transmigration was completed. Recently, in MDCK epithelial cells, it has been reported that a pool of surface E-cadherin molecules is constantly trafficked through an endocytic, clathrin-mediated recycling pathway (Le et al. 1999). It remains to be determined if such a pathway exists for VE-cadherin in endothelium. Future studies, however, are necessary to exclude a requirement for secreted or membrane-bound proteases, or their receptors, at the leading edge of migrating leukocytes. The expanding availability of murine models carrying targeted disruptions of such genes may allow testing in the near future.
Acknowledgments
We thank Kay Case and William Atkinson for isolation and primary culture of HUVEC, Han Ding for assistance with some flow experiments, Dr. Peter Newman (Milwaukee, WI) for mAb P1.1, and gratefully acknowledge Dr. Michael A. Gimbrone, Jr., for helpful discussions.
This work was supported by National Institutes of Health grants HL36028 and HL53993 (F.W. Luscinskas and J.R. Allport) and HL46849 (W.A. Muller). J.R. Allport is supported by a research fellowship from the Crohn's and Colitis Foundation of America, NY, NY. W.A. Muller is an Established Investigator of the American Heart Association.
Footnotes
Abbreviations used in this paper: [Ca2+]i, intracellular Ca2+ concentration; DPBS, Dulbecco's phosphate-buffered saline; HSA, human serum albumin; HUVEC, human umbilical vein endothelial cells; ICAM-1, intercellular adhesion molecule 1; PBMC, peripheral blood mononuclear cells; PECAM-1, platelet endothelial cell adhesion molecule 1; PMN, polymorphonuclear leukocytes; TNF-α, tumor necrosis factor α; U937L, U937 monocytic cells stably transfected with L-selectin (CD62L); U937L-Dif, differentiated U937L; VE-cadherin, vascular endothelial cell cadherin.
References
- Ali J., Liao F., Martens E., Muller W.A. Vascular endothelial cadherin (VE-cadherin)cloning and role in endothelial cell–cell adhesion. Microcirculation. 1997;4:267–277. doi: 10.3109/10739689709146790. [DOI] [PubMed] [Google Scholar]
- Allport J.R., Ding H., Collins T., Gerritsen M.E., Luscinskas F.W. Endothelial-dependent mechanisms regulate leukocyte transmigrationa process involving the proteasome and disruption of the vascular endothelial–cadherin complex at endothelial cell-to-cell junctions. J. Exp. Med. 1997;186:517–527. doi: 10.1084/jem.186.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman M.E., Muller W.A. Ligation of platelet/endothelial cell adhesion molecule-1 (PECAM-1/CD31) on monocytes and neutrophils increases binding capacity of leukocyte CR3 (CD11b/CD18) J. Immunol. 1995;154:299–307. [PubMed] [Google Scholar]
- Breviario F., Caveda L., Corada M., Martin-Padura I., Navarro P., Golay J., Introna M., Gulino D., Lampugnani M.G., Dejana E. Functional properties of human vascular endothelial cadherin (7B4/cadherin-5), an endothelium-specific cadherin. Arterioscler. Thromb. Vasc. Biol. 1995;15:1229–1239. doi: 10.1161/01.atv.15.8.1229. [DOI] [PubMed] [Google Scholar]
- Burns A.R., Walker D.C., Brown E.S., Thurmon L.T., Bowden R.A., Keese C.R., Simon S.I., Entman M.L., Smith C.W. Neutrophil transendothelial migration is independent of tight junctions and occurs preferentially at tricellular corners. J. Immunol. 1997;159:2893–2903. [PubMed] [Google Scholar]
- Carden D., Xiao F., Moak C., Willis B.H., Robinson-Jackson S., Alexander S. Neutrophil elastase promotes lung microvascular injury and proteolysis of endothelial cadherins. Am. J. Physiol. 1998;275:H385–H392. doi: 10.1152/ajpheart.1998.275.2.H385. [DOI] [PubMed] [Google Scholar]
- Chuluyan H.E., Issekutz A.C. VLA-4 integrin can mediate CD11/CD18-independent transendothelial migration of human monocytes. J. Clin. Invest. 1993;92:2768–2777. doi: 10.1172/JCI116895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E. Endothelial adherens junctionsimplications in the control of vascular permeability and angiogenesis. J. Clin. Invest. 1996;98:1949–1953. doi: 10.1172/JCI118997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E., Corada M., Lampugnani G. Endothelial cell-to-cell junctions. FASEB J. 1995;9:910–918. [PubMed] [Google Scholar]
- Dejana, E., O. Valiron, P. Navarro, and M.G. Lampugnani. 1998. Intercellular junctions in the endothelium and the control of vascular permeability. Ann. NY Acad. Sci. 36–43. [DOI] [PubMed]
- Del Maschio A., Zanetti A., Corada M., Rival Y., Ruco L., Lampugnani M.G., Dejana E. Polymorphonuclear leukocyte adhesion triggers the disorganization of endothelial cell-to-cell adherens junctions. J. Cell Biol. 1996;135:497–510. doi: 10.1083/jcb.135.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan G.S., Andrew D.P., Takimoto H., Kaufman S.A., Yoshida H., Spellburg J., de la Pompa J.L., Elia A., Wakeham A., Karan-Tamir B. Genetic evidence for functional redundancy of platelet/endothelial cell adhesion molecule-1 (PECAM-1)CD31-deficient mice reveal PECAM-1–dependent and PECAM-1–independent functions. J. Immunol. 1999;162:3022–3030. [PubMed] [Google Scholar]
- Geiger B. The cytoplasmic domain of adherens-type junctions. Cell Motil. Cytoskelet. 1991;20:1–6. doi: 10.1002/cm.970200102. [DOI] [PubMed] [Google Scholar]
- Gotsch U., Borges E., Bosse R., Boggemeyer E., Simon M., Mossmann H., Vestweber D. VE-cadherin antibody accelerates neutrophil recruitment in vivo. J. Cell Sci. 1997;110:583–588. doi: 10.1242/jcs.110.5.583. [DOI] [PubMed] [Google Scholar]
- Haselton F.R., Woodall J.H., Alexander J.S. Neutrophil–endothelial interactions in a cell-column model of the microvasculatureeffects of fMLP. Microcirculation. 1996;3:329–342. doi: 10.3109/10739689609148306. [DOI] [PubMed] [Google Scholar]
- Hixenbaugh E.A., Goeckler Z.M., Papaiya N.N., Wysolmerski R.B., Silverstein S.C., Huang A.J. Stimulated neutrophils induce regulatory myosin light chain kinase phosphorylation and isometric tension development in endothelial cells. Am. J. Physiol. 1997;273:H981–H988. doi: 10.1152/ajpheart.1997.273.2.H981. [DOI] [PubMed] [Google Scholar]
- Huang A.J., Furie M.B., Nicholson S.C., Fischbarg J., Liebovitch L.S., Silverstein S.C. Effects of human neutrophil chemotaxis across human endothelial cell monolayers on the permeability of these monolayers to ions and macromolecules. J. Cell Physiol. 1988;135:355–366. doi: 10.1002/jcp.1041350302. [DOI] [PubMed] [Google Scholar]
- Huang A.J., Manning J.E., Bandak T.M., Ratau M.C., Hanser K.R., Silverstein S.C. Endothelial cell cytosolic free calcium regulates neutrophil migration across monolayers of endothelial cells. J. Cell Biol. 1993;120:1371–1380. doi: 10.1083/jcb.120.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani M.G., Corada M., Caveda L., Breviario F., Ayalon O., Geiger B., Dejana E. The molecular organization of endothelial cell to cell junctionsdifferential association of plakoglobin, β-catenin, and α-catenin with vascular endothelial cadherin (VE-cadherin) J. Cell Biol. 1995;129:203–218. doi: 10.1083/jcb.129.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le T.J., Yap A.S., Stow J.L. Recycling of E-cadherin. A potential mechanism for regulating cadherin dynamics. J. Cell Biol. 1999;146:219–232. [PMC free article] [PubMed] [Google Scholar]
- Lennon P.F., Taylor C.T., Stahl G.L., Colgan S.P. Neutrophil-derived 5′-adenosine monophosphate promotes endothelial barrier function via CD73-mediated conversion to adenosine and endothelial A2B receptor activation. J. Exp. Med. 1998;188:1433–1443. doi: 10.1084/jem.188.8.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao F., Huynh H.K., Eiroa A., Greene T., Polizzi E., Muller W.A. Migration of monocytes across endothelium and passage through extracellular matrix involve separate molecular domains of PECAM-1. J. Exp. Med. 1995;182:1337–1343. doi: 10.1084/jem.182.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao F., Ali J., Greene T., Muller W.A. Soluble domain 1 of platelet-endothelial cell adhesion molecule (PECAM) is sufficient to block transendothelial migration in vitro and in vivo. J. Exp. Med. 1997;185:1349–1357. doi: 10.1084/jem.185.7.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y.-C., Snapp K., Kansas G.S., Camphausen R., Ding H., Luscinskas F.W. Important contributions of P-selectin glycoprotein ligand-1–mediated secondary capture to human monocyte adhesion to P-selectin, E-selectin, and TNF-α–activated endothelium under flow in vitro. J. Immunol. 1998;161:2501–2508. [PubMed] [Google Scholar]
- Lorenzon P., Vecile E., Nardon E., Ferrero E., Harlan J.M., Tedesco F., Dobrina A. Endothelial cell E- and P-selectin and vascular cell adhesion molecule-1 function as signaling receptors. J. Cell Biol. 1998;142:1381–1391. doi: 10.1083/jcb.142.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscinskas F.W., Brock A.F., Arnaout M.A., Gimbrone M.A., Jr. Endothelial-leukocyte adhesion molecule-1 (ELAM-1)–dependent and leukocyte (CD11/CD18)-dependent mechanisms contribute to polymorphonuclear leukocyte adhesion to cytokine-activated human vascular endothelium. J. Immunol. 1989;142:2257–2263. [PubMed] [Google Scholar]
- Luscinskas F.W., Kansas G.S., Ding H., Pizcueta P., Schleiffenbaum B.E., Tedder T.F., Gimbrone M.A., Jr. Monocyte rolling, arrest, and spreading on IL-4–activated vascular endothelium under flow is mediated via sequential action of L-selectin, β1-integrins, and β2-integrins. J. Cell Biol. 1994;125:1417–1427. doi: 10.1083/jcb.125.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscinskas F.W., Ding H., Lichtman A.H. P-selectin and VCAM-1 mediate rolling and arrest of CD4+ T-lymphocytes on TNF-α–activated vascular endothelium under flow. J. Exp. Med. 1995;181:1179–1186. doi: 10.1084/jem.181.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscinskas F.W., Ding H., Tan P., Cumming D., Tedder T.F., Gerritsen M.E. L-selectin and P-selectin but not VLA-4 mediate monocyte initial attachment and rolling on TNF-α activated vascular endothelium under flow in vitro . J. Immunol. 1996;156:326–335. [PubMed] [Google Scholar]
- Matsuyoshi N., Toda K., Horiguchi Y., Tanaka T., Nakagawa S., Takeichi M., Inamura S. In vivo evidence of the critical role of cadherin-5 in murine vascular integrity. Proc. Assoc. Am. Physicians. 1997;109:362–371. [PubMed] [Google Scholar]
- Meerschaert J., Furie M.B. Monocytes use either CD11/CD18 or VLA-4 to migrate across human endothelium in vitro . J. Immunol. 1994;152:1915–1926. [PubMed] [Google Scholar]
- Moll T., Dejana E., Vestweber D. In vitro degradation of endothelial catenins by a neutrophil protease. J. Cell Biol. 1998;140:403–407. doi: 10.1083/jcb.140.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W.A., Weigl S.A., Deng X., Phillips D.M. PECAM-1 is required for transendothelial migration of leukocytes. J. Exp. Med. 1993;178:449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman P.J. Switched at birtha new family for PECAM-1. J. Clin. Invest. 1999;103:5–9. doi: 10.1172/JCI5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfau S., Leitenberg D., Rinder H., Smith B.R., Pardi R., Bender J.R. Lymphocyte adhesion-dependent calcium signaling in human endothelial cells. J. Cell Biol. 1995;128:969–978. doi: 10.1083/jcb.128.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Minamiya Y., Kitamura M., Saito S., Enomoto K., Terada K., Ogawa J. Endothelial myosin light chain kinase regulates neutrophil migration across human umbilical vein endothelial cell monolayer. J. Immunol. 1998;161:1533–1540. [PubMed] [Google Scholar]
- Shen J., Luscinskas F.W., Connolly A., Dewey C.F., Jr., Gimbrone M.A., Jr. Fluid shear stress modulates cytosolic free calcium in vascular endothelial cells. Am. J. Physiol. 1992;262:C384–C390. doi: 10.1152/ajpcell.1992.262.2.C384. [DOI] [PubMed] [Google Scholar]
- Staddon J.M., Smales C., Schulze C., Esch F.S., Rubin L.L. p120, A p120-related protein (p100), and the cadherin/catenin complex. J. Cell Biol. 1995;130:369–381. doi: 10.1083/jcb.130.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittet V., Buchou T., Schweitzer A., Dejana E., Huber P. Targeted null-mutation in the vascular endothelial-cadherin gene impairs the organization of vascular-like structures in embryoid bodies. Proc. Natl. Acad. Sci. USA. 1997;94:6273–6278. doi: 10.1073/pnas.94.12.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Westlin W.F., Wang N., Ingber D.E., Rosenzweig A., Resnick N., Gimbrone M.A., Jr. Leukocyte adhesion to vascular endothelium induces E-selectin linkage to the actin cytoskeleton. J. Cell Biol. 1996;133:445–455. doi: 10.1083/jcb.133.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Szente B., Kiely J.-M., Rosenzweig A., Gimbrone M.A., Jr. Phosphorylation of the cytoplasmic domain of E-selectin is regulated during leukocyte–endothelial adhesion. J. Immunol. 1998;161:933–941. [PubMed] [Google Scholar]
- Ziegelstein R.C., Corda S., Pili R., Passaniti A., Lefer D., Zweier J.L., Fraticelli A., Capogrossi M.C. Initial contact and subsequent adhesion of human neutrophils or monocytes to human aortic endothelial cells releases an endothelial intracellular calcium store. Circulation. 1994;90:1899–1907. doi: 10.1161/01.cir.90.4.1899. [DOI] [PubMed] [Google Scholar]