Abstract
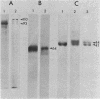
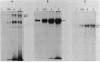
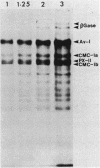
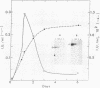
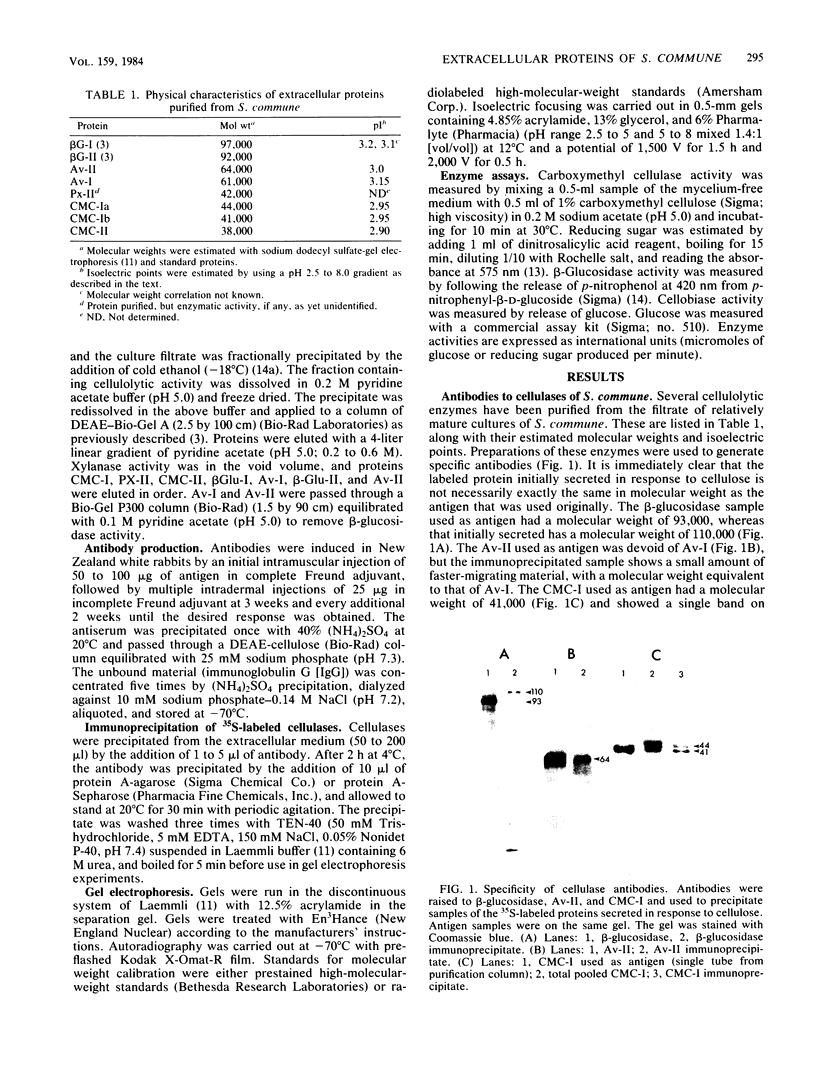
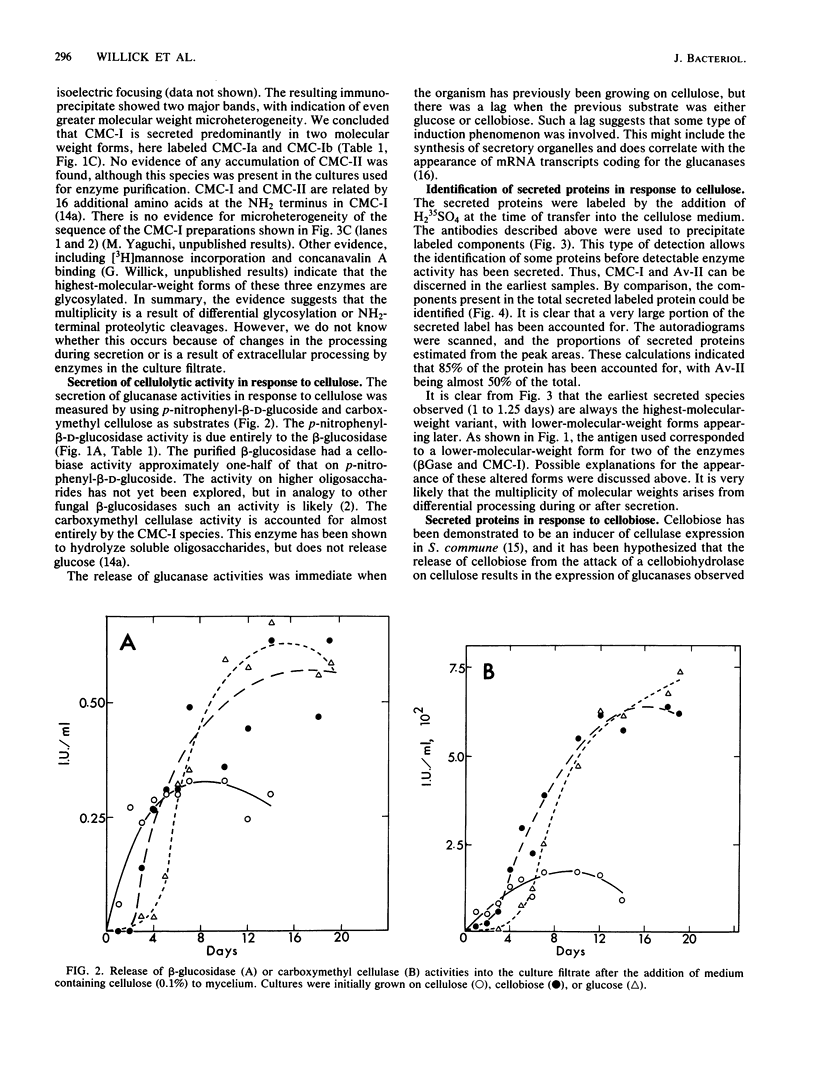
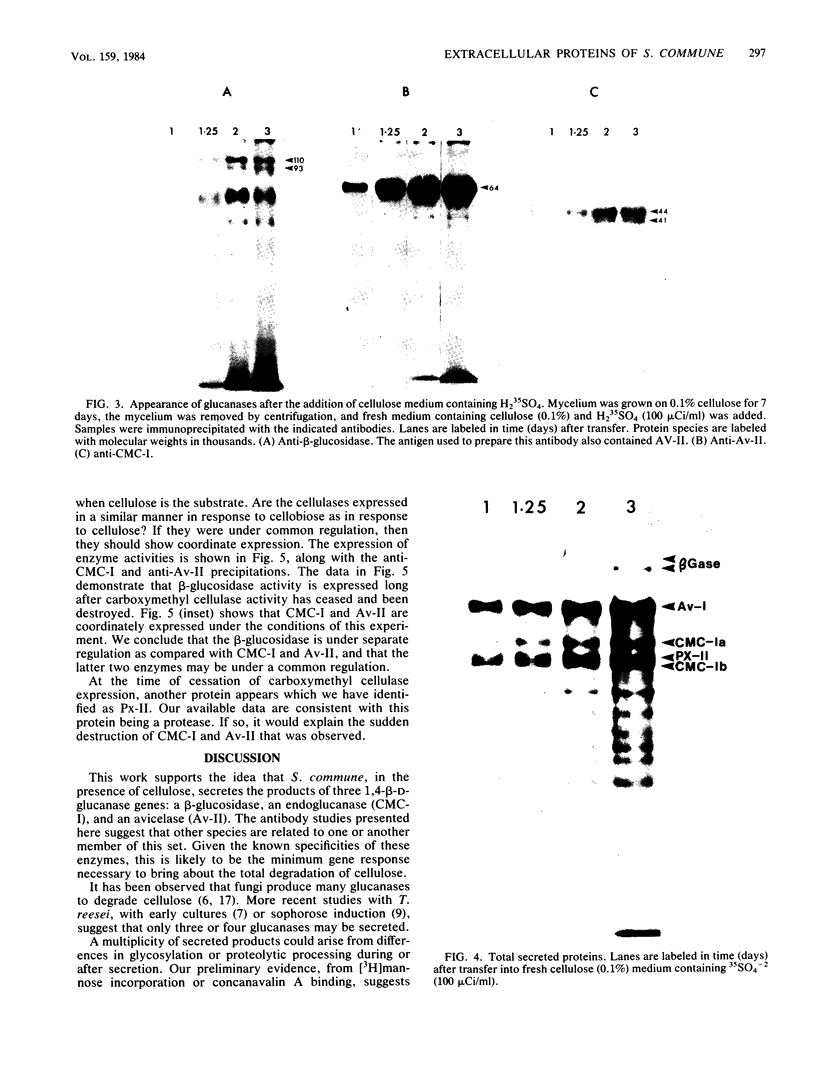
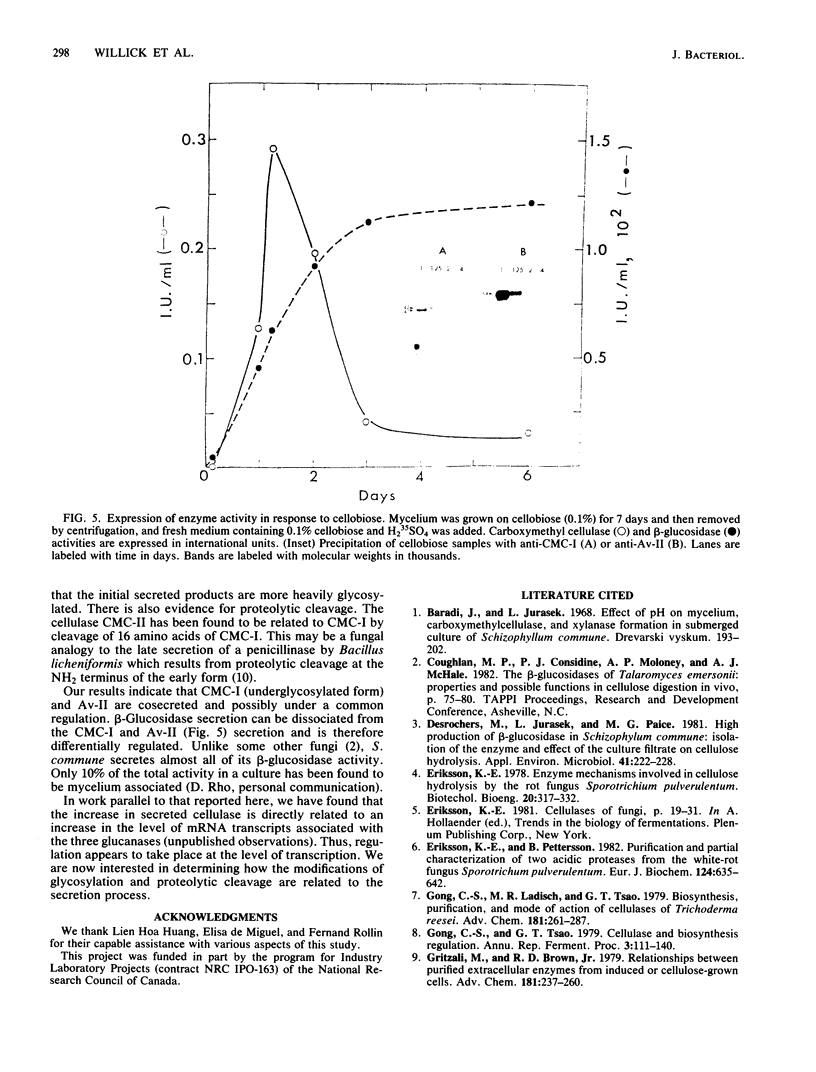
The secretion of 1,4-beta-D-glucanases by the basidiomycete Schizophyllum commune in response to cellulose or cellobiose has been studied. The proteins were labeled with 35S, and the secretion of enzymes was measured by beta-glucosidase and carboxymethyl cellulase activities and by immunoprecipitation with specific antibodies. The antigen proteins used were a beta-glucosidase (Mr, 93,000), an avicelase (avicelase II; Mr, 64,000), and a carboxymethyl cellulose (carboxymethyl cellulase I; Mr 41,000). The beta-glucosidase was initially secreted as an Mr 110,000 form, which was followed later by lower-molecular-weight (88,000 to 93,000) forms. The avicelase II, which accounted for about 50% of the secreted labeled protein, had an Mr of 64,000. Secretion of the related avicelase I (Mr 61,000) followed later. The carboxymethyl cellulose I was secreted in two molecular weight forms, Mr 44,000 and 41,000. The evidence is consistent with the idea that three genes account for the secreted glucanase activities. Other species result from different glycosylation or proteolytic cleavage processing, which may occur during or after secretion. The beta-glucosidase secretion appears to be regulated differently than that of avicelase II or carboxymethyl cellulase I; the latter two were regulated coordinately under the conditions used in this work. No common immune determinants between the three antigens were observed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Desrochers M., Jurasek L., Paice M. G. High Production of beta-Glucosidase in Schizophyllum commune: Isolation of the Enzyme and Effect of the Culture Filtrate on Cellulose Hydrolysis. Appl Environ Microbiol. 1981 Jan;41(1):222–228. doi: 10.1128/aem.41.1.222-228.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson K. E., Pettersson B. Purification and partial characterization of two acidic proteases from the white-rot fungus Sporotrichum pulverulentum. Eur J Biochem. 1982 Jun;124(3):635–642. doi: 10.1111/j.1432-1033.1982.tb06641.x. [DOI] [PubMed] [Google Scholar]
- Izui K., Nielsen J. B., Caulfield M. P., Lampen J. O. Large exopenicillinase, initial extracellular form detected in cultures of Bacillus licheniformis. Biochemistry. 1980 Apr 29;19(9):1882–1886. doi: 10.1021/bi00550a023. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mandels M., Andreotti R., Roche C. Measurement of saccharifying cellulase. Biotechnol Bioeng Symp. 1976;(6):21–33. [PubMed] [Google Scholar]
- Nisizawa T., Suzuki H., Nisizawa K. "De novo" synthesis of cellulase' induced by sophorose in Trichoderma viride cells. J Biochem. 1971 Sep;70(3):387–393. doi: 10.1093/oxfordjournals.jbchem.a129653. [DOI] [PubMed] [Google Scholar]
- Rho D., Desrochers M., Jurasek L., Driguez H., Defaye J. Induction of cellulose in Schizophyllum commune: thiocellobiose as a new inducer. J Bacteriol. 1982 Jan;149(1):47–53. doi: 10.1128/jb.149.1.47-53.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker S. P., Brown R. D., Jr Characterization of endo-1,4-beta-D-glucanases purified from Trichoderma viride. Biochim Biophys Acta. 1978 Mar 14;523(1):147–161. doi: 10.1016/0005-2744(78)90017-7. [DOI] [PubMed] [Google Scholar]