Abstract
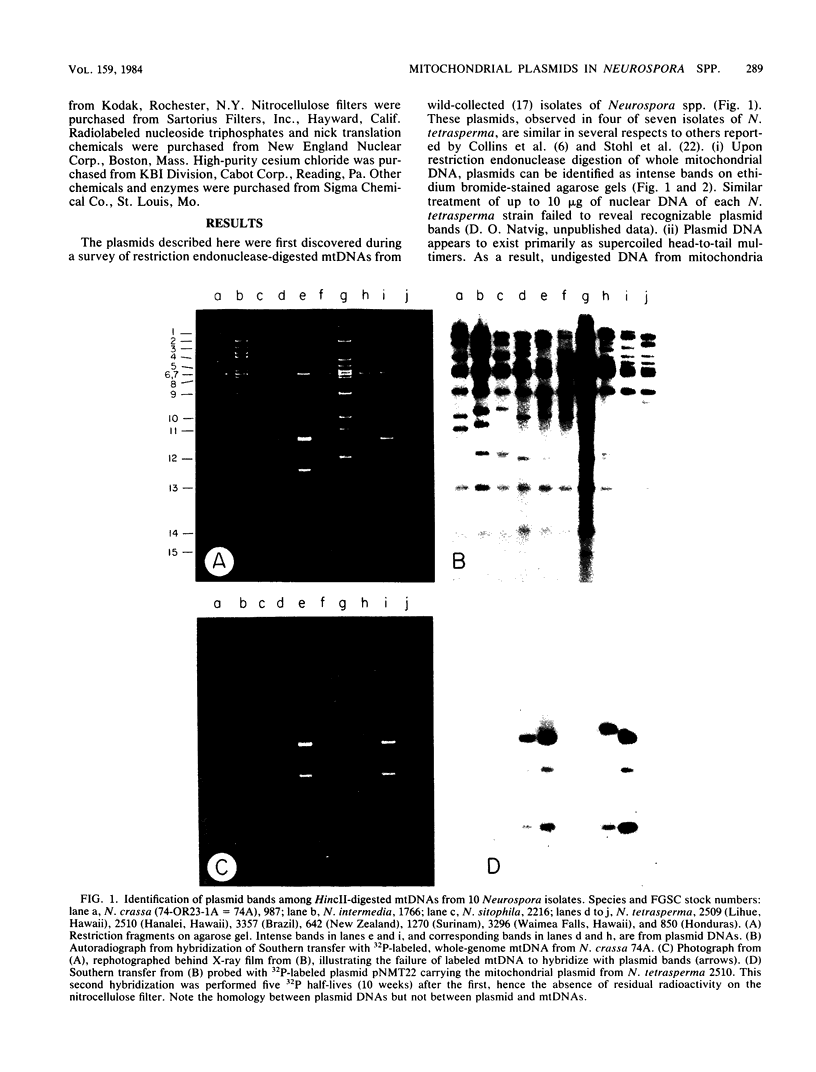
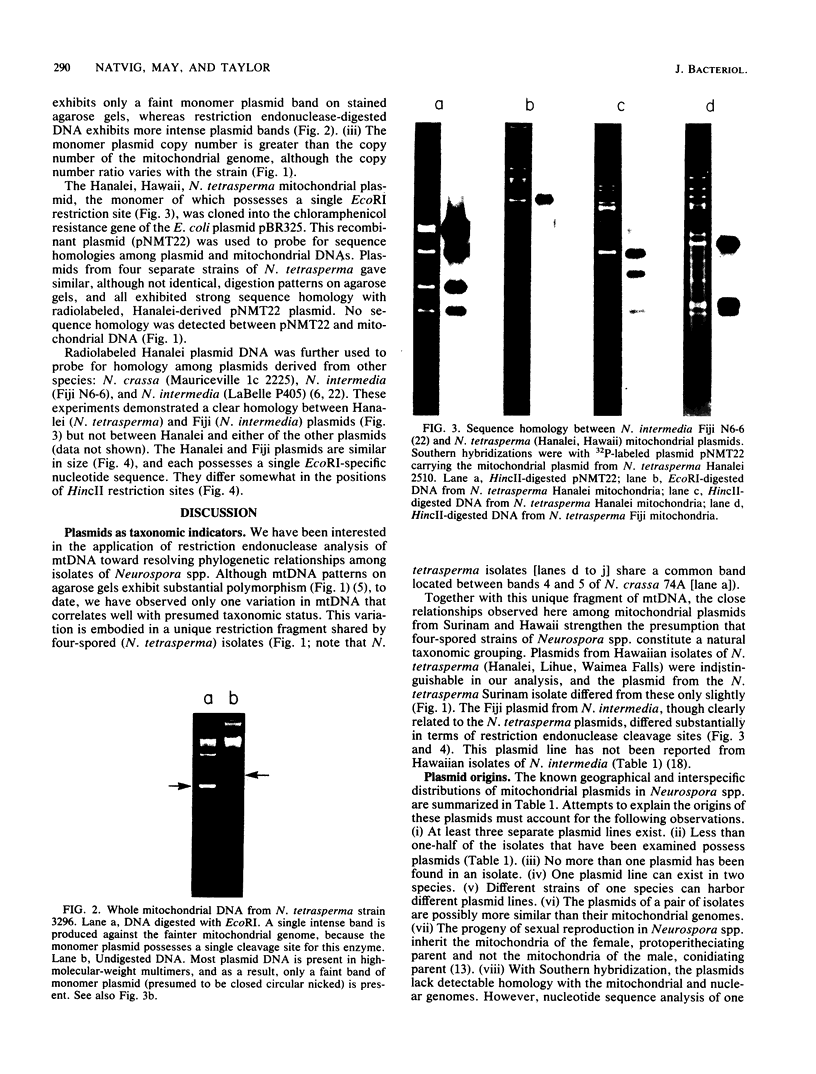
A mitochondrial plasmid line in the fungal genus Neurospora is geographically widely distributed and occurs in isolates of at least two species. On the basis of characterization with restriction endonucleases, it is apparent that plasmids from isolates of Neurospora tetrasperma are more closely related to one another than to an evolutionarily homologous plasmid from Neurospora intermedia; N. tetrasperma plasmids from Surinam and Hawaii differed from one another only slightly by our analysis, whereas the plasmid from N. intermedia (Fiji) exhibited substantial restriction site divergence from all N. tetrasperma plasmids. We believe these observations strengthen the presumption that four-spored isolates of Neurospora spp. represent a natural taxonomic grouping (N. tetrasperma). The plasmids from N. tetrasperma and N. intermedia (Fiji), although clearly related to each other as shown by hybridization studies, exhibited no detectable homology with either of two additional plasmid lines from isolates of Neurospora spp. Nor did they exhibit homology with the mitochondrial genome. Despite this lack of homology among three distinct plasmid lines, all the plasmids may possess a common mode of replication.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertrand H., Collins R. A., Stohl L. L., Goewert R. R., Lambowitz A. M. Deletion mutants of Neurospora crassa mitochondrial DNA and their relationship to the "stop-start" growth phenotype. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6032–6036. doi: 10.1073/pnas.77.10.6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Borst P., Grivell L. A. The mitochondrial genome of yeast. Cell. 1978 Nov;15(3):705–723. doi: 10.1016/0092-8674(78)90257-x. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Collins R. A., Lambowitz A. M. Structural variations and optional introns in the mitochondrial DNAs of Neurospora strains isolated from nature. Plasmid. 1983 Jan;9(1):53–70. doi: 10.1016/0147-619x(83)90031-8. [DOI] [PubMed] [Google Scholar]
- Collins R. A., Stohl L. L., Cole M. D., Lambowitz A. M. Characterization of a novel plasmid DNA found in mitochondria of N. crassa. Cell. 1981 May;24(2):443–452. doi: 10.1016/0092-8674(81)90335-4. [DOI] [PubMed] [Google Scholar]
- HOWE H. B., Jr Markers and centromere distances in Neurospora tetrasperma. Genetics. 1963 Jan;48:121–131. doi: 10.1093/genetics/48.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamet-Vierny C., Begel O., Belcour L. Senescence in Podospora anserina: amplification of a mitochondrial DNA sequence. Cell. 1980 Aug;21(1):189–194. doi: 10.1016/0092-8674(80)90126-9. [DOI] [PubMed] [Google Scholar]
- Lambowitz A. M. Preparation and analysis of mitochondrial ribosomes. Methods Enzymol. 1979;59:421–433. doi: 10.1016/0076-6879(79)59103-4. [DOI] [PubMed] [Google Scholar]
- Mannella C. A., Goewert R. R., Lambowitz A. M. Characterization of variant Neurospora crassa mitochondrial DNAs which contain tandem reiterations. Cell. 1979 Dec;18(4):1197–1207. doi: 10.1016/0092-8674(79)90232-0. [DOI] [PubMed] [Google Scholar]
- Mannella C. A., Lambowitz A. Interaction of wild-type and poky mitochondrial DNA in heterokaryons of Neurospora. Biochem Biophys Res Commun. 1978 Feb 14;80(3):673–679. doi: 10.1016/0006-291x(78)91621-2. [DOI] [PubMed] [Google Scholar]
- Mannella C. A., Pittenger T. H., Lambowitz A. M. Transmission of mitochondrial deoxyribonucleic acid in Neurospora crassa sexual crosses. J Bacteriol. 1979 Mar;137(3):1449–1451. doi: 10.1128/jb.137.3.1449-1451.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylyk O. M. Heterokaryon incompatibility genes in Neurospora crassa detected using duplication-producing chromosome rearrangements. Genetics. 1975 May;80(1):107–124. doi: 10.1093/genetics/80.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylyk O. M. Heteromorphism for Heterokaryon Incompatibility Genes in Natural Populations of NEUROSPORA CRASSA. Genetics. 1976 Jun;83(2):275–284. doi: 10.1093/genetics/83.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargang F. E., Bell J. B., Stohl L. L., Lambowitz A. M. A family of repetitive palindromic sequences found in Neurospora mitochondrial DNA is also found in a mitochondrial plasmid DNA. J Biol Chem. 1983 Apr 10;258(7):4257–4260. [PubMed] [Google Scholar]
- Rieck A., Griffiths A. J., Bertrand H. Mitochondrial variants of Neurospora intermedia from nature. Can J Genet Cytol. 1982;24(6):741–759. doi: 10.1139/g82-080. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stohl L. L., Collins R. A., Cole M. D., Lambowitz A. M. Characterization of two new plasmid DNAs found in mitochondria of wild-type Neurospora intermedia strains. Nucleic Acids Res. 1982 Mar 11;10(5):1439–1458. doi: 10.1093/nar/10.5.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohl L. L., Lambowitz A. M. Construction of a shuttle vector for the filamentous fungus Neurospora crassa. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1058–1062. doi: 10.1073/pnas.80.4.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Boogaart P., Samallo J., Agsteribbe E. Similar genes for a mitochondrial ATPase subunit in the nuclear and mitochondrial genomes of Neurospora crassa. Nature. 1982 Jul 8;298(5870):187–189. doi: 10.1038/298187a0. [DOI] [PubMed] [Google Scholar]