The Fruit Fly Bristle, a Thorn in Our Side
Fruit fly bristles have us tearing out our few remaining hairs. The puzzle is, in forming long bristles, how and why do flies assemble long actin bundles by gluing shorter bundles end-to-end? Fruit fly bristles extend posteriorly in a gentle curve over the fly's back like the hair on a well-groomed individual from the 60's. These bristles, which are cellular extensions of 70 μm in the microchaete and 400 μm in the macrochaete, are supported by a ring of 7–11 cross-linked, membrane-attached actin bundles that run the length of the extension. To generate such an extension and yet to allow curvature of the bristle, the bundles are composed of units or modules, which bend at their junction points (Tilney et al. 1996, Tilney et al. 1998). Fig. 1, a light micrograph of a macrochaete, reveals the segmentation of the long actin bundles into modules. The average length of the modules is 3 μm but there is quite a range of module lengths (1–5 μm). What is peculiar and more puzzling is that the modules in adjacent bundles in the same bristle, all of which are formed at the same time during bristle elongation, tend to be the same length. The result of this is that the modules in adjacent bundles tend to be in transverse register (see Fig. 1 and Tilney et al. 1996).
Figure 1.
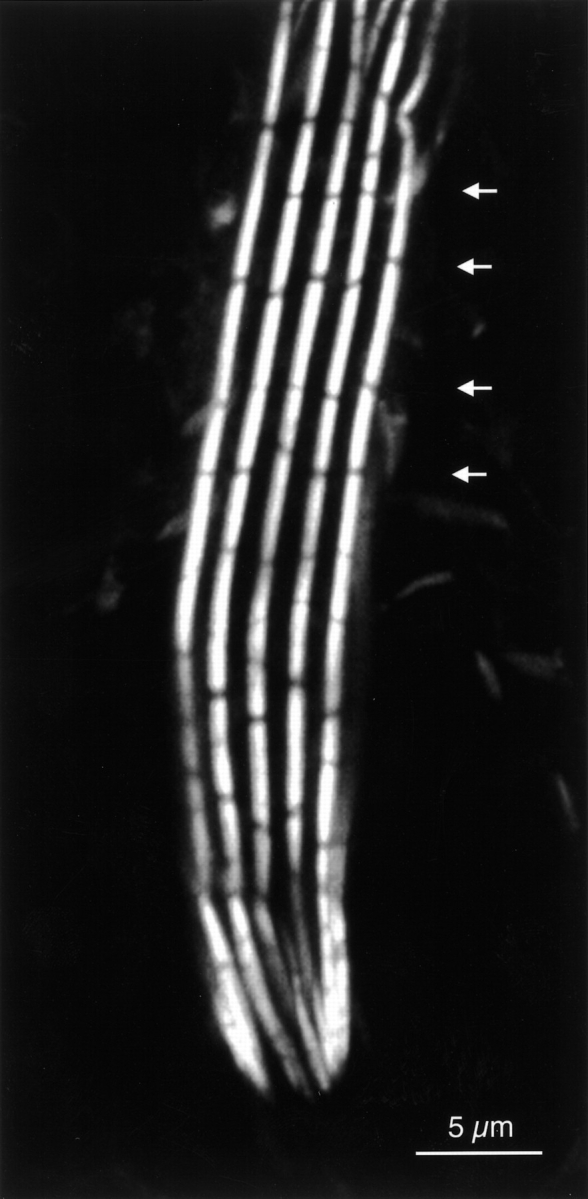
The actin bundles in a bristle of Drosophila were stained with fluorescent phalloidin and viewed with a confocal microscope. Each of the five bundles viewed in this plane is made up of smaller units or modules. Of interest is that the modules in adjacent bundles are in transverse register (see arrows). From Tilney et al., 1996.
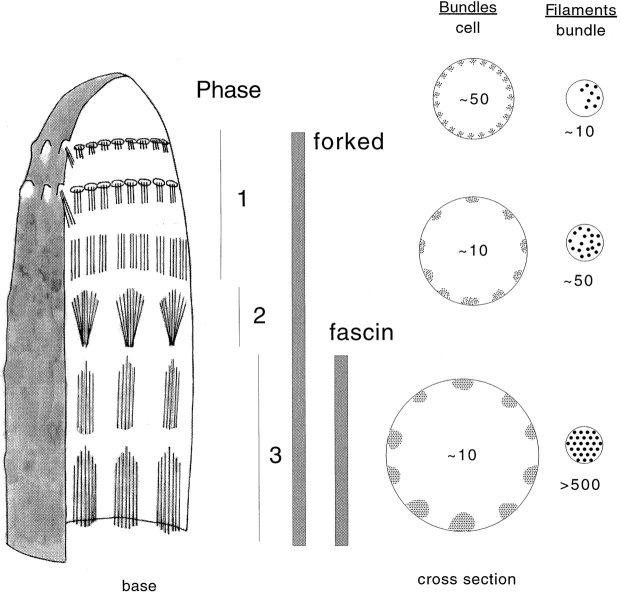
What are the steps used by the bristle cell in the assembly of bundles, as they may provide insights into the puzzle? Bristle elongation occurs by the assembly of modules at the elongating bristle tip. Modules form in three stages as indicated in Fig. 2. In stage 1, which occurs near the tip of the bristle, tiny bundles composed of 10–12 filaments appear attached to the plasma membrane both at their tips and laterally. In three dimensions each tiny bundle appears to extend basally from a small patch of dense material similar to that found at the tip of a microvillus. In fact, these tiny bundles look like tiny microvilli. The filaments in the bundle are polar with their barbed ends facing into a small patch of dense material. In stage 2, which occurs farther down the bristle, a number of these tiny bundles aggregate laterally in a polar manner into 7–11 larger bundles. In stage 3, the bundles are zippered up into hexagonally packed arrays by the addition of fascin. In both stage 1 and 2, the filaments are cross-linked by the forked protein, and finally in stage 3, fascin enters the bundles from the periphery and maximally cross-links the filaments. At the same time the bundles increase in width by the addition of more actin filaments. Four additional observations become germane here. First, as the bristle elongates, its rate of elongation increases but the module length remains the same. Second, both the 70-μm bristles of the microchaete and the 400-μm bristles of the macrochaete elongate in the same time period, and as they are different lengths, they must elongate at different rates. The module lengths in both, however, are the same. Third, the number of filaments present in each module does not effect module length. Fourth, the length of the module is independent of a specific type of cross-linker or the amount of cross-linker as determined by studying mutants (Tilney et al. 1996, Tilney et al. 1998).
Figure 2.
Drawing depicting the top of a Drosophila bristle at an early stage in its elongation. In stage 1 actin filaments are initiated on the cytoplasmic side of the plasma membrane in tiny pimple-like projections that resemble the tips of microvilli. Thin cross sections show that there are approximately 50 tiny bundles of roughly 10 filaments/bundle distributed evenly around the circumference of the plasma membrane. In stage 2 the tiny bundles aggregate into ∼10 large bundles each containing ∼50 filaments. The cross-linker forked is present in both stage 1 and 2. In stage 3 the cross-linker fascin enters these forked containing bundles to form along with forked hexagonally packed maximally cross-linked bundles. Additional filaments are added to the bundle and cross-linked into place. The apical end of each module is pointed, the basal end flat.
Just this description indicates there is something interesting going on. To repeat, neither the type nor amount of cross-bridging nor the rate of assembly seems to influence the length of the module. We are all familiar with hay baling machines. These machines take in hay at different rates (depending on the hay content in the fields) but these machines always tie them off at the same fixed length. Although the module length is not tightly regulated in bristles, there must be a machine like the hay baler that makes rectangular bundles of hay of approximately the same length independent of the rate at which the baler is travelling. The bristle cell has to accomplish the same feat. It requires more than just mixing together the components. If one tried to assemble modules by mixing actin and a cross-linker protein in a test tube, one would not produce uniform modules but rather bundles having a broad distribution of lengths and widths depending on the concentrations of components and the rates of assembly (Stokes and DeRosier 1991). In some cases, one might even get a gel instead of a bundle (Wachsstock et al. 1993).
To figure out the puzzle of modules and their assembly, we reexamined bundles and their assembly in a variety of cell types. (We should mention at the outset that we are defining bundles in this essay as closely packed, parallel filaments that all have the same polarity and are cross-linked by an actin-bundling protein. Thus, by the definition we are using here, stress fibers or muscle fibers are not bundles.) What we found is that all bundles share a common mechanism in morphogenesis which is related to bundle formation in microvilli. Based on our comparisons, we propose that there is a common factory inside cells that generates short bundles and that these are the building blocks out of which cells can make longer bundles.
This essay will first describe the characteristics of bundle formation in a variety of cell types and explain why we think all bundles are derived from microvilli, which in some cases are secondarily modified. We will then propose where and what we should look for in trying to understand the genesis of the bundles. At the end of this essay, we argue that although different cells make different kinds of bundles, they have a common feature, the small bundle factory.
The Microvillus: The Archetypal Factory of an Actin Bundle
Microvilli extend from the surfaces of most cells irrespective of origin, e.g., fibroblasts, cultured cells, growing neurons, epithelial cells, myoblasts, macrophages, and eggs. The best characterized are the microvilli which appear by light microscopy as a brush border on the apical surface of epithelial sheets such as those lining the lumen of the intestine or the proximal convoluted tubules of the kidney.
The Brush Border
An astounding 15,000 microvilli extend from the apical surface of each brush-border cell in vertebrate intestinal epithelium. Each microvillus contains an actin bundle of ∼20 filaments, all of which have their barbed ends facing into a small patch of dense material at the tip of each microvillus. In the intestine new epithelial cells are formed in the crypt and as they migrate out, the microvilli grow from a tiny dense patch on the plasma membrane and gradually increase in length from a fraction of a micron in the crypt to 3 or 4 μm (Fath et al. 1990). Three bundling proteins, villin, fimbrin, and espin, link together adjacent filaments (Shibayama et al. 1987; Ezzell et al. 1989; Bartles et al. 1998). Although villin appears first followed by fimbrin and then espin, microvilli of similar length and diameters are assembled even in the absence of villin (Ferrary et al. 1999).
Sea Urchin Egg Microvilli
The unfertilized sea urchin egg has on its surface tiny pimples. Each pimple seems to be the nub of a microvillus. It contains on the cytoplasmic surface of the plasma membrane some electron-dense material (Tilney and Jaffe 1980). Immediately after fertilization these pimples extend to form microvilli (Begg et al. 1982). Within each is a bundle of actin filaments that are cross-linked together by fascin. As in other microvilli, the filaments all have the same polarity with their barbed ends facing the electron-dense material. During the next 30 min the microvilli gradually elongate up to 10 μm (Begg et al. 1982).
Other Long Bundles Made from Microvillar-like Modules
Lest the reader think that bristles are unique in their use of modules or the manner in which the modules appear, we present two other instances in which long bundles are made of modules.
Nurse Cell Struts
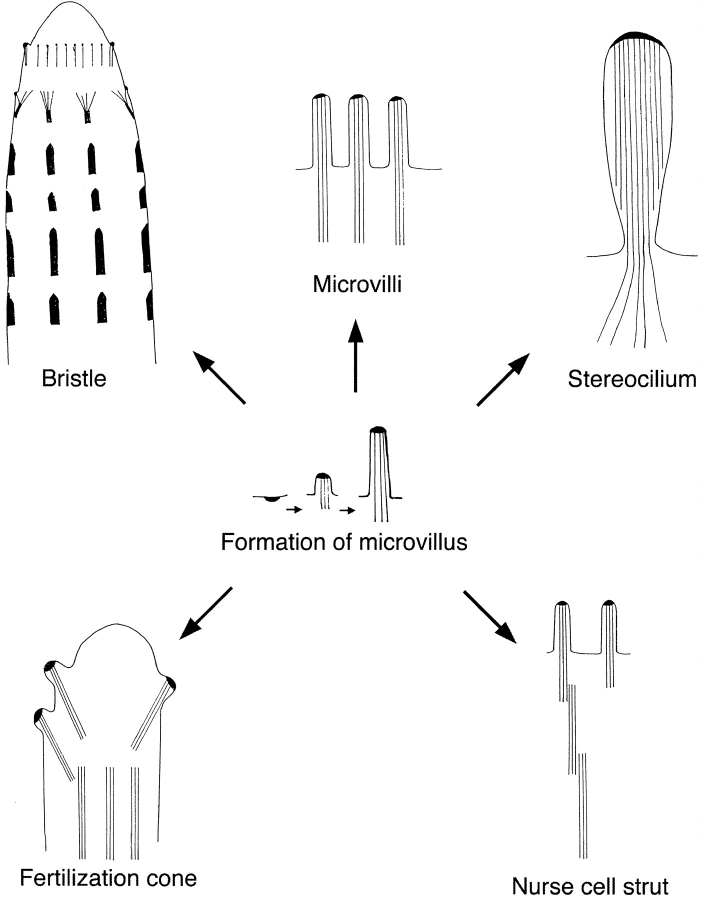
There is a second system in fruit flies in which long bundles are also composed of units or modules. In developing ovarian follicles the polyploid nurse cells at a late stage in oogenesis contract and in 30 min dump their contents into the oocytes via intercellular channels called ring canals. The nuclei are retained in the nurse cells and are kept from blocking the ring canals by a system of actin-containing struts that project from the plasma membrane toward the nucleus. Each strut is composed of overlapping units or modules each composed of ∼25 filaments cross-linked by two cross-links, fascin and villin. As dumping proceeds, the struts become progressively shorter and thicker due to the modules sliding past one another like the retraction of extension ladders when they are removed for storage (Guild et al. 1997). We have studied the formation of these struts. Each module is constructed out of a bundle derived from a microvillus (Fig. 3). The actin core of each microvillus elongates such that its base extends further into the cytoplasm. In our model for this process, the bundle is then released from its connection to the plasma membrane. A neighboring microvillus core binds to the newly released bundle. The neighboring core or elongation transports the first bundle towards the nucleus. The new bundle in turn becomes free from the membrane and the process is repeated with a third microvillus and then a fourth and so on, thereby producing a strut (Fig. 3). Since each bundle is derived from a microvillus, each module has the same polarity in the bundle with barbed ends of the filaments facing the cell surface (Guild et al. 1997) as they arose from the same kind of small patch of dense material seen in other microvilli.
Figure 3.
Examples of cross-linked bundles of actin filaments in a wide variety of cells from different organisms. All appear derived from the archetypal bundle, the microvillus. These include the bristle of Drosophila, the brush border of intestinal epithelial cell, the stereocilium of hair cells of the vertebrate ear, the nurse cell strut in Drosophila follicles and the fertilization cone of a sea urchin egg. An important feature of all these bundles is that the barbed ends of the actin filaments are embedded in a dense substance. Formation of a microvillus is depicted in the center and stages in the formation of a bristle are indicated. In this drawing all six parts, except the bristle (top left) are drawn to about the same size, or 1 cm equals 1 μm. The bristle is about three times smaller on this scale, or 3 mm equals ∼1 μm.
The Fertilization Cone
In marine eggs like the sea urchin the fertilization cone is generated from the egg surface and is used to engulf the sperm after fusion of the two gametes. This cone contains a large central actin filament bundle that is derived by the aggregation of numerous core bundles formerly in microvilli (Tilney and Jaffe 1980) that initially extended from the surface of the egg (Fig. 3). The fused bundles then elongate. As in the other examples, all the filaments in the core bundle have the same polarity as expected from their microvillar origin (Tilney and Jaffe 1980).
Of interest to our discussion here are the similarities in these three systems. First and most important is that long actin bundles are composed of shorter units or modules that bind to each other. Second, in all three systems these modules are derived from the core filament bundle in microvilli. Are similar strategies used in the assembly of other kinds of bundles? There are indeed obvious parallels between the bundles generated in the fruit fly with other systems. Below we enumerate a few of these.
Modified Microvilli
Some cell types also make long bundles but there is no need for modules. The two examples listed below are the result of secondary elaboration of microvilli. Other examples exist but for brevity we have just included these two.
The Stereocilium
Each stereocilium present in the hair cell of the ear is also derived from microvilli (Tilney and DeRosier 1986). Early in development, the surface of the hair cells and the adjacent supporting cells are covered with microvilli. The microvilli in the hair cells subsequently develop into stereocilia by the lateral addition of filaments to the microvillar bundle as well as elongation of the filaments in the bundle. The ends of the filaments at the stereociliary tips are embedded in a dense material (Fig. 3), and the polarity of the filaments is identical to its microvillar precursor (Tilney et al. 1983).
Acrosomal Processes of Marine Sperm
A more unusual kind of bundle assembly occurs in the production of the acrosomal bundles in marine sperm, but here again, they seem to be derivatives of microvilli.
Within the mature sperm of the edible blue mussel Mytilus edulis is a 2–5-μm-long maximally cross-linked actin bundle that contains 50 filaments (Tilney et al. 1987). Unlike the microvilli that extend from the cell surface the apical tip of this bundle is connected to the base of the acrosomal vacuole which is a secretory vacuole. Interestingly, the membrane of this vacuole will be continuous with the plasma membrane after activation. During spermiogenesis, this bundle is initiated at a small patch of dense material on the acrosomal surface and elongates posteriorly into the body of the sperm. The filaments in this bundle like those in the microvilli are unidirectionally polarized and crossbridged into a hexagonal bundle (Tilney et al. 1987).
In the sperm of the horseshoe crab Limulus there is a 60-μm-long bundle of actin filaments. The actin filaments in the bundle are stabilized and cross-linked by a unique cross-linking protein, scruin, that appears during spermiogenesis. This long bundle tapers, having 15 filaments at its tip and 85 filaments at its base. Studies on the formation of the bundle during spermiogenesis show that the basal end, containing the 85 filaments, assembles first and, as this bundle elongates basally, the number of filaments is gradually reduced, thereby producing the taper (Tilney et al. 1981). The bundle begins from a small patch of dense material that is attached to the acrosomal vacuole and then elongates. The elongation proceeds as one continuous process. It is as if the whole bundle is made as one long module. As expected, all the filaments have the same polarity with the barbed ends at the apical tip of the bundle.
From all these examples, e.g., in bristles, nurse cells, the fertilization cone, stereocilia, and the acrosomal processes, it appears that the microvillus or the microvillus-like structure initiates the formation of each bundle type. We have tried to depict these common mechanisms and their consequences in Fig. 3, which show how microvillus-like bundles are generated (see center portion), and then incorporated into other kinds of bundles that can be modified by the individual cell for its specific purpose. Thus, the microvillus seems to be the archetypal bundle factory. Accordingly we ask what are the characteristics of all these microvillus derivatives and how do they form?
Common Features of Bundles and Bundle Production
Having introduced, albeit briefly, bundle formation in microvilli, stereocilia, bristles, struts, fertilization cones, and acrosomal processes, we wonder what are the common features? (a) Bundles appear to be nucleated on a small patch of dense material located on a membrane. (b) Bundles taper, that is, the number of filaments decreases as one approaches the bundle tip. This statement is true of the bristle modules, microvilli of intestinal cells, the bundles in the fertilization cone and the situation in the acrosomal process of both Limulus and Mytilus. (c) Bundles can be produced roughly in synchrony. This is shown in the formation of a brush border, of a bristle, and of the fertilization cone. (d) Bundles are unidirectionally polarized with the barbed ends of the filaments located at the bundle tip nearest the plasma membrane. (e) The pointed ends of the filaments in bundles appear flat while the barbed end of the bundle often tapers to a point. (f) Bundle production is independent of a specific type of cross-linker. For example, in the bristle fascin and forked are present, in the nurse cell strut fascin and villin, in stereocilia fimbrin and espin, in the acrosomal process of Thyone sperm fascin, and in Limulus sperm scruin, in chick microvilli fimbrin, villin and espin, and in sea urchin eggs microvilli fascin. (g) The length of the filaments in a bristle module or in core bundles in a microvillus seems independent of filament number. Thus, intestinal epithelial cell microvilli can change in length with time or with stress, in struts the microvilli are long, and in bristles module length is independent of filament number or the speed of formation or position of the module.
The common features of all bundles and their apparent origins as microvilli suggest that all cells use some common mechanism to construct initial bundles. Cells, however, then modify these initial bundles to make larger assemblies. For example, stereocilia elongate and widen their bundles, and bristle cells and nurse cells glue bundles together to form long cables, and in bristles modules can at a later time period incorporate additional cross-links that will change filament packing (Fig. 2 and Tilney et al. 1998). Because of these similarities, we propose that there are in all these cell types common factories that generate bundles. These bundle-making factories can be reduced to two elements: a nucleating component, which specifies location and size, and a component that controls length.
Nucleating Component
Actin filaments appear to be nucleated near the plasma membrane, and it appears that elongation of the filaments occurs by the addition of monomers to the membrane-associated (barbed) ends of the filaments. In the cases we presented, the barbed end of the filaments are attached to membranes and are inserted in an electron-dense material of unknown composition. We suggest that this dense material may be the part of the nucleating component of our bundle factories and may also control the number of filaments on the bundle.
What might be the characteristics of this nucleating component? At present, the only system in which nucleation of actin is even vaguely understood is that involving the Arp2/3 complex. In Listeria-infected cells, for example, the Arp2/3 complex binds to G actin to form a nucleus. The idea is that other actin monomers can add to this complex to produce a filament. The Arp2/3 complex remains bound to the pointed end of the filament but detaches from the ActA, a Listeria-bound protein. Thus, as the filament elongates, the Arp2/3 complex, bound to the pointed end, is moved away from the site of initiation (for reviews, see Beckerle 1998; Machesky and Insall 1999). Several groups have visualized the Arp2/3 complex, showing that it localizes at Y junctions between filaments due to its capability both to bind to the side of a filament and/or nucleate a new branch (Mullins et al. 1998; Svitkina and Borisy 1999). In the case of a gel-like pseudopod this makes sense because one wants an open structure, and the Y junction promotes this (see Mullins et al. 1998; Svitkina and Borisy 1999), whereas in a bundle one wants a parallel set of filaments, which a Y junction would defeat. Accordingly, it is no surprise that by antibody staining the Arp2/3 complex is missing on bundles such as occur in filopodia (Welch et al. 1997) and in Drosophila bristles. The implication here is that bundles may be nucleated differently from gel-like actin structures in which Arp2/3 has been implicated.
Components that Control Elongation
After nucleation, there must be an elongation phase in which the filaments in the bundle grow. In the case of the brush border microvilli, the end result is a set of bundles that are of uniform length. In the bristle, the situation is more interesting. Recall that the 10 or so bundles in each cell are made of modules, which are glued end to end. The mean length of the modules is ∼3 μm, but there is variation with some modules being as short as 1 μm to as long as 5 or 6 μm. There is no obvious systematic variation of module length along the bundle even though the diameter decreases with distance along the bristle. Instead, there appears to be a correlation of module length between the bundles in the same portion of the bristle. It is as if the modules assembled at the same time are the same lengths. Moreover, the sizes of the bundles vary within the same portion of the cell so that the bundles closest to the fruit fly's body are the biggest. All this suggests to us that nucleation and elongation are independent, but they must be coordinated.
How Are These Two Processes Coordinated?
The question is what kinds of length-controlling mechanisms are possible? We consider several possible mechanisms. The first six do not appear to fit the observations. The seventh mechanism may be the answer.
One possibility is that a ruler defines length of the structure. For example, the length of the helical T4 phage tail is set by a protein, which runs up the center of the tail (Duda and Eiserling 1982). One would expect a similar mechanism to operate in muscle using nebulin to regulate thin filament length (Kruger et al. 1991). If there were a ruler, bundle length would not depend on rate of assembly or width of the bundle but the bundle length would equal the ruler length. Such a mechanism is unlikely, however, because the lengths of bundles in bristle cells vary from 1 to 5 μm. A second possibility is that random filament capping is employed to stop filament elongation. This kind of mechanism has been suggested to account for the short filaments in the tails of Listeria (Tilney et al. 1992; Mullins et al. 1997, Mullins et al. 1998). While such a mechanism could produce the variations in length seen in the short bundles comprising the bristle, it can't explain the correlation of module lengths in adjacent bundles. A third possibility is that one filament or one small bundle serves as a template for the other bundles made at about the same time. Thus, each round of bundle production begins with construction of a new template for that round. This would explain both the variation in module length and the correlation of lengths between modules made at the same time. This seems unlikely because modules made at the same time are made in different parts of the cell. A fourth possibility might involve some hanky-panky with the membrane in which the association of the bundle with the membrane somehow limits the module length. We could not think of how this might actually work nor how it would arrange that bundles made at the same time had the same length. Fifth, there may be some clock that starts and stops bundle formation. This does not explain why the rate of bristle elongation increases with bristle length whereas the module length remains unchanged. A sixth mechanism for determining bundle length involves a limitation in the concentration of the components available for assembly. Such a mechanism might work for the microvilli, but it does not account for the growth of the bristle because it does not explain why as the bundle size decreases, the module length is unaffected.
Our proposal for bundle length determination does involve a limitation in the concentration of components but it also couples the concentration of components to the concentration of nucleating sites. Thus, if the number of filaments specified in a nucleating site determines the concentration of components available for bundle formation, the average length of the bundles will be constant, independent of bundle width or rate of bristle elongation. In this sense, the bundle factory would work like the hay baler. Let us suppose for a moment that the generation of nucleating sites requires activation of a protein and that the concentration of activated protein determines the concentration of bundle components available for elongation. If the concentration of components is directly tied to the number of filaments nucleated, then the length of filaments will be constant. Thus, bundles made at the same time from the same pool of components will be the same length.
How a Bundle Factory Works
We suggest that construction of a bundle begins with the activation of a site of nucleation. These sites are seen as dense membrane-associated patches on the cell's membrane. The identity of this material is unknown, but from what we have stated earlier it is unlikely to be Arp2/3. Coupled to activation of the nucleating site is the activation of the bundle components so that the concentration of components is proportional to the concentration of nucleation sites. One possibility is that activation involves the release of a component from the nucleation site. The released component in turn determines the concentration of components in the bundle. The average length of the bundles would then be proportional to the ratio of the concentration of bundle components to the concentration of nucleating sites. After activation of the nucleating sites and the components, the bundles assemble on the cell surface in the form of a microvillus.
While this mechanism can explain how the average length of the bundle is independent of bundle diameter and rate of assembly in the case of the bristle, can it equally explain why bundles made at the same time are more uniform than bundles made at different times? Filaments assembled at the same time from a common pool of subunits will have lengths that are Poisson distributed. In a 3-μm-long filament, there are ∼1,000 actin subunits. If the average is 1,000, its standard deviation is ∼30, a few percent of the average. Thus, filaments assembled at the same time will have lengths within a few percent of one another. If the concentration of nucleating sites determines the concentration of components for bundle assembly, then the fluctuations in its concentration will determine the variation in the concentration of components. The number of such sites is small, and thus the fluctuations arising from the small number will be proportionately higher relative to the average. Thus we would expect the variation in lengths of bundles made at different times to be significantly greater than those made at the same time. Exactly what the actual numbers or concentrations are, and what the fluctuations are due to, requires a knowledge of the components.
We have argued for the existence of a small bundle–making factory that ties nucleation of the bundle to the activation of components for bundle assembly. We think that such a scheme can account for the common beginnings of all bundles in cells. We noted that all bundles initiate at a dense patch on a membrane. The identity of the components in such patches is completely unknown. Because microvilli typify best the components of this factory, we suggest it is time to turn our attention to identifying the components that make up the dense patch. Their identities should unlock the pathway of bundle assembly.
Acknowledgments
We would particularly like to thank the reviewers of this manuscript, especially to the one anonymous reviewer for his unfailing and constructive help in the reorganization of this paper.
This work is supported by grants NIH-GM26357 to David J. DeRosier and NIH-GM52857 to Lewis G. Tilney.
References
- Bartles J.R., Zheng L., Li A., Wierda A., Chen B. Small espina third actin-bundling protein and potential forked protein ortholog in brush border microvilli. J. Cell Biol. 1998;143:107–119. doi: 10.1083/jcb.143.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckerle M.C. Spatial control of actin filament assemblylessons from Listeria . Cell. 1998;95:741–748. doi: 10.1016/s0092-8674(00)81697-9. [DOI] [PubMed] [Google Scholar]
- Begg D.A., Rebhun L.I., Hyatt H. Structural organization of actin in the sea urchin egg cortexmicrovillar elongation in the absence of actin filament bundle formation. J. Cell Biol. 1982;93:24–32. doi: 10.1083/jcb.93.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda R.L., Eiserling F.A. Evidence for an internal component of the bacteriophage T4D tail corea possible length-determining template. J. Virol. 1982;43:714–720. [Google Scholar]
- Ezzell R.M., Chafel M.M., Matsudaira P.T. Differential localization of villin and fimbrin during development of the mouse visceral endoderm and intestinal epithelium. Development. 1989;106:407–419. doi: 10.1242/dev.106.2.407. [DOI] [PubMed] [Google Scholar]
- Fath K.R., Obenauf S.D., Burgess D.R. Cytoskeletal protein and mRNA accumulation during brush border formation in adult chicken enterocytes. Development. 1990;109:449–459. doi: 10.1242/dev.109.2.449. [DOI] [PubMed] [Google Scholar]
- Ferrary E., Cohen-Tannoudji M., Pehau-Arnaudet G., Lapillonne A., Athman R., Ruiz T., Boulouha L., El Marjou F., Doye A., Fontaine J.J. In vivo, villin is required for Ca2+-dependent F-actin disruption in intestinal brush borders. J. Cell Biol. 1999;146:819–829. doi: 10.1083/jcb.146.4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guild G.M., Connelly P.S., Shaw M.K., Tilney L.G. Actin filament cables in Drosophila nurse cells are composed of modules that slide passively past one another during dumping. J. Cell Biol. 1997;138:783–797. doi: 10.1083/jcb.138.4.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger M., Wright J., Wang K. Nebulin as a length regulator of thin filaments of vertebrate skeletal musclescorrelation of thin filament length, nebulin size, and epitope profile. J. Cell Biol. 1991;115:97–107. doi: 10.1083/jcb.115.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky L.M., Insall R.H. Signalling to actin dynamics. J. Cell Biol. 1999;146:267–272. doi: 10.1083/jcb.146.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins R.D., Heuser J.A., Pollard T.D. The interaction of Arp2/3 complex with actinnucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc. Natl. Acad. Sci. USA. 1998;95:6181–6186. doi: 10.1073/pnas.95.11.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins R.D., Stafford W.F., Pollard T.D. Structure, subunit topology, and actin-binding activity of the Arp2/3 complex from Acanthamoeba . J. Cell Biol. 1997;136:331–343. doi: 10.1083/jcb.136.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibayama T., Carboni J.M., Mooseker M.S. Assembly of the intestinal brush borderappearance and redistribution of microvillar core proteins in developing chick enterocytes. J. Cell Biol. 1987;105:335–344. doi: 10.1083/jcb.105.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes D.L., DeRosier D.J. Growth conditions control the size and order of actin bundles in vitro . Biophys. J. 1991;59:456–465. doi: 10.1016/S0006-3495(91)82239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina T.M., Borisy G.G. Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J. Cell Biol. 1999;145:1009–1026. doi: 10.1083/jcb.145.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L.G., Bonder E.M., DeRosier D.J. Actin filaments elongate from their membrane-associated ends. J. Cell Biol. 1981;90:485–494. doi: 10.1083/jcb.90.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L.G., Connelly P., Smith S., Guild G.M. F-actin bundles in Drosophila bristles are assembled from modules composed of short filaments. J. Cell Biol. 1996;135:1291–1308. doi: 10.1083/jcb.135.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L.G., Connelly P.S., Vranich K.A., Shaw M.K., Guild G.M. Why are two different cross-linkers necessary for actin bundle formation in vivo and what does each cross-link contribute? J. Cell Biol. 1998;143:121–133. doi: 10.1083/jcb.143.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L.G., DeRosier D.J. Actin filaments, stereocilia, and hair cells of the bird cochlea. IV. How the actin filaments become organized in developing stereocilia and in the cuticular plate. Dev Biol. 1986;116:119–129. doi: 10.1016/0012-1606(86)90048-5. [DOI] [PubMed] [Google Scholar]
- Tilney L.G., DeRosier D.J., Weber A., Tilney M.S. How Listeria exploits host cell actin to form its own cytoskeleton. II. Nucleation, actin filament polarity, filament assembly, and evidence for a pointed end capper. J. Cell Biol. 1992;118:83–93. doi: 10.1083/jcb.118.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L.G., Egelman E.H., DeRosier D.J., Saunder J.C. Actin filaments, stereocilia, and hair cells of the bird cochlea. II. Packing of actin filaments in the stereocilia and in the cuticular plate and what happens to the organization when the stereocilia are bent. J. Cell Biol. 1983;96:822–834. doi: 10.1083/jcb.96.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L.G., Fukui Y., DeRosier D.J. Movement of the actin filament bundle in Mytilus sperma new mechanism is proposed. J. Cell Biol. 1987;104:981–993. doi: 10.1083/jcb.104.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L.G., Jaffe L.A. Actin, microvilli, and the fertilization cone of sea urchin eggs. J. Cell Biol. 1980;87:771–782. doi: 10.1083/jcb.87.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachsstock D.H., Schwartz W.H., Pollard T.D. Affinity of alpha-actinin for actin determines the structure and mechanical properties of actin filament gels. Biophys. J. 1993;65:205–214. doi: 10.1016/S0006-3495(93)81059-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch M.D., DePace A.H., Verma S., Iwamatsu A., Mitchison T.J. The human Arp2/3 complex is composed of evolutionarily conserved subunits and is localized to cellular regions of dynamic actin filament assembly. J. Cell Biol. 1997;138:375–384. doi: 10.1083/jcb.138.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]