Abstract
There is an increased need for after-the-fact dosimetry because of the high risk of radiation exposures due to terrorism or accidents. In case of such an event, a method is needed to make measurements of dose in a large number of individuals rapidly and with sufficient accuracy to facilitate effective medical triage. Dosimetry based on EPR measurements of fingernails potentially could be an effective tool for this purpose. This paper presents the first operational protocols for EPR fingernail dosimetry, including guidelines for collection and storage of samples, parameters for EPR measurements, and the method of dose assessment. In a blinded test of this protocol application was carried out on nails freshly sampled and irradiated to 4 and 20 Gy; this protocol gave dose estimates with an error of less than 30%.
1. Introduction and background
After-the-fact dosimetry in isolated teeth and biological dosimetry have been proven to be very useful methods for dose reconstruction and investigation of incidents in which there may be significant exposure to radiation (Clairand et al., 2006). However, for the fast triage of a large number of casualties that is needed immediately after an exposure event, most existing dosimetric techniques require a significant delay before final results are available, while the one existing method that can provide immediate results, in vivo tooth dosimetry, may have limited throughput. Recent studies indicate that EPR-based dosimetry in fingernails or toenails can be an effective method for estimating acute dosimetry of a large number of subjects. Fingernails and toenails contain large amounts of α-keratin and the observed EPR signals appear to be from radiation-induced radicals formed in this component. Similarly, hair also has large amounts of α-keratin. Unfortunately, hair also contains melanin, which has a broad, intense EPR signal that can obscure the radiation-induced signal, making dose measurements in hair less accurate than in nails.
The potential benefits of EPR dosimetry with fingernails and toenails include:
Samples for dose measurements can be readily obtained.
The capability to make immediate dose assessment.
The dose measurements are in different locations in the body, i.e., hands and feet, which are very complementary to in vivo EPR tooth dosimetry.
The measurements can be made using conventional X-band EPR spectrometers, which are widely available.
The potential for having highly sensitive, small field-deployable X-band EPR spectrometers.
Based on prior studies there are several factors that need to be taken into account in establishing a protocol for EPR dosimetry with fingernails (Romanyukha et al., 2007; Wu et al., 1998; Symons et al., 1995; Dalgarno and Mcclymont, 1989):
Fading of the radiation-induced signal (RIS).
Presence of an intrinsic background signal (BKS).
Potential of a signal induced by mechanical stress during sample collection (MIS).
Effects of water content.
The possibility of interpersonal variability of several key factors: the decay of RIS and MIS, the magnitude of the BKS, and the dose-response to radiation.
It is essential to have pre-established guidelines or a protocol in order to manage the measurements of a large number of samples in a short time in an emergency scenario. The main objective of this paper is to propose and describe an operational protocol that could be used in emergency for dose estimation based on EPR measurements in fingernails. The protocol was developed using results from a recent study of the properties of EPR spectra in fingernails (Romanyukha et al., 2007).
2. Protocol for dosimetry
The protocol for fingernail dosimetry includes the following steps:
Sample collection and storage,
EPR measurements including sample preparation,
Dose assessment.
Three main spectral components can be identified in the EPR spectra of fingernails, i.e. a radiation induced signal (RIS), an intrinsic background signal (BKS), and a mechanically induced signal (MIS) (Symons et al., 1995). The structure of the RIS has not yet been fully elucidated, but there are some indications it has at least two components (Romanyukha et al., 2007). The main RIS line is a singlet that overlaps with the MIS and the BKS. Therefore to estimate the radiation dose it is necessary to correct for the contributions of the MIS and BKS.
The main RIS component has a characteristic rate of fading that depends on temperature and water content in fingernails. The simplest way to estimate the dose delivered, is to subtract the contributions of the MIS and BKS from the main singlet intensity and to correct the obtained RIS intensity for time decay as shown in Eq. (1) (Romanyukha et al., 2007):
| (1) |
where Stotal is the total intensity of the singlet line, SBKS is the intensity of the background signal, SMIS is the residual intensity of the MIS after humidification of cut edges, F is the fading correction factor, and D is the coefficient of radiation sensitivity. Linearity of the EPR dose response in fingernails has been observed between 3 and 100 Gy. In order to account for RIS fading, this process can be described by an exponential function of t (time after exposure), T (storage temperature) and H (humidity) as shown in Eq. (2):
| (2) |
3. Sample collection and storage
Separate collection of nails (left/right, hand and foot) is highly desirable because this can give information on the dose distribution of the exposure. The samples should be as large as possible and obtained with the minimum possible number of cuts. There is no need for any special treatment of the sample. Because contact with water can have a significant effect on the RIS (Romanyukha et al., 2007), it is important to note at the time of sample collection any hand washing or excessive hand sweating after exposure. Storage at low temperature (<0°C) significantly reduces the rate of decay of the RIS and, therefore, extends the time period after exposure when dose reconstruction can be done. For example, storage of irradiated fingernails at liquid nitrogen temperature makes dose measurements possible even months after exposure, whereas keeping them at −20 °C (home freezer) extends the time when accurate dose measurements are achievable by up to several weeks. In order to be able to correct measured RIS intensity for time fading, it is important to report accurately the temperature and time of storage. The following data on the subject should be recorded at the time of collection:
Identity information (name, age, gender, occupational activities).
Assumed time, date and type of exposure.
Approximate position of the subject relative to the radiation source.
The subject’s activities since irradiation (hand washing, shower, type, frequency, and length of physical activities).
The collection of nail parings should be done as follows:
Subjects should not wash their hands, if they do it should be noted.
To reduce the intensity of the MIS the nail parings should be as large as possible and obtained with a minimum of cuts.
Separately collect and label nail clippings from fingers and toes on both sides.
Freshly cut nails should be stored without any special treatment into a clearly labeled small tube or container which should be sealed.
If possible, before sealing each sample container, accurately weigh each sample and record it.
Containers should be stored at the lowest available temperature. Record the time, temperature, and type of storage device.
The variations of temperature during storage and transportation (time and temperature) should be reported.
If nail parings are dirty, cleaning without water may be done, but abrasion should be minimized because of the possibility of increasing the MIS. The sample should be weighed initially and also at the end of the protocol when drying is completed; this provides a measurement of the water removed from the sample.
4. Sample preparation
Samples should be obtained in a way to minimize the intensity of the MIS. Since its intensity directly depends on the amount of cut edges, samples should be as large as possible obtained with a minimum amount of cutting. Because the MIS decays much faster than the RIS, it is essential to record the time that the sample was obtained and, if feasible, it may be beneficial to wait to perform the EPR measurements for several hours after the samples are obtained. The decay rate of the MIS is strongly affected by the water content. A method for chemically treating nail parings to selectively eliminate the MIS is in progress, but a protocol for this approach has not been yet finalized (Romanyukha et al., 2007). Because the MIS are mainly located on the cut edges of fingernails, whereas the RIS are distributed over the whole sample, application of a wet brush to the cut edges can be used to selectively eliminate MIS on the edges. Such treatment can be easily performed and it allows almost complete MIS removal, whereas reduction of the RIS is less than 5%. For samples of large size with a minimum of cuts, a sample mass of 15–30 mg is enough to provide accurate dose measurements.
5. EPR measurements
As an example, the following parameters were used for spectra acquisition with a Bruker EMX spectrometer equipped with a high Q cavity: (incident microwave power 0.5 mW, modulation amplitude 0.2 mT, and time constant of 163.8 ms). The number of repeated acquisitions and the number of scans for each acquisition should be optimized according to the intensity of the measured signals, the number of samples, as well as a compromise between the accuracy of the measurements and the urgency to have the results available. As an example, 3 to 6 scans are usually used for each acquisition and measurements can be repeated two or three times.
6. Dose estimation
Under emergency conditions, an initial rapid estimate of dose in nails can be carried out using Eqs. (1) and (2) with average values of H, D, and BKS intensity determined from previous measurements of nails taken from the largest possible sample of donors. This preliminary rough dose estimate can be performed in less than 10 min, including the time of measurement. Variations in these average values due to age, ethnic origin, gender, dietary factors and season of the year have not been yet studied. If future studies will show such influence, different sets of average values then can be determined and tabulated.
The accuracy of the dose assessment can be improved by determining the real individual value of BKS, H, and D of samples with additional steps in the protocol. The following protocol is outlined in Fig. 1.
Fig. 1.
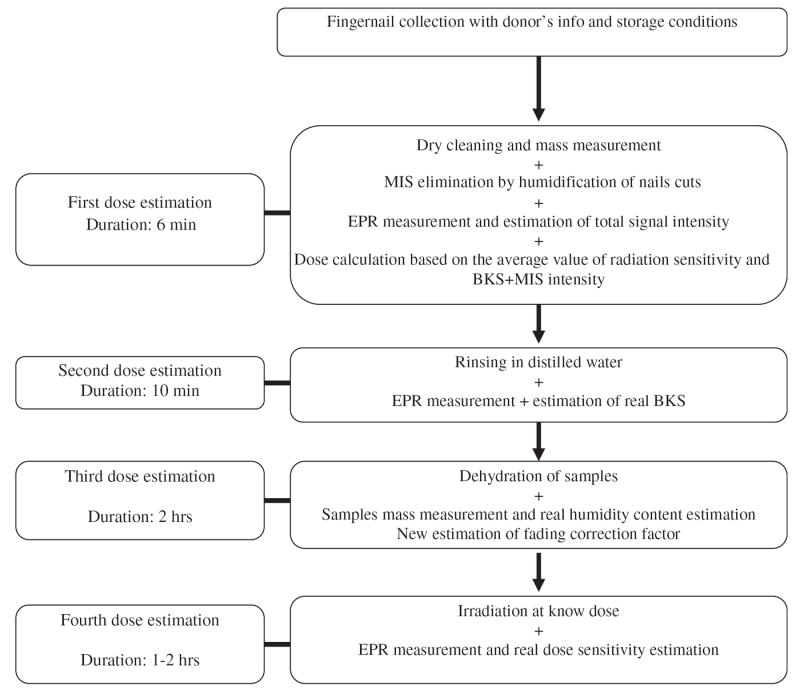
The workflow diagram for the proposed protocol shows 4 repeated measurements to separate the BKS, MIS and RIS signals. The fourth measurement determines the radiation dose sensitivity by irradiating to a known dose.
Determination of BKS intensity: Rinsing samples for 10 s in distilled water can completely eliminate the RIS and residual MIS. Then the EPR measurement provides the value of the BKS. This additional step can be done in less than 10 min.
Determination of water content of the sample: Samples are weighed, then dried and then weighed again. The difference in mass before and after drying is an estimate of the water content; the average value for freshly cut fingernails is estimated at 5.5% and values range between 3% and 8%. In our case, samples were dried at 55 °C for 2 h in an oven used for thermoluminescence dosimeter annealing. The use of this information allows one to correct for fading due to the water content.
Determination of the individual dose-response of individual samples: After measurement of the water content, the sample can then be irradiated to a known dose for determination of the dose–response to radiation. As an example, a dose of 40 Gy of 60Co gamma rays was used for the results reported here. Measurement was performed after the same time delay and with the same water content (dry samples) that had been used to determine the initial estimate of dose.
A first blind test of this protocol was carried out on freshly sampled nails that were irradiated to doses of 4 or 20Gy. For measurements performed 2 h after irradiation, from the first step, this protocol delivered dose estimates with an error of less than that 30% using averaged parameters. Extended tests are scheduled to assess the accuracy of this protocol for various time delays between irradiation and storage at low temperature. The dose detection limit for fingernails was previously estimated between 2 and 5 Gy (Romanyukha et al., 2007; Wu et al., 1998; Symons et al., 1995; Dalgarno and Mcclymont, 1989). Clearly it is desirable to carry out additional experiments to more firmly establish the dose–response relationship and limits of detectability.
7. Limits of applicability
One of the main limiting factors is the delay between the irradiation and the collection of the sample. In real conditions, this delay could range from 1–2 h to days. Because the increase of temperature or humidity accelerates the decay of the signal, it might be difficult to estimate accurately the fading correction factor because of difficulty in estimating these conditions before sample collection.
The ability to implement this technique in the field depends on developments which, while quite achievable, have not yet been accomplished. This includes the development of techniques to obtain the samples in a standardized physical form and to make the measurements with an appropriate field-deployable EPR spectrometer.
As noted above, it would be very desirable to improve knowledge of the parameters that affect the dose–response relationship. These range from the need to make measurements in samples of different sizes as well as assessing inter-individual variation regarding the BKS, MIS generation, dose–response of the RIS, and the rate of signal fading. Nevertheless, even with the present state of development, the results obtained in the first blinded test of the protocol were very encouraging and would be applicable for immediate use in an emergency.
8. Conclusion
Using our current knowledge of nail dosimetry, it was possible to write a protocol for collection, storage, and measurement by EPR of fingernails or toenails in emergency situations. Rapid estimation of dose in the range of a few Gy to several tens of Gy can be made quickly and for a large number of samples, with an estimated accuracy of 30% if samples are properly collected in a delay of a few hours after irradiation. If the exposure is asymmetric it can provide data on some critical aspects of the dose distribution. The same protocol may be also used for incidents involving orphan sources or unsecured gamma radiography sources, which can deliver potentially very high doses to the extremity. If only a few subjects are involved, it would be feasible to carry out additional steps that would significantly increase the accuracy of the measurements.
Acknowledgments
This study was partially supported in part by NIH Grant U19 AI067733, and by a Department of Defence Grant, DA905-02-011 (DTRA).
References
- Clairand I, Trompier F, Bottollier-Depois J-F, Gourmelon P. Ex vivo ESR measurements associated with Monte Carlo calculations for accident dosimetry: application to the 2001 Georgian accident. Radiat Prot Dosim. 2006;120:500–505. doi: 10.1093/rpd/nci516. [DOI] [PubMed] [Google Scholar]
- Dalgarno BG, Mcclymont JD. Evaluation of ESR as a radiation accident dosimetry technique. Appl Radiat Isotopes. 1989;40:1013–1020. [Google Scholar]
- Romanyukha A, Trompier F, LeBlanc B, Calas C, Clairand I, Mitchell C, Smirniotopoulos JG, Swartz H. EPR dosimetry in chemically treated fingernails. Radiat Meas. 2007 doi: 10.1016/j.radmeas.2007.05.026. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons M, Chandra H, Wyatt JL. Electron paramagnetic resonance spectra of irradiated fingernails: a possible measure of accidental exposure. Radiat Prot Dosim. 1995;58:11–15. [Google Scholar]
- Wu K, Guo L, Cong JB, Sun CP, Hu JM, Zhou ZS, Wang S, Zhang Y, Zhang X, Shi YM. Researches and applications of ESR dosimetry for radiation accident dose assessment. Radiat Prot Dosim. 1998;77:65–67. [Google Scholar]


