Abstract
Recent studies of tissue culture cells have defined a widespread system of oxygen-regulated gene expression based on the activation of a heterodimeric transcription factor termed hypoxia-inducible factor-1 (HIF-1). To determine whether the HIF-1 transcriptional response is activated within solid tumors and to define the consequences, we have studied tumor xenografts of a set of hepatoma (Hepa-1) cells that are wild type (wt), deficient (c4), and revertant (Rc4) for an obligatory component of the HIF-1 heterodimer, HIF-1β. Because HIF-1β is also essential for the xenobiotic response (in which it is termed the aryl hydrocarbon receptor nuclear translocator), we also studied c31 cells, which have a different defect in the xenobiotic response and form the HIF-1 complex normally. Two genes that show different degrees of HIF-1-dependent hypoxia-inducible expression in cell culture were selected for analysis—the glucose transporter, GLUT3, and vascular endothelial growth factor (VEGF). In situ hybridization showed intense focal induction of gene expression in tumors derived from wt, Rc4, and c31 cells, which was reduced (VEGF) or not seen (GLUT3) in those derived from c4 cells. In association with these changes, tumors of c4 cells had reduced vascularity and grew more slowly. These findings show that HIF-1 activation occurs in hypoxic regions of tumors and demonstrate a major influence on gene expression, tumor angiogenesis, and growth.
Hypoxia is an important component of many pathological processes including tumor formation, where it has been associated with resistance to radiotherapy, malignant progression, and metastasis formation (1–3). Changes in gene expression accompanying tumor hypoxia are well recognized (4), but the underlying mechanisms and precise consequences are still poorly understood. One recent insight into the regulation of gene expression by hypoxia has come from the definition of a widely operative transcriptional response to hypoxia that is dependent on hypoxia-inducible factor-1 (HIF-1) (for review see ref. 5). This inducible transcription factor was initially defined in studies of the oxygen-regulated expression of the hematopoetic growth factor erythropoietin (6). Subsequently it was recognized that this mechanism of transciptional activation is not confined to erythropoietin-producing cells but appears to be a universal property of mammalian cells (7). Functionally critical HIF-1 binding sites have now been defined in the control sequences of a wide variety of genes, all of which show oxygen-regulated expression in cell culture (8–13). In addition to erythropoietin, these genes include examples with metabolic functions, such as glucose transport and metabolism, and angiogenic growth factors—suggesting that activation of HIF-1 may be involved in the regulation of vascular growth and cellular metabolism.
Following the cloning of cDNAs encoding HIF-1 (14), a new opportunity for the study of this system was recognized in the existence of mutant cells that are unable to form a functional HIF-1 complex. Molecular analysis of HIF-1 revealed that the DNA-binding complex consists of a heterodimer of two basic helix–loop–helix proteins, HIF-1α and HIF-1β (14). HIF-1α was a newly described protein, but HIF-1β had already been recognized as the dimerization partner of the aryl hydrocarbon receptor in the xenobiotic response, where it was termed the aryl hydrocarbon receptor nuclear translocator (ARNT) (15). Mutant cell lines with a defective xenobiotic response have previously been derived from mouse Hepa-1 (Hepa-1c1c7) hepatoma cells (16). These have provided an important tool for analysis of HIF-1, because one complementation group is functionally deficient in HIF-1β/ARNT (17) and is unable to form the HIF-1 heterodimer (18). Examination of oxygen-regulated gene expression in these cells revealed reduced or absent induction by hypoxia of genes encoding glucose transporters, glycolytic enzymes, and vascular growth factors (18–22). This confirmed that HIF-1β/ARNT is critically involved in the induction of these genes in hypoxic cell culture, and suggested the utility of these mutant cells in defining further the consequences of HIF-1 activation.
Ischemic hypoxia occurs frequently in tumors, raising an important question as to whether these conditions are appropriate for the activation of HIF-1 and, if so, what role such transcriptional activation might have in determining the behavior of the tumor. To address these questions we grew the Hepa-1 cells and selected derivatives as xenografts in immunodeficient mice. We report here the existence of large differences in gene expression, vascularity, and growth between the tumor xenografts, which correlated with the ability of the cells to generate a HIF-1 complex. The findings indicate that the microenvironment in hypoxic regions of tumors is appropriate for HIF-1 activation, and that this has an important function in determining tumor angiogenesis and growth.
METHODS
Cell Lines and Culture.
The wild-type (wt) Hepa-1 cells (Hepa-1c1c7) and derivatives c4, c31, and Rc4 have been described previously (16, 17, 23, 24). In outline, c4 and c31 were selected for loss of the xenobiotic response by survival in the presence of benzo[a]pyrene. c4 lacks HIF-1β/ARNT by Western blot analysis and does not form a HIF-1 complex on electrophoretic mobility-shift assay (18). c31 expresses a dominantly acting repressor preventing transcriptional activation of the xenobiotic response but not the hypoxic response (18, 24). Rc4 is a revertant line derived from c4, with wild-type levels of HIF-1β/ARNT activity (23). For measurement of growth rates as tissue culture monolayers, cell lines were inoculated at 50,000 cells per 60-mm dish in MEM alpha medium with 10% fetal calf serum in ambient air, or 1% oxygen (balance nitrogen), with 5% CO2. Each day cells from two dishes of each line were harvested and counted in a Coulter counter, correcting for doublets and triplets with a hemacytometer. Growth rates were determined between 4 and 7 days after inoculation, when growth was exponential. Maximal cell density occurred on about day 9. When culture medium was changed every 2 days (instead of leaving the cells in the original medium), growth rate was not affected, but maximum cell number increased approximately 2-fold. Results are presented for the nonfed dishes.
Growth of Xenografts in Nude Mice.
Tumors were initiated by injection of 106 cells in 50 μl phosphate buffered saline (PBS) under the dorsal skin of NuNu mice. Three independent series of experiments were performed, in which aliquots of the cell types were implanted in parallel as follows. Series 1: wt Hepa-1 and c4, grown to approximately 400 mm3; series 2: wt Hepa-1, c4, Rc4, and c31, grown to 400–800 mm3; and series 3: wt Hepa-1, c4, Rc4, and c31, grown to 200–400 mm3. Every 2nd day tumor size was measured using calipers. Tumors were excised when ulceration occurred or when they reached the predetermined size. They were then frozen in liquid nitrogen or fixed in formaldehyde.
RNA Probes.
cDNA fragments of mouse GLUT3 (nucleotides 736–883, accession no. M75135) and mouse vascular endothelial growth factor (VEGF; nucleotides 177–345, accession no. M95200) were cloned into pSP72 (Promega). Labeled probes were generated with SP6 (antisense) and T7 (sense) polymerase and [35S]UTP, >1,000 Ci/mmol (1 Ci = 37 GBq; Amersham).
In Situ Hybridization.
Ten-micrometer frozen sections were cut onto slides coated with Vectabond (Vector Laboratories), briefly air-dried, and stored at −80°C. Prior to use they were fixed in 4% paraformaldehyde in PBS for 20 min, washed in PBS, and treated with proteinase K (0.0005%) in 0.1 M Tris/0.05 M EDTA, pH 8.0 for 5 min at 37°C. Slides were rinsed in 0.2% glycine in water, postfixed in 4% paraformaldehyde/0.1 M NaOH/0.1 M NaAc for 5 min, rinsed in 0.1 M triethanolamine (TEA), pH 8.0 for 3 min, acetylated for 10 min in 0.25% acetic anhydride/0.1 M TEA, pH 8.8, washed in 2× SSC, and dehydrated. RNA probe was then hybridized to the sections at 60°C for 16 hr in 50% formamide/10% dextran sulfate/0.15 M NaCl/1× Denhardt’s solution/0.01 M Tris⋅Cl, pH 8.0/0.01 M DTT with 0.5 mg/ml tRNA. Sections from each tumor were always hybridized to sense probes as a control for specificity. The slides were next rinsed in 4× SSC and incubated at 37°C for 30 min with 0.1 mg/ml RNaseA in 0.5 M NaCl/0.01 M Tris⋅Cl, pH 8.0/1 mM EDTA. They were then desalted, dehydrated through graded ethanols, and coated with emulsion (Kodak NTB-2, Eastman Kodak). Following exposure at 4°C for 5 days (VEGF) or 2 weeks (GLUT3), emulsion was developed (Kodak D19 developer) and fixed (Kodak Fixer), and sections were stained with hematoxylin and eosin.
Assessment of Vascularity.
Five-micrometer frozen sections were fixed in acetone for 10 min. Endogenous peroxidase was blocked with 0.3% hydrogen peroxide and 0.1% sodium azide in PBS. Sections were then incubated with rat monoclonal antibody to PECAM/CD31 (MEC13.3; ref. 25) followed by peroxidase-conjugated goat anti-rat immunoglobulins (Dako). 3′3′-Diaminobenzidine was used as substrate for peroxidase.
Microvessel density in the most vascular regions of each section was assessed with a Chalkley 25-point eyepiece graticule (Graticules, Tonbridge, UK) as described previously (26). Regions of maximum vascularity were identified at low power. Then, using a 20× objective, the graticule was orientated so that the maximum number of points overlay CD31/PECAM staining or lay within a microvessel. This score was recorded for five fields, and the mean of the highest three scores was calculated.
The concentric circles method (27) was used to assess vascularity throughout the section. An eyepiece graticule with 10 concentric circles of 1–10 mm diameter (Graticules) and a 40× objective were used to measure the distance of the central point of the field to the nearest capillary. The slide was moved in 250-μm steps across the stage (so that the outer circle moved one diameter across the section). Fields were excluded if the central point lay within a region of necrosis or was closer to the edge of the tumor (or an area of necrosis) than to the nearest labeling for CD31. For analysis of the resultant frequency distribution, observations within each circle were assigned the mean of the diameter of that circle and the next smaller circle.
Statistical Analysis.
Differences between means were examined using unpaired t tests, and the Bonnferoni correction was used for multiple comparisons.
RESULTS
Gene Expression in Xenografts.
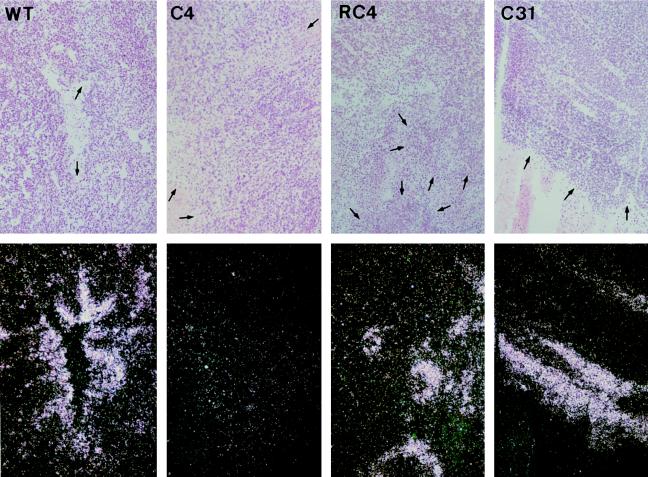
To determine whether HIF-1-dependent changes in gene expression occur in solid tumors we initially examined xenografts of wt Hepa-1 cells and the mutant line c4, which is deficient in HIF-1β/ARNT and does not form HIF-1. First, we studied expression of the glucose transporter GLUT3. We selected this gene for analysis because in cultured wt Hepa-1 cells it is expressed only at a low level in normoxia but shows a high degree of HIF-1β/ARNT-dependent induction by hypoxia (21). We reasoned that if the HIF-1 system of gene regulation were activated by hypoxia in the tumors there would be a large contrast in the expression of GLUT3 mRNA between wild-type and c4 xenografts. In every wt Hepa-1 tumor examined, areas of intense focal expression of GLUT3 mRNA were observed and these were often adjacent to necrotic regions (Fig. 1), with the intensity of signal reducing over a distance of approximately 50–100 μm. In contrast, in the c4 tumors, the signal throughout each tumor was similar to the level of background hybridization. In a minority of the c4 tumors some regional increase in GLUT3 expression was evident around areas of necrosis. However, when this was observed it was much less marked than that seen in the wt Hepa-1 tumors.
Figure 1.
In situ hybridization for GLUT3 mRNA. Bright-field (Upper) and dark-field (Lower) views of sections of wt Hepa-1, c4, Rc4, and c31 xenografts. Regions of high-intensity signal are seen in the dark-field views of wt Hepa-1, Rc4, and c31 tumors, but not in the c4 tumors. Each of the four views includes areas of necrosis. Arrows within necrotic areas in the bright-field views point toward the boundary with viable tumor cells. In the wt, Rc4, and c31 tumors regions of high-intensity signal border on these areas of necrosis. Final magnification, ×80.
Next, xenografts of Rc4 and c31 cells were examined. Rc4 is a revertant line derived from c4 cells that contain HIF-1β/ARNT (23). c31 is another mutant derivative of Hepa-1 cells that manifests dominant interference with the xenobiotic response but expresses HIF-1β/ARNT, and in tissue culture it exhibits unimpaired induction of gene expression by hypoxia (18, 24). In situ hybridization studies of GLUT3 mRNA in tumors derived from c31 cells and from Rc4 cells showed a pattern of gene expression very similar to that observed in wt Hepa-1 tumors. This pattern, which was observed in wt Hepa-1, c31, and Rc4 tumors but not in c4 tumors, demonstrates the importance of HIF-1β/ARNT in the induction of gene expression in these tumors and strongly suggests that this arises from the function of HIF-1β/ARNT in the HIF-1-dependent response to hypoxia rather than from its role in the xenobiotic response.
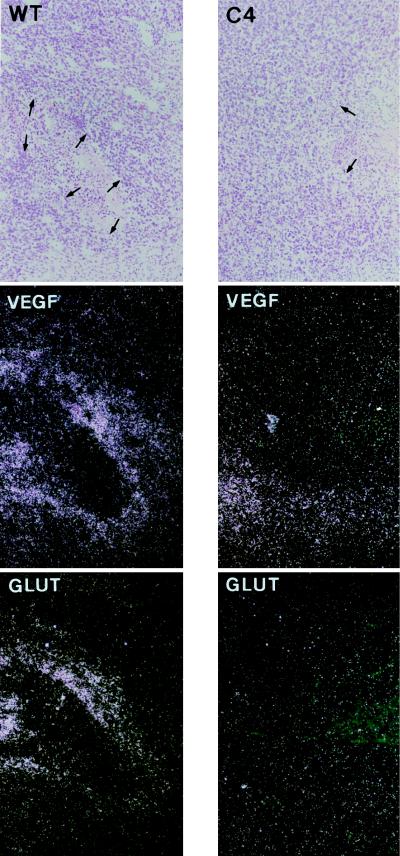
To examine further the importance of HIF-1β/ARNT in the regulation of gene expression in these tumors, sections were analyzed for VEGF mRNA. VEGF gene expression is regulated by several mechanisms involving both transcriptional and posttranscriptional responses (28–32). In keeping with this, tissue culture studies of wt Hepa-1 and c4 cells have shown that hypoxic induction of VEGF mRNA under these conditions is only partly dependent on HIF-1β/ARNT (18, 19). When we examined VEGF mRNA in the xenografts, the level of signal was generally higher than that for GLUT3 mRNA. As with GLUT3 mRNA, there was regional variation in the wt Hepa-1 tumors. When semi-serial sections were examined the regions of high-level VEGF mRNA expression correlated with those showing high-level GLUT3 mRNA expression (Fig. 2), which was consistent with induction of both genes by the same stimulus. In the c4 tumors regional induction of VEGF mRNA expression was clearly less intense than in the wt Hepa-1 tumors, although this difference between the two tumor types was less pronounced than for GLUT3. In c31 and Rc4 tumors, VEGF expression was very similar to that seen in wt Hepa-1 tumors (data not shown).
Figure 2.
In situ hybridization for VEGF and GLUT3 mRNA in wt Hepa-1 (Left) and c4 (Right) tumors. Bright-field (Top) and dark-field (Middle) views of sections hybridized to the antisense VEGF probe. In the wt Hepa-1 tumor high-level VEGF expression borders on an area of necrosis (arrows). Semi-serial sections hybridized for GLUT3 mRNA are also shown (Bottom). In the wt Hepa-1 tumor the same regions express high levels of GLUT3 and VEGF mRNA. Final magnification, ×80.
Thus the regional increase in expression of these two genes in wild-type but not c4 tumors demonstrates that HIF-1β/ARNT-dependent induction of gene expression occurs in extensive regions of wild-type tumors.
Vascularity in Hepa-1 Xenografts.
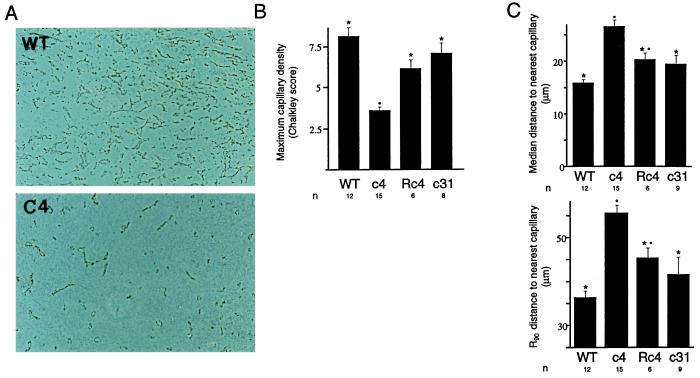
Because hypoxia has been proposed to be an important stimulus for tumor angiogenesis, we sought to determine the role of HIF-1β/ARNT in this process. Blood vessels in tumor sections were identified by indirect immunolabeling of PECAM/CD31 using a rat monoclonal antibody, MEC13.3 (25). Qualitative assessment suggested that vascularity was significantly less in c4 tumors than in tumors derived from wild-type Hepa-1 cells (Fig. 3A). Tumor vascularization was then assessed quantitatively in two different ways in each of the four tumor types. First, maximal capillary density in the most vascular regions of each tumor was assessed using a Chalkley random dot array (26). Results are given in Fig. 3B. In each of the wt Hepa-1, c31, and Rc4 tumors, the maximum capillary density was higher than in c4 tumors, with little overlap between the values obtained. Minor differences between values obtained for c31 and Rc4 tumors compared with wt Hepa-1 tumors were not statistically significant. We next performed an overall assessment of tumor vascularity using the concentric circles method (27). This evaluates the distance of each of an array of points from the nearest identifiable microvessel. By moving an eyepiece graticule over the entire section, the distance from a complete array of points spaced at 250-μm intervals throughout each tumor to the nearest identifiable labeling for PECAM/CD31 was measured (see Methods). From the resulting frequency distribution, the median and 90th centile distances from points within each tumor to the nearest capillary in the section were obtained. Results are given in Fig. 3C. The median and 90th centile distances were both significantly greater in c4 tumors than in wt Hepa-1 tumors. Furthermore, these distances were also substantially greater in c4 tumors than in Rc4 tumors and c31 tumors. The Rc4 and c31 tumors were not quite as well vascularized as the wt Hepa-1 tumors. Within each tumor type, there was no significant correlation between size at the time of excision and vascularity. Thus, the striking difference between tumor types was reduced vascularization of c4 xenografts, with quantitative differences over the whole range from the best to the least vascularized parts of each tumor.
Figure 3.
Immunoperoxidase labeling for the vascular endothelial marker CD31/PECAM. (A) Representative sections through wt Hepa-1 (Upper) and c4 (Lower) xenotransplants viewed with phase contrast. Vascular density is greater in the wt Hepa-1 xenograft. Final magnification, ×80. (B) Histogram showing maximum microvessel density assessed by Chalkley counting. The mean Chalkley score for tumors of each cell type is given (±SEM). n, number of tumors assessed. ∗, significant difference from c4; •, significant difference from wt (P < 0.05). (C) Histograms showing the median and 90th centile distances to the nearest labeling for CD31/PECAM from an array of points within each tumor. Mean values for the tumors of each cell type are given (±SEM). ∗, significant difference from c4; •, significant difference from wt (P < 0.05).
Infiltration and Necrosis.
Infiltrating host macrophages were identified by labeling with the monoclonal antibody F4/80 (33). Areas of necrosis were evaluated in paraffin-embedded sections stained with hematoxylin and eosin. Comparison of xenografts of the different cell lines did not reveal clear differences in either of these parameters. The proportion of each tumor section labeling with F4/80 was between 15% and 40%.
Differences in Xenograft Growth.
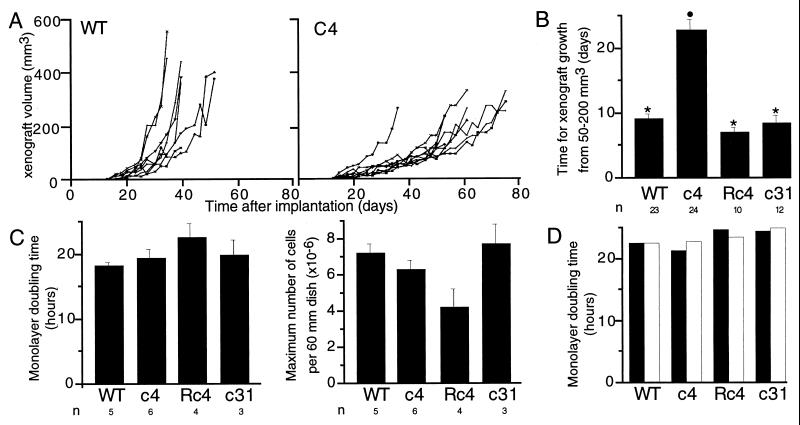
To investigate whether changes in hypoxia-inducible gene expression were associated with altered growth, rates of growth were compared for the four different types of tumor xenograft. Growth curves for individual wt Hepa-1 and c4 tumors from a single series of implantations are shown in Fig. 4A. Overall, growth in the c4 tumors was clearly retarded, although occasional exceptions were seen, one of which can be observed in Fig. 4A. Fig. 4B shows the time taken for a 4-fold increase in tumor size (from 50 to 200 mm3) for each of the four cell types. This time was substantially prolonged for c4 tumors compared with wt Hepa-1. Rc4 and c31 tumors also exhibited more rapid growth than c4 tumors, the rate being similar to wt Hepa-1 tumors.
Figure 4.
Growth of cells in tissue culture monolayers and as xenografts. (A) Growth curves for wt and c4 xenografts from the first series of tumors. Growth of the c4 tumors is seen to be generally slower than the wt tumors. One of the wt Hepa-1 implants did not form a detectable tumor. (B) Histogram showing the time taken for growth from 50 to 200 mm3 for tumors of each cell type from all three series of experiments (mean ± SEM). ∗, significant difference from c4; •, significant difference from wt (P < 0.05). (C) Histograms showing the doubling time and maximum number of cells per 60-mm dish for each of the four cell types in normoxic culture (mean of 3–6 observations, ±SEM). The differences between cell types were not statistically significant. (D) Histograms showing doubling times for cells cultured in parallel in normoxia (solid bars) and 1% oxygen (open bars). The mean from two experiments is shown.
To determine whether these differences in tumor growth might reflect intrinsic differences between the cell lines, growth rates were compared in tissue culture monolayers. Results for normoxic cells are shown in Fig. 4C. Doubling times during the logarithmic phase of growth were similar for wt Hepa-1, c4, Rc4, and c31 cells. The maximal cell density achieved was also similar for wt Hepa-1, c4, and c31 but was somewhat reduced for Rc4 cells.
To consider further the mechanism of reduced xenograft growth, monolayers were cultured in parallel in normoxia (20% oxygen) and hypoxia (1% oxygen). Cell growth was not impaired at 1% oxygen, and no differences were seen between the cell types under these hypoxic conditions (Fig. 4D).
DISCUSSION
Solid tumors contain regions of hypoxia, and tumor hypoxia is an important determinant of clinical prognosis (1–3, 34). Although mechanisms by which severe hypoxia renders tumor cells resistant to radiotherapy and chemotherapy have been defined, it is likely that cellular hypoxia has other important consequences. Recent attention has focused on alterations in gene expression and the possibility that these may induce significant changes such as enhanced angiogenic and metastatic behavior (4, 35, 36).
To determine whether the oxygen-regulated transcription factor HIF-1 is activated in solid tumors, we grew tumor xenografts from Hepa-1 cells that were wild type, defective (c4), and revertant (Rc4) for HIF-1β/ARNT. To provide an assay for the activation of HIF-1-dependent gene expression in the tumors, we first analyzed the expression of GLUT3 mRNA. Hepa-1 cells in normoxic culture express this gene at a low level that is strongly induced by hypoxia (21). In hypoxic tissue culture, induction was not seen in c4 cells but was fully restored by a transfected HIF-1β/ARNT gene, demonstrating dependence on HIF-1β/ARNT (21). Therefore, the focal expression of GLUT3 mRNA in regions of tumors derived from Hepa-1, but not c4, strongly suggested that HIF-1 was activated in these regions of the tumor. Because HIF-1β/ARNT is also critical for the xenobiotic response, it might be argued that these differences could have arisen from functions of HIF-1β/ARNT other than in oxygen-regulated gene expression. Two lines of evidence argue against this. First, tumors of c31 cells (which contain HIF-1β/ARNT but have a defective xenobiotic response; ref. 24) show a similar focal induction of GLUT3 mRNA to wild-type xenografts. Second, focal expression of GLUT3 mRNA was observed bordering on regions of necrosis, in keeping with induction by hypoxia (4, 35).
A similar analysis was performed for VEGF mRNA, and identical arguments suggest that activation of HIF-1 contributes very substantially to the focal pattern of increased expression observed for this gene. Such a pattern of increased expression around necrotic regions of tumors has been described previously in naturally occurring tumors (4, 35). Our results support the view that hypoxia is responsible for the effect. Furthermore, despite the complexity of the biochemical disturbance in ischemic tumors and the multiple regulatory mechanisms operating on genes such as VEGF (28–32), our results demonstrate that, at least in Hepa-1 tumors, HIF-1 or a closely related heterodimer involving HIF-1β/ARNT (37) is the major mediator of this pattern of gene expression.
These findings raise the further question of the role of HIF-1 activation in tumor biology. Vascularization of c4 tumors was reduced compared with wild-type, Rc4, and the hypoxia-responsive but xenobiotic-unresponsive c31 tumors. This suggests that hypoxia, acting via HIF-1-dependent changes in gene expression, is an important factor in the angiogenic process. The observed differences in vascularization were not confined to restricted regions; rather, quantitative differences were observed throughout the frequency distribution of distance to the nearest capillary.
In addition to reduced vascularity, c4 tumors in general grew more slowly than those derived from the other cell types. Interestingly, an occasional c4-derived tumor grew more quickly and at a rate similar to the tumors derived from wild-type cells (Fig. 4A). Although reversion to HIF-1β/ARNT expression is a potential explanation, the reversion rate of these cells in tissue culture is extremely low (23), suggesting other escape mechanisms. In contrast to growth as tumors, no differences in cell growth as monolayers were observed, indicating that differences in growth are not intrinsic to these cells. Though 1% oxygen is a sufficient stimulus for induction of HIF-1-dependent gene expression, it did not impair monolayer growth, suggesting that impaired xenograft growth arose under more severe or tumor-specific conditions. Interestingly, we have found that growth of c4 cells as multicellular spheroids is unimpaired (G.U.D., unpublished data), supporting the importance of impaired tumor vascularity.
Although our experiments indicate that some aspect of HIF-1β/ARNT-dependent gene expression is responsible for the observed difference in tumor phenotype, we cannot determine the extent to which the HIF-1β/ARNT-dependent changes in gene expression of GLUT3 and VEGF might contribute. Nevertheless, VEGF is a clear candidate, because studies with blocking antibodies have demonstrated an important effect on tumor growth (38, 39). However, such studies are not directly analagous to the current experiments because both basal and inducible VEGF would be blocked, irrespective of whether the source was the tumor cells themselves or infiltrating cells. If impairment of VEGF expression is responsible for the altered phenotype of c4 tumors, then our experiments indicate that the HIF-1-inducible component in the tumor cells is responsible for the large difference in vascularization that we observed. Alternatively, other HIF-1-inducible genes such as inducible nitric oxide synthase (which can influence tumor vascularization; ref. 40) or as yet unrecognized HIF-1-inducible genes could contribute to this difference.
Vascularization is vital to tumors, and the development of angiogenic characteristics is believed to be a key event in tumor formation. What is less clear is the extent to which the angiogenic phenotype is dependent on mutational activation of angiogenic mechanisms or driven by environmental stimuli (such as hypoxia) developing within the tumor. Our demonstration of the critical importance of HIF-1β/ARNT, a physiological mediator of the response to hypoxia, strongly suggests that the latter is important.
Acknowledgments
We thank D. Ferguson and T. Hacker for histology, E. Minehart for technical assistance, and S. M. Wood and J. O’Rourke for their contribution to this work. This work was supported by The Wellcome Trust, The Medical Research Council, and the National Cancer Institute, National Institutes of Health.
ABBREVIATIONS
- HIF-1
hypoxia-inducible factor-1
- ARNT
aryl hydrocarbon receptor nuclear translocator
- GLUT3
glucose transporter-3
- VEGF
vascular endothelial growth factor
- wt
wild type
References
- 1.Nordsmark M, Overgaard M, Overgaard J. Radiother Oncol. 1996;41:31–39. doi: 10.1016/s0167-8140(96)91811-3. [DOI] [PubMed] [Google Scholar]
- 2.Höckel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Cancer Res. 1996;56:4509–4515. [PubMed] [Google Scholar]
- 3.Brizel D M, Scully S P, Harrelson J M, Layfield L J, Bean J M, Prosnitz L R, Dewhirst M W. Cancer Res. 1996;56:941–943. [PubMed] [Google Scholar]
- 4.Shweiki D, Itin A, Soffer D, Keshet E. Nature (London) 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 5.Bunn H F, Poyton R O. Physiol Rev. 1996;76:839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- 6.Semenza G L, Wang G L. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maxwell P H, Pugh C W, Ratcliffe P J. Proc Natl Acad Sci USA. 1993;90:2423–2427. doi: 10.1073/pnas.90.6.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Firth J D, Ebert B L, Pugh C W, Ratcliffe P J. Proc Natl Acad Sci USA. 1994;91:6496–6500. doi: 10.1073/pnas.91.14.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semenza G L, Roth P H, Fang H-M, Wang G L. J Biol Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- 10.Ebert B L, Firth J D, Ratcliffe P J. J Biol Chem. 1995;270:29083–29089. doi: 10.1074/jbc.270.49.29083. [DOI] [PubMed] [Google Scholar]
- 11.Levy A P, Levy N S, Wegner S, Goldberg M A. J Biol Chem. 1995;270:13333–13340. doi: 10.1074/jbc.270.22.13333. [DOI] [PubMed] [Google Scholar]
- 12.Norris M L, Millhorn D E. J Biol Chem. 1995;270:23774–23779. doi: 10.1074/jbc.270.40.23774. [DOI] [PubMed] [Google Scholar]
- 13.Melillo G, Musso T, Sica A, Taylor L S, Cox G W, Varesio L. J Exp Med. 1995;182:1683–1693. doi: 10.1084/jem.182.6.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang G L, Jiang B-H, Rue E A, Semenza G L. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reyes H, Reisz-Porszasz S, Hankinson O. Science. 1992;256:1193–1195. doi: 10.1126/science.256.5060.1193. [DOI] [PubMed] [Google Scholar]
- 16.Hankinson O. Proc Natl Acad Sci USA. 1979;76:373–376. doi: 10.1073/pnas.76.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffman E C, Reyes H, Chu F-F, Sander F, Conley L H, Brooks B A, Hankinson O. Science. 1991;252:954–958. doi: 10.1126/science.1852076. [DOI] [PubMed] [Google Scholar]
- 18.Wood S M, Gleadle J M, Pugh C W, Hankinson O, Ratcliffe P J. J Biol Chem. 1996;271:15117–15123. doi: 10.1074/jbc.271.25.15117. [DOI] [PubMed] [Google Scholar]
- 19.Gradin K, McGuire J, Wenger R H, Kvietikova I, Whitelaw M L, Toftgard R, Tora L, Gassmann M, Poellinger L. Mol Cell Biol. 1996;16:5221–5231. doi: 10.1128/mcb.16.10.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H, Ko H P, Whitlock J P., Jr J Biol Chem. 1996;271:21262–21267. doi: 10.1074/jbc.271.35.21262. [DOI] [PubMed] [Google Scholar]
- 21.O’Rourke J F, Pugh C W, Bartlett S M, Ratcliffe P J. Eur J Biochem. 1996;241:403–410. doi: 10.1111/j.1432-1033.1996.00403.x. [DOI] [PubMed] [Google Scholar]
- 22.Salceda S, Beck I, Caro J. Arch Biochem Biophys. 1996;334:389–394. doi: 10.1006/abbi.1996.0469. [DOI] [PubMed] [Google Scholar]
- 23.Van Gurp J R, Hankinson O. Mol Cell Biol. 1984;4:1597–1604. doi: 10.1128/mcb.4.8.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watson A J, Weir-Brown K I, Bannister R M, Chu F-F, Reisz-Porszasz S, Fujii-Kuriyama Y, Sogawa K, Hankinson O. Mol Cell Biol. 1992;12:2115–2123. doi: 10.1128/mcb.12.5.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vecchi A, Garlanda C, Lampugnani M G, Resnati M, Matteucci C, et al. Eur J Cell Biol. 1994;63:247–254. [PubMed] [Google Scholar]
- 26.Fox S B, Leek R D, Weekes M P, Whitehouse R M, Gatter K C, Harris A L. J Pathol. 1995;177:275–283. doi: 10.1002/path.1711770310. [DOI] [PubMed] [Google Scholar]
- 27.Kayar S R, Archer P G, Lechner A J, Banchero N. Microvasc Res. 1982;24:342–353. doi: 10.1016/0026-2862(82)90021-8. [DOI] [PubMed] [Google Scholar]
- 28.Ikeda E, Achen M G, Breier G, Risau W. J Biol Chem. 1995;270:19761–19766. doi: 10.1074/jbc.270.34.19761. [DOI] [PubMed] [Google Scholar]
- 29.Forsythe J A, Jiang B H, Iyer N V, Agani F, Leung S W, Koos R D, Semenza G L. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levy A P, Levy N S, Loscalzo J, Calderone A, Takahashi N, Yeo K-T, Koren G, Colucci W S, Goldberg M A. Circ Res. 1995;76:758–766. doi: 10.1161/01.res.76.5.758. [DOI] [PubMed] [Google Scholar]
- 31.Stein I, Neeman M, Shweiki D, Itin A, Keshet E. Mol Cell Biol. 1995;15:5363–5368. doi: 10.1128/mcb.15.10.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levy A P, Levy N S, Goldberg M A. J Biol Chem. 1996;271:2746–2753. doi: 10.1074/jbc.271.5.2746. [DOI] [PubMed] [Google Scholar]
- 33.Austyn J M, Gordon S. Eur J Immunol. 1981;11:805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- 34.Höckel M, Knoop C, Schlenger K, Vorndran B, Baussmann E, Mitze M, Knapstein P G, Vaupel P. Radiother Oncol. 1993;26:45–50. doi: 10.1016/0167-8140(93)90025-4. [DOI] [PubMed] [Google Scholar]
- 35.Plate K H, Breier G, Weich H A, Risau W. Nature (London) 1992;359:845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- 36.Shweiki D, Neeman M, Itin A, Keshet E. Proc Natl Acad Sci USA. 1995;92:768–772. doi: 10.1073/pnas.92.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tian H, McKnight S L, Russell D W. Genes Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- 38.Kim K J, Li B, Winer J, Armanini M, Gillett N, Phillips H S, Ferrara N. Nature (London) 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 39.Borgstrom P, Hillan K J, Sriramarao P, Ferrara N. Cancer Res. 1996;56:4032–4039. [PubMed] [Google Scholar]
- 40.Jenkins D C, Charles I G, Thomsen L L, Moss D W, Holmes L S, Baylis S A, Rhodes P, Westmore K, Emson P C, Moncada S. Proc Natl Acad Sci USA. 1995;92:4392–4396. doi: 10.1073/pnas.92.10.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]