Abstract
The identification of potentially useful immune-based treatments for prostate cancer has been severely constrained by the scarcity of relevant animal research models for this disease. Moreover, some of the most critical mechanisms involved in complete and proper antitumoral T cell activation have only recently been identified for experimental manipulation, namely, components involved in the costimulatory pathway for T cell activation. Thus, we have established a novel syngeneic murine prostate cancer model that permits us to examine two distinct manipulations intended to elicit an antiprostate cancer response through enhanced T cell costimulation: (i) provision of direct costimulation by prostate cancer cells transduced to express the B7.1 ligand and (ii) in vivo antibody-mediated blockade of the T cell CTLA-4, which prevents T cell down-regulation. In the present study we found that a tumorigenic prostate cancer cell line, TRAMPC1 (pTC1), derived from transgenic mice, is rejected by syngeneic C57BL/6 mice, but not athymic mice, after this cell line is transduced to express the costimulatory ligand B7.1. Also, we demonstrated that in vivo antibody-mediated blockade of CTLA-4 enhances antiprostate cancer immune responses. The response raised by anti-CTLA-4 administration ranges from marked reductions in wild-type pTC1 growth to complete rejection of these cells. Collectively, these experiments suggest that appropriate manipulation of T cell costimulatory and inhibitory signals may provide a fundamental and highly adaptable basis for prostate cancer immunotherapy. Additionally, the syngeneic murine model that we introduce provides a comprehensive system for further testing of immune-based treatments for prostate cancer.
Keywords: CTLA-4, B7.1, CD28, tumor rejection, cellular immunity
In both its prevalence and incidence, prostate cancer ranks as one of the most common cancers among males in the United States. Rates of death attributable to prostate cancer are steadily rising, estimated at more than 40,000 in 1997 alone, vying with colorectal cancer as the second most common cause of cancer-related death in males (1, 2). Although the mainstay of therapy for patients with the advanced form of this disease is androgen withdrawal or suppression, this treatment is seldom, if ever, curative.
Using a variety of means, it has been shown that a host antitumoral immune response can be raised against a number of different cancers. This has prompted a keen interest in immune-based strategies intended for definitive or adjunctive treatment of prostatic adenocarcinoma. Studies using the Copenhagen rat challenged with syngeneic rat prostate cancer (Dunning) cells, modified to secrete xenotypic cytokines including human or murine granulocyte/macrophage colony-stimulating factor (3) or interleukin 2 (4, 5), or murine INF-gamma (5), suggest these cytokines can elicit an antiprostate cancer immune response presumably by improving presentation of putative tumor antigens and/or by directly augmenting cytolytic T cell activation and proliferation. Further progress in prostate cancer immunotherapy, however, has been markedly hindered by the scarcity, until recently, of wholly syngeneic, immunologically intact murine models replete with their species-consistent immunomodulatory genes and gene products. However, the development of the inbred transgenic adenocarcinoma mouse prostate (TRAMP) mouse (6, 7), which has been engineered to spontaneously develop prostate cancer, and the isolation of syngeneic transplantable epithelial prostate cancer cell lines derived from TRAMP prostate tumors, has provided a much needed model system to comprehensively test various experimental, immune-based prostate cancer therapies, including the induction of a specific antitumor T cell response.
The most essential mechanisms involved in regulation of T cell activation and inhibition have only recently been recognized. It is now evident that complete T cell activation requires two signals. The first is provided by antigen-specific signals arising from interactions between the T cell receptor (TCR) and antigen/major histocompatibility complex (MHC). The second signal arises from antigen-independent interactions between the CD28 molecule on the T cell surface with the costimulatory B7 family of ligands (CD80 and CD86), which are commonly expressed on professional antigen-presenting cells (APC). We and others have demonstrated that provision of these costimulatory B7 ligands to cancer cells from diverse tissue origins bypasses the requirement for exogenous APC help, culminating in potent and tumor-specific CD8+ T cell activation (8–10).
The CD28 homolog, CTLA-4, also binds to B7, but with an approximately 20-fold higher affinity than CD28 (11). In contrast to the B7/CD28 interaction, however, the B7/CTLA-4 interaction delivers an inhibitory signal to T cells (12–14). Therefore, the outcome of TCR signaling is dependent on the competing stimulatory and inhibitory interactions of B7 with CD28 and CTLA-4, respectively. In vivo, antibody-mediated blockade of inhibitory interactions between B7 and CTLA-4 can augment host antitumor responses, as has been demonstrated using experimental murine B7+ or B7− colorectal carcinoma (51BLim10) or murine fibrosarcoma (Sa1N) raised in their syngeneic murine hosts (15).
In the present study, we examine whether these newly elucidated mechanisms regulating T cell costimulation can be manipulated to elicit an antiprostate cancer response. To perform these studies, we established a novel syngeneic model in which an early-passage prostate cancer cell line derived from TRAMP mice (6, 7), or genetically modified derivatives of this cell line, is introduced into normal inbred C57BL/6 mice. The current experiments provide the basis for future trials of immunological treatment of localized and metastatic prostate cancers that develop in TRAMP mice and that closely parallel the biology and progression of human prostate cancer.
MATERIALS AND METHODS
Cell Lines.
TRAMPC1 (pTC1) is an early-passage murine prostate cancer cell line derived from TRAMP mice that spontaneously develop prostate cancer due to prostate-specific simian virus 40 (SV40) large T tumor antigen (Tag) expression (6, 7). The medium for growth of this line in culture is Dulbecco’s Modified Eagle Medium (DMEM; GIBCO/BRL) supplemented with 5% fetal calf serum (FCS; HyClone), 5% characterized FCS (NuSerum, Collaborative Biomedical Products, Bedford, MA), 5 μg/ml insulin (Sigma), 0.01 nM dihydrotestosterone (Sigma), and penicillin/streptomycin (BioWhittaker). This line, as well as modified derivatives, were maintained at 37°C in 5% CO2 and were passaged weekly in 10-cm dishes. Cells in these studies typically were used between passages 9 and 20. Cells to be used in animal injections were washed with 10 ml of DMEM × 3 per dish. Animal injections were performed using the specified number of viable cells (determined by trypan blue exclusion) suspended in a final volume of 0.1 ml of either PBS or DMEM injected through a 19-gauge needle.
Stable Transduction of pTC1.
pTC1 was stably transduced to express the costimulatory murine B7.1 (B7-TC1) using an ecotropic retrovirus (16) containing the murine B7.1 (B7) gene. For controls, pTC1 was also transduced with the empty vector lacking the murine B7 gene (vTC1). This retroviral vector also contains a hygromycin resistance gene for positive selection in vitro, as well as the herpes simplex virus thymidine kinase gene (HSVTK) to permit negative selection in vivo following ganciclovir administration (16). After about 1 month in hygromycin selection, B7 is expressed by greater than 95% of the cells.
Flow Cytometry.
Murine B7.1 expression was evaluated by staining with the B7-binding CTLA-4-immunoglobulin fusion protein, followed by FITC-conjugated goat antihuman immunoglobulin (Caltag, South San Francisco, CA). MHC class I and II expression was evaluated by flow cytometry after staining the cells with an anti-pan MHC class I (M1/42.3.9.8.HLK), anti-pan class II MHC (N22), anti-Db (28–11-5S), or anti-Kb (Y-3; American Type Culture Collection), followed by fluorescein isothiocyanate (FITC)-conjugated goat anti-hamster immunoglobulin or goat anti-rat immunoglobulin (Caltag) second-step reagents. Flow cytometry was performed using Coulter Epics Elite ESP flow cytometer.
Isolation and Analysis of SV40 Large Tumor T Antigen (Tag) mRNA.
Analyses for Tag mRNA expression in pTC1 and its derivatives, from tumors raised in BALB/C mice or cells grown in culture, were performed as previously described (6).
Anti-CTLA-4 Production, Purification, and Titering.
Anti-CTLA-4 antibody used in these studies was protein G-purified from nine supernatants from the 9H10 hybridoma line raised in either a Cell Pharm System 2000 (UniSyn, Hopkinton, MA) or Cellco Bioreactor (Cellco, Kensington, MD). Antibody concentrations were quantified by ultraviolet spectrophotometry (15).
Animal Studies.
Animal experiments were conducted in the Laboratory of Kidney and Electrolyte Metabolism, National Heart, Lung, and Blood Institute (National Institutes of Health), and the Cancer Research Laboratory at the University of California at Berkeley according to National Institutes of Health animal care and use guidelines. Six- to 8-week-old male C57BL/6, C57BL/6 nu/nu, and BALB/C nu/nu mice, were obtained from The Jackson Laboratory, Charles River Breeding Laboratories, or Taconic Farms. Mice receiving subcutaneous injections of tumor cells (at the doses specified) received Metophane (Mallinckrodt) inhalational anesthetic at the time of tumor cell injection. Tumor growth was monitored by measuring bisecting diameters of the tumor base with vernier calipers.
RESULTS
MHC I Expression by pTC1.
Cytofluorimetric analysis of pTC1 grown in vitro reveals that approximately 50–70% of early passage (passage ≤10) pTC1 cells express low but detectable MHC class I molecules (Fig. 1). Further passaging of these cells, however, is associated with a decline in MHC I expression, and by passage 15, ≤5% of pTC1 cells express MHC I (Fig. 1). Among those pTC1 cells positive for MHC I, both Kb and Db are expressed at equivalent levels (data not shown). No MHC II is detectable as determined by cytofluorimetric analysis (data not shown).
Figure 1.
Percentage of pTC1 cells expressing MHC class I as a function of cell passage. pTC1 cells, in vitro, were harvested and then evaluated by flow cytometry after staining the cells with an anti-MHC class I antibody followed by FITC-conjugated goat anti-rat immunoglobulin (Caltag) second-step reagents. For each histogram, the pTC1 cell passage number is given first and is followed by the percentage of pTC1 cells that stain positive for MHC class I molecules.
Tag Is Not Expressed by pTC1 or Its Modified Derivatives in Vitro or in Vivo.
To eliminate the possibility that Tag could serve as a potential antigen in our model, pTC1 and its derivatives were analyzed for Tag mRNA by reverse transcription–polymerase chain reaction followed by Southern blot analysis. No Tag mRNA is apparent in pTC1 and derivative cells maintained in vitro (Fig. 2A) or obtained from tumors raised in BALB/C nu/nu mice (Fig. 2B).
Figure 2.
(A and B) mRNA analysis for SV40 large tumor T antigen (Tag) of pTC1, v-TC1, or B7-TC1 grown in vitro (A) or in vivo (B) in BALBc nu/nu mice. (A) Total RNA was isolated from cells in culture, DNase treated, and reverse transcribed; PCR was performed; and the products were visualized by ethidium bromide staining in agarose gel. L-19 was used as an internal control for the PCR. Tag* is the above gel transferred to membrane and probed with a 32P-labeled internal oligonucleotide sequence. Lanes: 1, late-stage TRAMP tumor from TRAMP mouse; 2, molecular weight markers; 3, TC1; 4, vTC1; 5, B7-TC1. (B) Same analysis as above using RNA from: 1, late-stage TRAMP tumor; 2, molecular weight markers; 3, TC1 tumor; 4, vTC1 tumor; and 5, B7-TC1 tumor.
Murine B7 Expression by Murine Prostate Cancer Cells Is Sufficient to Cause Their Rejection in Vivo.
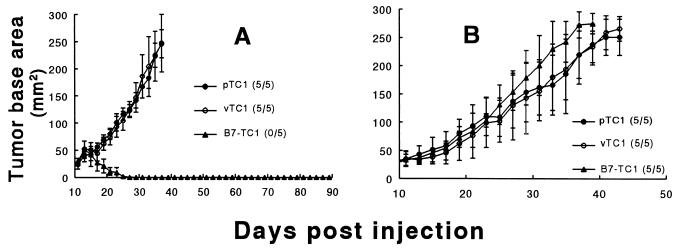
To test whether B7 expression by prostate cancer cells can provide sufficient direct costimulation to elicit an immune response that results in the rejection of these cells, passage 9 pTC1 was modified to express the murine costimulatory ligand B7 (B7-TC1) or the empty transduction vector (vTC1). The stably transduced B7-TC1 and vTC1 cells used in these studies contain a mixed population of cells, of which approximately 30% are MHC I+ in vitro (data not shown). Fig. 3A shows that, despite an early interval of growth, a subcutaneous challenge of 5 × 106 B7-TC1 results in complete rejection of these cells in 100% of C57BL/6 mice tested. In contrast to B7-TC1, pTC1 and vTC1 are uniformly tumorigenic in syngeneic C57BL/6 mice (Fig. 3A). In additional experiments (data not shown) complete rejection also occurred following challenges with 1 × 106 cells up to 15 × 106 cells, and this response was durable given that no tumors were detected after more than 90 days following their rejection. However, B7-TC1 is tumorigenic in male C57BL/6 nu/nu (Fig. 3B) and BALB/C nu/nu mice (data not shown) at doses ranging from 1 to 5 × 106 cells and grows at a rate comparable to pTC1 and vTC1 in these athymic mice (Fig. 3B). These results demonstrate that the failure of B7-TC1 to grow in intact syngeneic mice is a result of a host immune response and not due to loss of inherent tumorigenicity of the modified pTC1 line.
Figure 3.
(A and B) Rejection or growth of B7-TC1, and growth of pTC1 and vTC1 tumors, in male syngeneic C57BL/6 mice (A) or C57BL/6 nu/nu mice (B). (A) Male C57BL/6 mice received subcutaneous injections to the back on day 0 with 5 × 106 cells of p-, v-, or B7-TC1. (B) Male C57BL/6 nu/nu mice received similar injections on day 0 with 1 × 106 pTC1 cells or its derivatives. Data are presented as the mean tumor base area (mm2) ± SD from a single representative experiment that was repeated three times. Each group contained 5–7 mice. The fraction of animals in each group that formed tumors is provided in parentheses next to corresponding marker designations. Animals were euthanized when their tumors achieved 250 mm2.
In Vivo Blockade of CTLA-4 by Anti-CTLA-4 (9H10) Antibody in C57BL/6 Mice Slows Wild-Type pTC1 Growth or Causes Its Rejection.
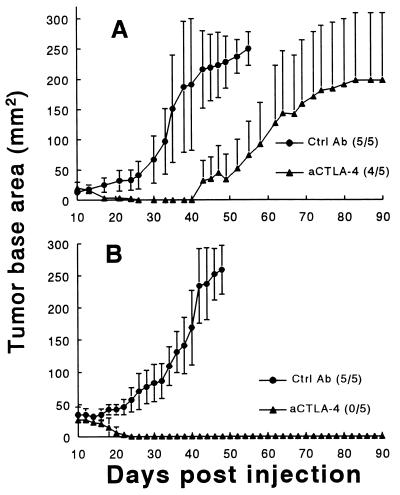
To test whether blockade of CTLA-4 augments an antitumoral immune response against wild-type pTC1, the following experiments were performed. Early-passage unmanipulated pTC1 cells (passages 9, 10, or 11) were injected at three different challenge doses (1.25, 2.5, and 5.0 × 106 cells) subcutaneously into the backs of male inbred C57BL/6 mice. Subsequently, mice received 100-μg intraperitoneal injections of either an irrelevant hamster antibody or anti-CTLA-4 antibody (Ctrl Ab or aCTLA-4, respectively; see Fig. 4) on days 7, 10, and 13 following tumor cell injection. In the representative experiment depicted in Fig. 4A, administration of anti-CTLA-4 significantly delayed the growth and in one animal induced the complete rejection of tumor cells in mice challenged with 2.5 × 106 pTC1 cells. Similar responses to anti-CTLA-4 administration were observed following challenges with either 1.25 or 5 × 106 pTC1 cells, with a slightly greater effect being observed at the lower pTC1 challenge dose (data not shown). Interestingly, however, in two separate experiments using 2 × 106 pTC1 cells of a higher passage (passage >15), which express far less MHC I in vitro, a more complete response was observed (Fig. 4B). In a total of five experiments (including those shown in Fig. 4 A and B), 21 of 50 (42%) anti-CTLA-4-treated mice exhibited complete rejection of their tumors, while the majority of the remaining mice demonstrated delayed growth of pTC1 tumors. In contrast, pTC1 was uniformly tumorigenic in animals treated with the control hamster antibody.
Figure 4.
(A and B) Effects of intraperitoneal anti-CTLA-4 administration on growth of early-passage pTC1 (A, passages 9, 10, and 11) and late passage pTC1 (B, passages >15). C57BL/6 mice were injected with 2.0 × 106 (B) or 2.5 × 106 (A) wild-type pTC1 cells. Data are mean tumor areas from a single, representative experiment in which groups of five mice received either 100 μg of a control hamster antibody (Ctrl Ab) or 100 μg of anti-CTLA-4 antibody (aCTLA-4) on days 7, 10, and 13 following tumor injection. The fraction of animals in each group that formed tumors is provided in parentheses next to corresponding marker designations. Experiments in A were performed three times, and experiments in B were performed two times.
DISCUSSION
We describe a new syngeneic model to investigate whether recently identified mechanisms involved in the costimulatory pathway of T cell activation can be appropriately manipulated for immunotherapy of prostate cancer. To perform these experiments, we used a transplantable prostate cancer cell line, pTC1, that does not express Tag and is readily tumorigenic in the nontransgenic, syngeneic C57BL/6 mouse. Our studies demonstrate that when pTC1 is modified to express the murine B7.1 costimulatory ligand, a host immune response is elicited leading to the complete rejection of these cells in the syngeneic C57BL/6 mouse. Given that B7-TC1, but not pTC1 or vTC1, is rejected by syngeneic C57BL/6 mice (Fig. 3A) and that B7-TC1 is as tumorigenic as pTC1 and vTC1 in athymic mice (Fig. 3B), the mechanism for the rejection of B7-TC1 is likely T cell-mediated. It should be noted, however, that in two experiments, B7-TC1 failed to be completely rejected due to poor initial B7 expression by these cells (data not shown). As has been proposed for other MHC I+ B7-expressing cancer cells, rejection of B7-TC1 likely involves direct, antigen-specific costimulation and activation of CD8+ T cells (8, 9) by these modified prostate cancer cells.
We also demonstrate that in vivo antibody-mediated blockade of CTLA-4 can “tip the balance,” favoring immune-mediated rejection of unmodified prostate tumor cells presumably by removing inhibitory signals in the costimulatory pathway that down-regulate T cell responses. CTLA-4 blockade is capable of slowing the growth of unmanipulated wild-type pTC1 tumors (Fig. 4A). Because these tumor cells do not express costimulatory ligands, it is likely that the effect is at the level of cross-priming of T cells by host APCs rather than by the tumor cells themselves (15, 17, 18). The enhancement by CTLA-4 blockade may be a result of more efficient recognition of antigenic peptides by T cells whose threshold for activation has been lowered or by more extensive proliferation of activated T cells, or both (19).
The effectiveness of transduction with B7 or of CTLA-4 blockade in inducing regression of those pTC1 cells that express little or no MHC I is perhaps surprising. One possibility is that, despite expression at levels below that detectable by flow cytometry, MHC I is present at sufficient levels to support both the inductive and effector phases of the response under the potentiating effects provided by B7 expression or CTLA-4 blockade. Also, MHC I expression might be induced to higher levels in vivo as a result of cytokines released in an initial inflammatory response. In any event, cross-priming of T cells by host APCs with antigens derived from MHC I− tumor cells is well documented (17, 18), and the fact that CTLA-4 blockade would enhance the rejection process would not be at all unexpected.
Also, the mechanism whereby MHC I− tumor cells might be eliminated is not readily apparent, because such cells would not be expected to serve as targets for direct cytolysis by CD8+ killer cells. MHC I− cells could be eliminated as a result of bystander effects by T cells activated by adjacent APCs. In addition, natural killer (NK) cells could play a role in the elimination of MHC I− cells (20), at least in the case of the B7-transfected TC1 prostate cancer cell line. It has been shown that B7-mediated stimulation can enhance the activity of NK cells (21, 22), and NK cells have been directly implicated in the elimination of B7-transfected tumor cells in another system (18). On the other hand, no role of CTLA-4 in NK cell activation or function has yet been established. Studies are underway to elucidate more precisely the mechanisms by which B7 and anti-CTLA-4 enhance antitumor immunity in the current prostate cancer model, particularly when no MHC expression is apparent.
In summary, using a novel murine model for prostate cancer, we have shown that two independent but related limbs of the T cell costimulatory pathway can be manipulated to enhance the immune response against prostate cancer. Future studies will address the application of these therapeutic strategies for the prevention of prostate cancer formation in TRAMP mice and the primary or adjunctive treatment of autochthonous localized or metastatic prostate cancer in these mice (6).
Acknowledgments
We thank Dr. Dana R. Leach for his assistance with preparation of anti-CTLA-4 antibody and maintenance of the 9H10 hybridoma. We also thank Martha R. Kirby for her technical expertise and generous assistance with cytofluorometric analysis, Dr. Sergei Apasov for his helpful discussions, and Santana Flores for his help with animal care. This work was supported in part by National Institutes of Health/National Cancer Institute Grants CA57986, CA64851, and CA40041 (J.P.A), CA09179 (J.P.A, AAH), and a Specialized Program of Research Excellence Prostate Research Grant (CA58204) (N.M.G), and a generous donation to the National Heart, Lung, and Blood Institute Gift Fund by Dr. Stephen M. Quinlan. A.A.H. is the recipient of a Department of Defense Breast Cancer Initiative Fellowship. E.D.K. is an American Foundation for Urologic Diseases (Pfizer) Scholar and American Cancer Society Clinical Oncology Fellow.
ABBREVIATIONS
- MHC
major histocompatibility complex
- APC
antigen-presenting cell
- B7
B7.1
- TRAMP
transgenic adenocarcinoma mouse prostate
- SV40
simian virus 40
- Tag
SV40 large T tumor antigen
References
- 1.Parker S L, Tong T, Bolden S, Wingo P A. Ca Cancer J Clin. 1997;47:5–27. doi: 10.3322/canjclin.47.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Keetch D W, Andriole G L. Monogr Urol. 1996;17:31–48. [Google Scholar]
- 3.Sanda M G, Ayyagari S R, Jaffee E M, Epstein J I, Clift S L, Cohen L K, Dranoff G, Pardoll D M, Mulligan R C, Simons J W. J Urol. 1994;151:622–628. doi: 10.1016/s0022-5347(17)35032-2. [DOI] [PubMed] [Google Scholar]
- 4.Fearon E R, Pardoll D M, Itaya T, Golumbeck P, Levitsky H I, Simons J W, Karasuyama H, Vogelstein B, Frost P. Cell. 1990;60:397–403. doi: 10.1016/0092-8674(90)90591-2. [DOI] [PubMed] [Google Scholar]
- 5.Vieweg J, Rosenthal F M, Bannerji R, Heston W D W, Fair W R, Gansbacher B, Gilboa E. Cancer Res. 1994;54:1760–1765. [PubMed] [Google Scholar]
- 6.Greenberg N M, DeMayo F, Finegold M J, Medina D, Tilley W D, Aspinall J O, Cunha G R, Donjacour A A, Matusik R J, Rosen J M. Proc Natl Acad Sci USA. 1995;92:3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gingrich J R, Greenberg N M. Toxicol Pathol. 1996;24:502–504. doi: 10.1177/019262339602400414. [DOI] [PubMed] [Google Scholar]
- 8.Townsend S E, Allison J P. Science. 1993;259:368–370. doi: 10.1126/science.7678351. [DOI] [PubMed] [Google Scholar]
- 9.Chen L, Ashe S, Brady W A, Hellström I, Hellström K E, Ledbetter J A, McGowan P, Linsley P S. Cell. 1992;71:1093–1102. doi: 10.1016/s0092-8674(05)80059-5. [DOI] [PubMed] [Google Scholar]
- 10.Townsend S E, Su F W, Atherton J M, Allison J P. Cancer Res. 1994;54:6477–6483. [PubMed] [Google Scholar]
- 11.Linsley P S, Greene J L, Brady W, Bajorath J, Ledbetter J A, Peach R. Immunity. 1994;1:793–801. doi: 10.1016/s1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- 12.Krummel M F, Allison J P. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krummel M F, Sullivan T J, Allison J P. Int Immunol. 1996;8:519–523. doi: 10.1093/intimm/8.4.519. [DOI] [PubMed] [Google Scholar]
- 14.Walunas T L, Lenschow D J, Bakker C Y, Linsley P S, Freeman G J, Green J M, Thompson C B, Bluestone J A. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 15.Leach D R, Krummel M F, Allison J P. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 16.Lupton S D, Brunton L L, Kalberg V A, Overell R W. Mol Cell Biol. 1991;11:3374–3378. doi: 10.1128/mcb.11.6.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang A Y C, Golumbeck P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Science. 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 18.Levitsky H I, Lazenby A, Hayashi R J, Pardoll D M. J Exp Med. 1994;179:1215–1224. doi: 10.1084/jem.179.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chambers C A, Krummel M F, Boitel B, Hurwitz A A, Sullivan T J, Fournier S, Cassell D, Brunner M, Allison J P. Immunol Rev. 1996;153:27–46. doi: 10.1111/j.1600-065x.1996.tb00919.x. [DOI] [PubMed] [Google Scholar]
- 20.Lanier L L. Immunity. 1997;6:371–378. doi: 10.1016/s1074-7613(00)80280-0. [DOI] [PubMed] [Google Scholar]
- 21.Nandi D, Gross J A, Allison J P. J Immunol. 1994;152:3361–3369. [PubMed] [Google Scholar]
- 22.Chambers B J, Salcedo M, Ljunggren H. Immunity. 1996;5:311–317. doi: 10.1016/s1074-7613(00)80257-5. [DOI] [PubMed] [Google Scholar]