Abstract
The nerve growth factor precursor (proNGF) may function as a death-inducing ligand that mediates its apoptotic effects via p75NTR. ProNGF-induced apoptosis is postulated to be dependent upon membrane expression of the sortilin receptor, which interacts with p75NTR to promote a high affinity-binding site for proNGF. Here, we explore the expression of proNGF, sortilin and p75NTR in the mouse lumbar dorsal root ganglion (DRG) to understand the potential for this trimeric signaling complex to function in injury-induced neuronal death of DRG neurons. Our results reveal the expression of all 3 components within the DRG and that a subpopulation of neurons coexpresses sortilin and p75NTR. Following sciatic nerve transection, the expression of these proteins appears insensitive to injury; however, the majority of small p75NTR-sortilin coexpressing neurons are lost 25 days after sciatic nerve transection. These results propose proNGF-induced, p75NTR-sortilin mediated neuronal death as a critical aspect of nerve-injury induced death in the DRG.
Keywords: cell death, DRG, mouse, proNGF, sortilin, p75NTR, peripheral nerve
Introduction
Traumatic nerve injury frequently leads to loss of function due to neuronal degeneration and death. The molecular mechanisms involved in injury-induced neuronal death are poorly understood, but are thought to involve apoptosis, or programmed cell death (Ekstrom, 1995). Recently, several molecules have been identified as key players in the induction of neuronal death. Sortilin, a type I membrane receptor expressed in neuronal tissues and thought to be involved in membrane trafficking, has been shown to interact with the p75 neurotrophin receptor (p75NTR) in the induction of apoptosis upon binding unprocessed nerve growth factor (NGF), also known as proNGF (Nykjaer et al., 2004).
Neurotrophins are growth factors released by target tissues that can regulate cell survival and death signaling. Neurotrophins, such as NGF, are synthesized as precursors known as proneurotrophins. These pro-forms are susceptible to intracellular as well as extracellular cleavage by numerous proteolytic enzymes including furin, plasmin and MMP (Lee et al., 2001). Proneurotrophins have long been thought to be inactive precursors to their mature counterparts, but recent evidence has identified proNGF as the predominant form of NGF in many tissues, recognizing it as a potentially biologically important molecule (Lee et al., 2001; Yardley et al., 2000; Fahnestock et al., 2001; Pedraza et al., 2005; and Reinshagen et al., 2000). Neurotrophins are capable of mediating profound diverse biological effects depending on processing of the pro-forms and their interaction with two cell surface receptors, p75NTR and the trk family of tyrosine kinase receptors. For example, mature NGF preferentially binds to trk A and promotes cell survival. However, recent reports have demonstrated that proneurotrophins, such as proNGF, selectively bind to p75NTR and activate its pro-apoptotic signaling cascade (Nykjaer et al., 2004). The formation of a signaling complex in which the sortilin receptor interacts with p75NTR is required for proNGF-mediated neuronal death to occur (Nykjaer et al., 2004). ProNGF simultaneously binds sortilin (via the pro-domain) and p75NTR (via the mature domain) and thereby acts as a crosslinker, creating a tertiary complex that results in the activation of neuronal apoptosis.
Evidence identifying proNGF as a physiological ligand for p75NTR under pathological conditions continues to build. Recent reports demonstrate that proNGF expression is induced after brain injury and causes death of adult corticospinal neurons upon binding p75NTR in vivo (Harrington et al., 2004). Likewise, proNGF mediates cell death of oligodendrocytes following spinal cord injury in vivo (Beattie et al., 2002) and has been suggested to be responsible for the neurodegeneration found in the brains of Alzheimer’s disease patients (Pedraza et al., 2005).
In this report, we investigate whether members of the proNGF-p75NTR-sortilin signaling complex could potentially be responsible for the injury-induced apoptosis of primary sensory neurons in the lumbar L4/5 dorsal root ganglia (DRG). The results presented here demonstrate that members of this signaling complex are present in the L4/5 DRG and their expression is not significantly altered soon after injury. Moreover, there is a population of L4/5DRG neurons that coexpress both sortilin and p75 NTR and that these neurons may be susceptible to injury-induced neuronal death 25 days following sciatic nerve transection. These data propose this signaling complex as a potential mechanism driving injury-induced apoptosis in certain L4/5 DRG neurons.
Results
Sortilin is present in the mouse L4/5 DRG and its expression is not responsive to injury
Immunocytochemistry was performed to identify L4/5 DRG neurons that express sortilin. Cell counts of sortilin-immunoreactive neurons revealed that 73.8% +/− 4.82 of L4/5 DRG neurons express the sortilin receptor (Fig. 1). Neurons that displayed sortilin immunoreactivity displayed a diffuse cytoplasmic distribution of sortilin, as well as superficial membrane staining. (Fig. 1). The cytoplasmic distribution of sortilin observed here is consistent with previous studies that localize over 90% of sortilin expression to intracellular vesicles including the golgi apparatus and endo- and lysosomal compartments (Petersen et al., 1997 and Mazella et al., 1998). Next, we evaluated the responsiveness of sortilin expression to injury. L4/5 DRG were examined for sortilin expression 3 days following sciatic nerve transection. At this time point, injury results in little neuronal death but effects the expression of many neuropeptides (Shi et al., 2001 and McKay Hart et al., 2002). Cell counts of sortilin-immunoreactive neurons after injury revealed a similar patter on staining and 72.8% +/− 3.50 of neurons were sortilin-positive (Fig. 1). An unpaired t-test determined that there was no significant difference in the number sortilin-expressing neurons between injured and uninjured groups (p = 0.3573). To substantiate this result, we used Western blot analysis to quantify sortilin peptide expression. Using the same antisera, we were able to detect a band of 95 kDa, which is consistent with the molecular weight of sortilin (Petersen et al., 1997) (Fig. 2A). Peptide expression was quantified by analyzing band intensity and no significant difference in the number of neurons expressing sortilin was observed between injured and uninjured groups (p = 0.8643). Collectively, these findings support the view that sortilin protein expression is not altered in response to injury of peripheral axons of L4/5 DRG neurons.
Figure 1.

Immunocytochemistry was performed on L4/5 DRG of uninjured and injured C57BL/6 mice to identify neurons that express sortilin (red, top panels) or p75NTR (green, middle panels). Top left) On average, 73% of neurons express sortilin in uninjured mice. Top right) Similarly, 72.8% of neurons express sortilin 3 days following sciatic nerve transection. Middle left) 66.1% of neurons express p75NTR in uninjured mice. Middle right) Similarly, 67.2% of neurons express p75NTR three days after sciatic nerve transection. Bottom left) Merged image illustrating the overlap of sortilin and p75 with no injury (left) and following injury (right) Scale bars equal 100μm for each panel.
Figure 2.

Immunoblotting analysis was performed using L4/5 DRG removed 3 days following no injury (lane 1) or sciatic nerve transection (lane 2). A) A polyclonal antibody against sortilin recognized a band of 95 kDa that was determined to be the sortilin receptor. All protein loading was normalized to cyclophilin A (18 kDa). An unpaired t-test performed on band intensity revealed no significant difference in sortilin expression between injured and uninjured groups (p = 0.8643), suggesting that sortilin expression is not altered in DRG neurons following sciatic nerve injury.
B) Immunoblotting analysis was performed using L4/5 DRG removed 3 days following no injury (lane 1) or sciatic nerve transection (lane 2). A polyclonal antibody designed to detect p75NTR recognized a band of 75 kDa that was identified as the p75 receptor. Bands of ~55, ~135, and ~155 kDa represent possible cleaved or post-translationally modified forms of p75NTR, respectively. All protein loading was normalized to cyclophilin A (18 kDa). An unpaired t-test performed on band intensity revealed no significant difference in p75NTR expression between injured and uninjured groups (p = 0.7836), suggesting that p75NTR expression is not altered in DRG neurons following sciatic nerve injury.
p75NTR is present in the L4/5 DRG and its expression is not responsive to injury
DRG neurons expressing p75NTR were identified using a primary polyclonal antibody and neuronal counts revealed that 66.1% +/− 2.83 of L4/5 DRG neurons from uninjured mice express p75NTR (Fig. 1). According to the literature, p75NTR expression is upregulated in response to injury, throughout various disease states, and during development (King et al., 2000; Syroid et al., 2000; and Crockett et al., 2000). However, these studies were carried out in the central nervous system. Similarly, we assessed if p75NTR expression was altered by sciatic nerve transection. Analysis of L4/L5 DRGs 3 days following sciatic nerve transection revealed a similar pattern of p75NTR and counts of 67.2% +/− 4.43 p75NTR-positive neurons (Fig. 1). An unpaired t-test confirmed no significant difference in the average number of neurons expressing p75NTR between groups (p = 0.8248). To confirm these results, we employed Western blot analysis to re-examine the effects of injury upon p75NTR expression. Application of the primary polyclonal antibody resulted in the presence of a ~75 kDa band along with bands of approximately ~55, ~135, and ~155 kDa in size (Fig. 2B). These multiple bands were present in samples taken from both injured and uninjured mice. Band intensity was analyzed to detect any alteration in peptide expression. An unpaired t-test did not detect any significant difference in p75NTR expression between groups (p = 0.7836), confirming our immunocytochemical results.
Sortilin is expressed in several neuronal subpopulations within the lumbar DRG
Next, we sought to determine if sortilin expression is confined to a specific neuronal subpopulation within the DRG. These neurons can be divided into 3 broad subcategories based on expression of neuronal markers. The first class consists of neurons that do not express the classic neuropeptides generally associated with pain transmission; however, they will bind the isolectin IB4, which is specific for small, non-peptidergic sensory neurons (Kashiba et al., 2001). The second subpopulation consists of neurons that produce calcitonin gene-related peptide (CGRP), which exclusively identifies the small- and medium-sized peptidergic sensory neurons (Silverman et al., 1988). Finally, there is a class of neurons that can be identified using the neuronal marker N52, which is specific for large, myelinated neurons. Uninjured L4/5 DRG neurons that express both the sortilin receptor and a specific neuronal marker were counted. Immunocytochemistry revealed 13.6% +/− 1.16 of sortilin-positive neurons express IB4. Alternatively, 31.7% +/− 3.69 of IB4-positive neurons expresses the sortilin receptor (Table 1, Fig. 3). Approximately 20.6% +/− 1.47 of sortilin-expressing neurons coexpress CGRP, while 44.1% +/− 3.10 of CGRP-positive neurons express sortilin (Table 1, Fig. 3). Finally, approximately 18.2% +/− 2.57 of neurons that express sortilin also coexpresses N52. On the contrary, 52.8% +/− 4.95 of N52-expressing neurons display sortilin coexpression (Table 1, Fig. 3). In conclusion, sortilin expression is found within the small non-peptidergic neurons, the small-medium sized peptidergic neurons, and finally the large, myelinated neurons. These data indicate that sortilin expression is not confined strictly to one primary sensory neuronal population, but rather it is found within all classes of L4/5 DRG neurons.
Table 1.
| Total No. of Immunopositive Neurons | No. of Sortilin Expressing Neurons that Coexpress CGRP, IB4, or N52 | |
|---|---|---|
| Sortilin | 778 | |
| CGRP | 334 | 106 (13.6 %) |
| IB4 | 365 | 161 (20.6 %) |
| N52 | 269 | 142 (18.2%) |
Values represent the percentage of immunopositive neurons in L4/5 DRGs from 6-week-old mice (n = 3)..
Figure 3.
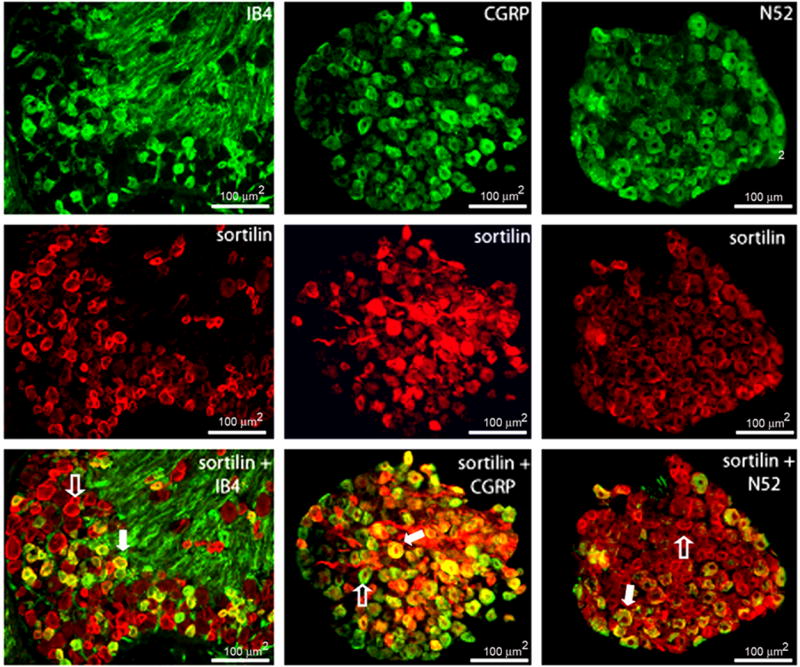
Immunocytochemistry was performed on L4/5 DRG from uninjured C57BL/6 mice using a primary polyclonal sortilin (red) antibody in conjunction with the neuronal markers (green) IB4, CGRP, and N52. 13.6% of sortilin-expressing neurons coexpress IB4. Alternatively, 31.7% of IB4-expressing neurons coexpress the sortilin receptor. 20.6% of sortilin-expressing neurons coexpress CGRP while 44.1% of CGRP-expressing neurons coexpress sortilin. 18.2% of sortilin-expressing neurons coexpress N52; 52.8% of N52-expresing neurons coexpress sortilin. Filled arrows represent neurons that coexpress sortilin and a specific neuronal marker; open arrows identify neurons that express sortilin in the absence of neuronal markers.
proNGF is the predominant form of NGF present in the mouse L4/5 DRG
Next, we sought to determine if the pro-form of nerve growth factor is present in the mouse DRG and if its expression is responsive to injury. To date, this was the first assessment of proNGF expression in the mouse L4/5 DRG. Western blot analysis using a polyclonal antibody designed to detect the pro-form of proNGF detects bands of approximately ~35, ~40, ~44, and ~87 kDa in size (Fig. 4A). Each band is present in all of the treatment groups (no injury and sciatic nerve crush or transection). The ~ 35kDa band closely resembles the molecular weight of the unglycosylated NGF precursor, as detected by Yardley and colleagues (Yardley et al., 2000). The ~40, ~44, and ~80 kDa bands most likely represent post-translationally modified forms of proNGF (Yiangou et al., 2002 and Lobos et al., 2005). To address the effects of injury upon proNGF expression, band intensity was analyzed between treatment groups. ANOVA did not reveal a significant effect of group on band intensity, indicating that proNGF expression is not altered in mouse L4/5 DRG neurons in response to peripheral sciatic nerve injury (p = 0.9990). Next, we inquired if the bands detected by our proNGF antibody truly represented peptides of proNGF nature. We used a polyclonal antibody aimed at identifying the mature domain of NGF. First, using a commercial source of recombinant NGF peptide, we ensured that this particular antibody would indeed detect mature NGF (Fig. 4B). Similarly, the same bands of ~35, ~40, ~44, and ~87 kDa were again detected in each treatment group. On the contrary, a band of ~91 kDa was also present which was not detectable with the use of proNGF antisera, which suggested the band represented at ~91 kDa to not be of true NGF origin (Fig. 4C). Interestingly, we were not able to distinguish a band for mature NGF (~13 kDa), signifying that mature NGF is not present in detectable quantities under injured or uninjured conditions in the mouse L4/5 DRG. Again, band intensity was analyzed and no significant difference in peptide expression was detected between groups using one way ANOVA (p = 0.9868).
Figure 4.
A) Immunoblotting analysis was performed using L4/5 DRG from the following groups of mice: no injury (lane 1), three day post-sciatic nerve crush (lane 2), or transection (lane 3). A polyclonal antibody specific for the pro-domain of proNGF detected multiple bands of ~35, ~40, ~44, and ~80 kDa. The ~ 35 kDa band was determined to identify the unglycosylated NGF precursor. All higher molecular weight bands likely represent post-translationally modified forms of proNGF. Statistical analysis using ANOVA revealed no significant difference in band intensity amongst groups (p = 0.999), suggesting that proNGF expression is not altered in response to injury in the DRG. A polyclonal antibody specific for mature NGF detected bands of similar size, including a band of ~ 91 kDa (see panel C). Because this band was not detectable when using the pro-NGF antibody, this band was determined to not be of true NGF origin. B) Commercially prepared NGF (20ng/lane) was used to test the specificity of the mature antibody. C) Mature NGF (12 kDa) was not present in detectable concentrations. No significant change in peptide expression was detected by ANOVA between treatment groups (p = 0.9868). All protein loading was normalized to cyclophilin A (18kDa).
Certain DRG neurons co-express sortilin and p75NTR
Next, we examined if there exists within the L4/5 DRG a unique population of neurons that coexpress the sortilin and p75NTR receptors, making them particularly vulnerable to proNGF-induced neuronal death. To examine this matter, we used immunocytochemistry to identify which neurons were positive for both sortilin and p75NTR. First, the total number of neurons expressing either the sortilin or the p75NTR receptor was determined. Next, we assessed the percentage of sortilin-positive neurons coexpressing p75NTR and vice versa, the number of p75NTR-positive neurons coexpressing sortilin in uninjured animals (Table 2). Analysis revealed 52.5% +/− 3.77 of sortilin-positive neurons express p75NTR. Alternatively, 50.7% +/−3.84 of p75NTR-positive neurons coexpress the sortilin receptor. Three days following sciatic nerve transection, 54.5% +/− 2.82 of sortilin-positive neurons continue to coexpress p75NTR and 49.9% +/− 0.83 of p75NTR- positive neurons maintain sortilin expression. These data suggest that there is no significant change in the number of neurons coexpressing sortilin and p75NTR 3 days after injury.
Table 2.
| Sortilin Expressing Neurons | p75NTR Expressing neurons | Sortilin+/p75 NTR Expressing neurons | % Sortilin that Express p75NTR | % p75NTR that Express Sortilin | |
|---|---|---|---|---|---|
| No Injury | 780 | 806 | 409 | 52.5 % | 507 % |
| Injury | 759 | 829 | 414 | 54.5 % | 49.9 % |
Values represent the percentage of positively labeled neurons in L4/5 DRGs from 6-week-old mice (n = 3). No significant differences were observed between non-injured and injured groups.
To determine if this neuronal subpopulation was vulnerable to death following injury, we extended the time frame at which neuronal death was assessed and examined the number of sortilin-p75NTR coexpressing neurons 25 days following sciatic nerve transection. We observed a 59.4% loss of sortilin-p75NTR coexpressing neurons compared to uninjured animals 25 days following sciatic nerve axotomy.
In addition, we sought to define which particular class of neurons among the sortilin-p75NTR coexpressing neuronal population is particularly susceptible to injury-induced neuronal death. To identify this population, we measured the size of sortilin-p75NTR coexpressing neurons present in uninjured mice but absent 25 days following sciatic nerve axotomy (Fig. 5). These data indicate that small neurons (0–200μm2) comprise 58.03% of the total number of neurons found in uninjured mice. However, neurons of an equivalent area were completely absent in the injured group. Likewise, medium-sized neurons (200–400μm2) compose 22.79% of the uninjured population while only 1.84% of neurons measured were found of the same area within the injured group. Finally, 19.18% of the total neuronal population can be classified as large (<400μm2) in uninjured mice while this size class accounts for the majority of the total neuronal population (98.16%) in injured mice. These results are represented in a histogram-based model by a large shift to the right, indicating that the percentage of small and medium-sized neurons is drastically reduced in injured mice while the large-sized neuronal population is maintained (Fig. 8). These data suggest that among sortilin-p75NTR coexpressing DRG neurons, the small and medium-sized neuronal populations are included in those vulnerable to injury-induced neuronal death.
Figure 5.
Size-frequency histogram illustrating the distribution of neuronal area (μm2) in uninjured (black bars) and sciatic nerve transected (grey bars) L4/5 DRG 25-days following sciatic nerve axotomy. Small neurons (0–200μm2) comprise over half (58.03%) of the L4/5 DRG neuronal population in uninjured mice (black bars). This population of neurons is completely lost 25-days following sciatic nerve transection (grey bars).
Discussion
Peripheral nerve injuries are very common, with several hundred thousand cases occurring each year within the United States alone (Wiberg et al., 2003). Neurodegeneration occurs as a result of traumatic nerve injury. To date, the mechanisms underlying this pronounced neuron loss remain to be revealed. It is clear that a better understanding of the molecular mechanisms involved in injury-induced neuronal death is critical to the creation of new therapeutic strategies aimed at preventing the sensory deficits that result from peripheral nerve injuries.
The purpose of this study was to identify a possible mechanism for the neuronal death that occurs amongst the primary sensory DRG neurons following sciatic nerve injury. Recently, several key players have been implicated in the induction of neuronal death; including the sortilin and p75NTR receptors and the death-inducing ligand, proNGF. Sortilin and p75NTR cooperate to promote a high-affinity binding site for proNGF (Nykjaer et al., 2004). These 3 individual components thereby interact to create a trimeric signaling complex that is capable of inducing apoptosis. Our first goal was to confirm the existence of these pro-apoptotic components in the L4/5 DRG in order to determine if these participants might indeed be responsible for the injury-induced neurodegeneration observed here.
proNGF
The proneurotrophins, such as proNGF, have long been thought to be inactive precursors to their mature counterparts. However, recent publications show that proneurotrophins are detectable in the extracellular environment, indicating that they may have biologically important roles (Lee et al., 2001). In fact, proNGF is the dominant form of NGF present in many tissues (Chen et al., 1997; Harrington et al., 2004; Yardley et al., 2000; Fahnestock et al., 2001; Pedraza et al., 2005; Fahnestock et al., 2004, Seidah et al., 1996 and Reinshagen et al., 2000). Recently, it has been reported that proNGF induces apoptosis in cells coexpressing sortilin and p75NTR in vivo, identifying proNGF as a pathological ligand (Pedraza et al., 2005; Harrington et al., 2004; Beattie et al., 2002; and Volosin et al., 2006). The many isoforms represent the multiple cleavage and glycosylation sites available, which render proNGF susceptible to post-translational modification. Here, all of the isoforms were present in each of the treatment groups; no injury, and sciatic nerve crush or transection. Furthermore, none of the isoforms displayed any modification in response to injury.
Interestingly, we were not able to detect the presence of mature NGF (~13 kDa) in the C57Bl/6 mouse L4/5 DRG. This finding is not altogether surprising since several other studies have reported the presence of proNGF but not mature in tissues such as rat DRG, brain, spermatid, prostate and skin (Chen et al., 1997; Delsite and Djakiew, 1999; Reinshagen et al., 2000; Yardley et al., 2000; Fahnestock et al., 2001). Taken together, these data suggest that proNGF is present as the predominant form of NGF in the mouse L4/5 DRG and furthermore, that proNGF expression is not modified following sciatic nerve injury, in contrast to other neurotrophins such as brain derived neurotrophic factor (BDNF) (Karchewski et al., 2002).
Sortilin
Sortilin, a member of the Vps10p family of receptors, cooperates with p75NTR to promote high-affinity binding of proNGF. We first sought to determine if the sortilin receptor was expressed in the mouse DRG and our results indicate that the majority of L4/5 DRG neurons express sortilin (~73%). Furthermore, a similar percentage of neurons continue to express sortilin 3 days after injury. Since there is no significant neuronal loss in L5 mouse DRG three days following sciatic nerve axotomy (Shi et al., 2001), these results suggest that sortilin peptide expression is not altered in response to injury.
Our studies also revealed that sortilin is expressed by the majority of L4/5 DRG neurons and is generally expressed by all 3 neuronal subpopulations examined, including IB4, CGRP, and myelinated N52+ large neurons. Although sortilin expression was found in all subpopulations of DRG neurons, it is unclear why sortilin expression is not found within all neurons of the DRG. One explanation is that sortilin might define a target specific pattern of expression. For example, neurons which project to muscle and skin might express sortilin, but not neurons navigating to viscera. These questions can be addressed in the future using retrograde labeling studies to help determine if sortilin expression is target dependent.
p75NTR
p75NTR is a member of the tumor necrosis factor (TNF) receptor superfamily (Roux and Barker, 2002). To date, there has been no enzymatic activity associated with p75NTR, but its associations with several cytoplasmic interactors, such as NRIF, TRAF6, and NRAGE, allow it to induce apoptosis via a cytoplasmic signaling cascade (Kenchappa et al., 2006). The exact molecular events involved in activation of the p75NTR apoptotic pathway are not completely understood but are thought to involve the activation of c-Jun N-terminal kinase (JNK). Further downstream events include phosphorylation of c-jun, and activation of p53 as well as the “BH3-domain only” family members Bad and Bid, which results in mitochondrial release of cytochrome c and the consequent activation of caspases (Nykjaer et al., 2005). p75NTR is a requirement for proNGF-induced neuronal death to occur. This study demonstrates the presence of p75NTR in a majority of DRG neurons (66%), localizing the third and final component of the death-inducing complex to the DRG. Furthermore, the data presented here establish that p75NTR expression is found in 67% of neurons following injury, indicating the p75NTR expression is not altered following peripheral sciatic nerve injury in the DRG. This result was surprising as previous studies have described dramatic alterations in p75NTR expression after injury (Byers et al., 1992, Arendt et al., 1995, Zhou et al., 1996; Syroid et al., 2000, King et al., 2000, Xie et al., 2003). One possible explanation for these inconsistencies is the timeframe at which p75NTR expression was assessed after injury. Although the expression of many peptides is modified 3 days after injury, the discrepancies could potentially be attributed to the designated stage of examination in this study. Importantly, however, studies by Zhou et al., 1996 revealed that increases p75 after injury is due to selective upregulation within glial cells in the DRG, and that neuronal expression of p75 concomitantly decreases. Overall, these responses culminate in an overall lack of change in p75 expression in the DRG (Zhou et al., 1996), thus supporting our findings that overall p75 protein levels do not change after injury.
We found 4 distinct forms of the p75NTR receptor by western blot analysis. The band of ~ 75 kDa represents the full-length receptor, while the band of ~ 55 kDa most likely represents the intracellular domain (ICD) which is cleaved by γ–secretase in response to binding proapoptotic ligands in neurons (Kenchappa et al., 2006 and Humpert et al., 2007). Higher molecular weight bands of ~155 and ~135 kDa potentially represent a p75NTR dimer and accompanying post-translational modifications, respectively.
Previous studies have shown that p75NTR expression is confined to specific subclasses of neurons within the DRG. p75NTR is coexpressed in almost all of the neurons that express the trk A or trk B receptor (CGRP-positive subpopulation) while only approximately 50% of the trk C-expressing neurons (N52-positive neuronal subpopulation) are positive for p75NTR expression. Interestingly, p75NTR expression is not found in the small, non-peptidergic sensory neurons (IB4-positive neuronal subpopulation), theoretically rendering this class of neurons resistant to proNGF-induced neuronal death (Wright and Snider 1995). Results presented here indicate that small sortilin-p75NTR coexpressing neurons (< 200μm2) are lost 25 days after injury. Reportedly, small non-peptidergic neurons do not express p75NTR. Therefore, our results suggest that the small peptidergic DRG neurons are included in those that are lost after injury.
Sortilin-p75NTR coexpressing neurons
Here we define a unique DRG neuronal subpopulation coexpressing sortilin and p75NTR, making this distinct neuronal class particularly vulnerable to proNGF-induced neuronal death. Previous studies have established a 54% loss of DRG neurons amongst mice 28 days following sciatic nerve transection (Shi et al., 2001). We report in this study that neither p75NTR nor sortilin peptide expression is altered three days following sciatic nerve transection. Therefore, we are able to attribute a reduction in p75NTR and sortilin immunoreactivity to a loss of neurons due to injury-induced apoptosis. In our study, injury to the peripheral sciatic nerve induced a 60% loss of sortilin-p75NTR coexpressing neurons, suggesting that these neurons are vulnerable to injury-induced death. That injury did not lead to death of all of sortilin-p75 NTR coexpressing neurons may be explained by the fact that these surviving neurons are supported by mechanisms rendering them insensitive to proNGF-induced cell death. However, our results do suggest proNGF is a contributing factor to injury-induced neuronal cell death in adult mouse DRG. Related to this point, a recent paper studying the ability of proNGF to induce cell death in retinal ganglion neurons reported that proNGF induced cell death during development, but does not promote injury-induced neuronal death in retinal ganglion neurons, suggesting that this apoptotic mechanism is not active in all settings (Nakamura et al. 2007). Finally, data from Nykjaer et al. 2004 assessed the need for both receptors by culturing neurons only expressing sortilin or p75 to determining the apoptotic effects of pro NGF upon these specific signaling molecules. In addition, these authors transfected neurons with either sortilin, p75, or both receptors and found that proNGF actions require both receptors, suggesting that both receptors are required for proNGF-induced apoptosis. Future studies in the mouse DRG will be necessary to confirm the importance of both receptors in injury-induced cell death of these neuronal subpopulations. Collectively, these results indicate that it will be very important to define proNGF’s actions in each model and stage of development.
Conclusion
In conclusion, we have shown that proNGF, sortilin, and p75NTR are present in mouse DRG neurons, identifying them as potential mediators of neuronal death. Furthermore, we have demonstrated that the number of sortilin-p75NTR coexpressing neurons is reduced dramatically in response to injury. These data suggest a pathological role for proNGF in vivo in mediating apoptosis of L4/5 DRG neurons following sciatic nerve injury. By identifying a likely mechanism for cell death in a particularly vulnerable population, we can now focus on potential therapeutic strategies aimed at preventing the death of these neurons thereby preventing loss of sensory function.
Experimental Procedure
Materials
Phenylmethylsulfonyl fluoride, leupeptin, pepstatin A, aprotinin, antipain, sodium fluoride, sodium orthovanadate, ethylenediaminetetraacetic acid disodium dihydrate and all other reagent grade chemicals were purchased from Sigma (St. Louis, MO). Normal donkey/goat sera, as well as porcine gelatin, were obtained from Jackson Immunoresearch (West Grove, PA). 12.5% Tris-HCl gels and 0.4 μm nitrocellulose membranes were purchased from Bio Rad (Hercules, CA).
The anti-sortilin rabbit polyclonal antibody, specific for the extracellular (luminal) domain of sortilin, was generously donated by Dr. Claus Munck Petersen, University of Aarhus, Denmark. The polyclonal antibody specific to the extracellular domain of the p75NTR was a gift from Dr. Louis Reichardt, Howard Hughes Medical Center. The anti-nerve growth factor beta and the anti-pro-NGF polyclonal antibodies were purchased from Chemicon International (Temecula, CA) and Sigma, respectively. Primary antibodies for the neuronal markers CGRP and N52 were purchased from Chemicon and Sigma, respectively. The secondary antibodies, Alexa Fluro 488/555 and horseradish peroxidase conjugated affinity purified secondary antibody, were purchased from Molecular Probes and Chemicon, respectively. IB4 was purchased from Molecular Probes (Eugene, OR).
Animal Model
The experiments were carried out on six-week old, male C57BL/6 mice (Charles River, Wilmington, MA) and were conducted according to the guidelines from the National Institutes of Health and the University of Kansas Medical Center Animal Care and Use Protocol. The animals were housed in 12/12-h light/dark cycle and allowed free access to food and water. Anesthesia was induced by an intraperitoneal injection of a 1.25% avertin solution (2.5 g of 2,2,2-tribromoethyl alcohol and 5 ml tert-amyl alcohol [Sigma] dissolved in 200 ml deionized water, 0.2 ml/10 g body weight). Sciatic nerves were bilaterally exposed at the mid-thigh level (between 18 and 22 mm distal to the DRG) and transected or not injured. Following transection, a 5 mm portion of the sciatic nerve was resected to prevent regeneration. The sciatic nerves of mice in the uninjured group were viewed anatomically without further intrusion. Sciatic nerves of the crush and transected groups received either mild pressure applied with forceps for approximately five seconds or were completely severed, respectively. The surgical incision was closed by applying Nexaband® liquid tissue adhesive (Webster Veterinary Supply, Raleigh, NC). Mice were allowed to survive for three days following surgical treatment of the sciatic nerves for western blot or immunocytochemical analysis. For neuronal survival assessment, L4/5 DRG were bilaterally removed 25 days following sciatic nerve transection.
Western blot Analysis
L4/5 dorsal root ganglia (DRG) (n = four mice per treatment group) were pooled together for optimal protein concentration. Tissue was placed in liquid nitrogen upon dissection and homogenized using a PRO250 homogenizer (PRO Scientific, Monroe, CT) in 500μL ice cold RIPA buffer (1% triton, 1% deoxycholic acid, 0.1% SDS, 15 M NaCl, 50 mM Tris pH 7.5, 1% NP-40, and 1 mM PMSF) for 30 seconds. Samples were then centrifuged for 30 minutes at 15,000 rpm at +4°C. Total protein concentration was determined using the Bradford Assay method (Bradford, 1976). Samples were denatured for 10 minutes at 100°C in bromophenol blue. Protein samples (50 μg/lane) were separated by SDS-page on 12.5% gels for the detection of mature NGF, proNGF, sortilin, and p75NTR. Recombinant mouse NGF (R&D Systems, 20ng/lane) was loaded as a positive control to test the specificity of the anti-mature NGF antibody. All protein loading was normalized to cyclophilin A (1:1,000). Primary antibodies were diluted as follows: anti-sortilin (1:4,000), anti-p75NTR (1:2,000), anti-mature NGF (1:2,000), and anti-proNGF (1:2,000). Membranes were incubated in the secondary antibody (HRP-conjugated affinity-purified secondary antibody; 1:20,000) for one hour at +4°C. The proteins were detected using an enhanced chemilluminescence reagent kit (Pierce, Rockford, IL) and imaged using a ChemiDoc™ XRS Imager (Bio Rad). Relative band density was analyzed with Quantity One software (Bio Rad) by applying the following equation:
Immunocytochemistry
Sortilin and p75NTR expression was assessed in L4/5 DRG obtained from mice with bilaterally uninjured or transected sciatic nerves (n = 10/treatment group). Mice were deeply anesthetized with avertin (0.2 ml/10 g body weight) and transcardially perfused with ice cold saline followed by 4% paraformaldehyde solution in 0.1 M phosphate buffer (PBS) (pH 7.4). L4/5 DRG were dissected and allowed to post-fix in the same solution for one hour. Following post-fixation, the tissue was cryoprotected using 30% sucrose. 12 μm sections were cut using a cryostat maintained at −20°C and mounted onto Superfrost Plus microscope slides (Fisher, Chicago, IL). Immunocytochemistry was performed using previously published protocols (Christianson et al., 2003). Briefly, sections were blocked for 1 hour in 1.5% normal goat/donkey serum, 0.5% porcine gelatin, and 0.2% Triton X-100 in Superblock buffer (pH 7.4, Pierce, Rockford, IL). Sections were incubated overnight with primary antisera to sortilin (1:5,000) or p75NTR (1:2,000) at +4°C under humidified conditions. The primary antibody was removed by two washes in PBS-triton (0.5%) and sections were then incubated with secondary antibodies (Alexa Fluro 488 and 555; 1:2,000) for one hour at +4°C. Fluorescently labeled sections were rinsed and coverslipped with PBS and viewed using a Nikon E800 microscope attached to a Magnafire digital camera.
Sortilin expression within DRG neuronal subpopulations
Sortilin expression in DRG neuronal subpopulations was defined by removing L4/5 DRG bilaterally from uninjured C57Bl/6 mice (n = 5). A polyclonal primary sortilin antibody (1:5,000) was applied to 14 μm sections in conjunction with the individual neuronal markers anti-CRGP (1:500), IB4 (1:20), and N52 (1:400). Immunocytochemistry was performed as described above.
Sortilin-p75NTR-coexpressing neurons
To identify DRG neurons that coexpress sortilin and p75NTR, L4/5 DRG from C57BL/6 mice (n = 5) were removed and perfused three days following no injury or sciatic nerve transection. Primary polyclonal sortilin and p75NTR antibodies were used together on 14 μm DRG sections. The number of neurons coexpressing both receptors was counted in random, non-sequential sections of DRG. Approximately ten sections per DRG per animal were counted to arrive at an average number of neurons coexpressing both receptors.
Analysis of Neuronal Survival
The number of neurons in L4/5 DRG coexpressing the sortilin and p75NTR receptors was assessed in C57BL/6 mice (n = 7/treatment group) that were perfused 25 days following no injury or bilateral sciatic nerve transection. To identify a subpopulation of neurons vulnerable to sortilin-p75NTR-mediated proNGF-induced neuronal death, immunocytochemistry was performed on DRG sections using primary antisera specific for the sortilin and p75NTR receptors. The number of neurons coexpressing sortilin and p75NTR were counted in both injured and uninjured groups of mice. The area of neurons was measured, in square microns (μm2), using NIH Image J software. Neurons were categorized into different size-based groups based on their area as follows: small neurons (0–200μm2), medium neurons (200–400μm2), and large neurons (<400μm2). The number of neurons in each group was counted and regarded as a percentage of the total number of neurons within the overall treatment group (no injury or 25 days post-sciatic nerve transection).
Statistical Analysis
Statistical analysis was performed using either ANOVA or an unpaired t-test to determine a significant difference between treatment groups. Statistical significance was set at P < 0.05. The variations in number of neurons are given as mean +/− S.E.M.
Acknowledgments
The authors would like to thank Drs. Claude Munck Petersen and Louis Reichardt for the contribution of sortilin and p75NTR antibodies, respectively. We are also grateful to Dr. Susan Smittkamp for help with statistical analysis as well as members of the Wright lab for their roles in proofreading the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beattie MS, Harrington AW, Lee R, Kim JY, Boyce SL, Longo FM, Bresnahan JC, Hempstead BL, Yoon SO. ProNGF induces p75-mediated death of oligodendrocytes following spinal cord injury. Neuron. 2002;36:375–386. doi: 10.1016/s0896-6273(02)01005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Dicou E, Djakiew D. Characterization of nerve growth factor precursor protein expression in rat round spermatids and the trophic effects of nerve growth factor in the maintenance of Sertoli cell viability. Molecular and Cellular Endocrinology. 1997;127:129–136. doi: 10.1016/s0303-7207(96)04001-4. [DOI] [PubMed] [Google Scholar]
- Christianson JA, Ryals JM, McCarson KE, Wright DE. Beneficial actions of neurotrophin treatment on diabetes-induced hypoalgesia in mice. J Pain. 2003;4:493–504. doi: 10.1016/j.jpain.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Christianson JA, Ryals JM, McCarson KE, Wright DE. Beneficial actions of neurotrophin treatment on diabetes-induced hypoalgesia in mice. J Pain. 2003;4:493–504. doi: 10.1016/j.jpain.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Crockett DP, Harris SL, Egger MD. Neurotrophin receptor (p75) in the trigeminal thalamus of the rat: development, response to injury, transient vibrissa-related patterning, and retrograde transport. The Anatomical Record. 2000;259:446–460. doi: 10.1002/1097-0185(20000801)259:4<446::AID-AR80>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Delsite R, Djakiew D. Characterization of nerve growth factor precursor protein expression by human prostate stromal cells: a role in selective neurotrophin stimulation of prostate epithelial cell growth. Prostate. 1999;41:39–48. doi: 10.1002/(sici)1097-0045(19990915)41:1<39::aid-pros6>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Fahnestock M, Michalski B, Xu B, Coughlin MD. The precursor pro-nerve growth factor is the predominant form of nerve growth factor in brain and is increased in Alzheimer’s disease. Molecular and Cellular Neurosciences. 2001;18:210–220. doi: 10.1006/mcne.2001.1016. [DOI] [PubMed] [Google Scholar]
- Harrington AW, Leiner B, Blechschmitt C, Arevalo JC, Lee R, Morl K, Meyer M, Hempstead BL, Yoon SO, Giehl KM. Secreted proNGF is a pathophysiological death-inducing ligand after adult CNS injury. Proceedings of the National Academy of Sciences of the USA. 2004;101:6226–6230. doi: 10.1073/pnas.0305755101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempstead BL. Dissecting the diverse actions of pro- and mature neurotrophins. Current Alzheimer Research. 2006;3:19–24. doi: 10.2174/156720506775697061. [DOI] [PubMed] [Google Scholar]
- Karchewski LA, Gratto KA, Wetmore C, Verge VM. Dynamic patterns of BDNF expression in injured sensory neurons: differential modulation by NGF and NT-3. The European Journal of Neuroscience. 2002;16:1449–1462. doi: 10.1046/j.1460-9568.2002.02205.x. [DOI] [PubMed] [Google Scholar]
- Kenchappa RS, Zampieri N, Chao MV, Barker PA, Teng HK, Hempstead BL, Carter BD. Ligand-dependent cleavage of the P75 neurotrophin receptor is necessary for NRIF nuclear translocation and apoptosis in sympathetic neurons. Neuron. 2006;50:219–232. doi: 10.1016/j.neuron.2006.03.011. [DOI] [PubMed] [Google Scholar]
- King VR, Bradbury EJ, McMahon SB, Priestley JV. Changes in truncated trkB and p75 receptor expression in the rat spinal cord following spinal cord hemisection and spinal cord hemisection plus neurotrophin treatment. Experimental Neurology. 2000;165:327–341. doi: 10.1006/exnr.2000.7480. [DOI] [PubMed] [Google Scholar]
- Klein R, Silos-Santiago I, Smeyne RJ, Lira SA, Brambilla R, Bryant S, Zhang L, Snider WD, Barbacid M. Disruption of the neurotrophin-3 receptor gene trkC eliminates la muscle afferents and results in abnormal movements. Nature. 1994;368:249–251. doi: 10.1038/368249a0. [DOI] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Morris NJ, Ross SA, Lane WS, Moestrup SK, Petersen CM, Keller SR, Lienhard GE. Sortilin is the major 110-kDa protein in GLUT4 vesicles from adipocytes. The Journal of Biological Chemistry. 1998;273:3582–3587. doi: 10.1074/jbc.273.6.3582. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Namekata K, Harada C, Harada T. Intracellular sortilin expression pattern regulates proNGF-induced naturally occurring cell death during development. Cell Death Differ. 2007;14:1552–1554. doi: 10.1038/sj.cdd.4402173. [DOI] [PubMed] [Google Scholar]
- Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, Jacobsen C, Kliemannel M, Schwarz E, Willnow TE, Hempstead BL, Petersen CM. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427:843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- Nykjaer A, Willnow TE, Petersen CM. p75NTR--live or let die. Current Opinion in Neurobiology. 2005;15:49–57. doi: 10.1016/j.conb.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Pedraza CE, Podlesniy P, Vidal N, Arevalo JC, Lee R, Hempstead B, Ferrer I, Iglesias M, Espinet C. Pro-NGF isolated from the human brain affected by Alzheimer’s disease induces neuronal apoptosis mediated by p75NTR. The American Journal of Pathology. 2005;166:533–543. doi: 10.1016/S0002-9440(10)62275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CM, Nielsen MS, Nykjaer A, Jacobsen L, Tommerup N, Rasmussen HH, Roigaard H, Gliemann J, Madsen P, Moestrup SK. Molecular identification of a novel candidate sorting receptor purified from human brain by receptor-associated protein affinity chromatography. The Journal of Biological Chemistry. 1997;272:3599–3605. doi: 10.1074/jbc.272.6.3599. [DOI] [PubMed] [Google Scholar]
- Reinshagen M, Geerling I, Eysselein VE, Adler G, Huff KR, Moore GP, Lakshmanan J. Commercial recombinant human beta- nerve growth factor and adult rat dorsal root ganglia contain an identical molecular species of nerve growth factor prohormone. Journal of Neurochemistry. 2000;74:2127–2133. doi: 10.1046/j.1471-4159.2000.0742127.x. [DOI] [PubMed] [Google Scholar]
- Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Progress in Neurobiology. 2002;67:203–233. doi: 10.1016/s0301-0082(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Sarret P, Krzywkowski P, Segal L, Nielsen MS, Petersen CM, Mazella J, Stroh T, Beaudet A. Distribution of NTS3 receptor/sortilin mRNA and protein in the rat central nervous system. The Journal of Comparative Neurology. 2003;461:483–505. doi: 10.1002/cne.10708. [DOI] [PubMed] [Google Scholar]
- Shi TJ, Tandrup T, Bergman E, Xu ZQ, Ulfhake B, Hokfelt T. Effect of peripheral nerve injury on dorsal root ganglion neurons in the C57 BL/6J mouse: marked changes both in cell numbers and neuropeptide expression. Neuroscience. 2001;105:249–263. doi: 10.1016/s0306-4522(01)00148-8. [DOI] [PubMed] [Google Scholar]
- Syroid DE, Maycox PJ, Soilu-Hanninen M, Petratos S, Bucci T, Burrola P, Murray S, Cheema S, Lee KF, Lemke G, Kilpatrick TJ. Induction of postnatal schwann cell death by the low-affinity neurotrophin receptor in vitro and after axotomy. J Neurosci. 2000;20:5741–5747. doi: 10.1523/JNEUROSCI.20-15-05741.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volosin M, Song W, Almeida RD, Kaplan DR, Hempstead BL, Friedman WJ. Interaction of survival and death signaling in basal forebrain neurons: roles of neurotrophins and proneurotrophins. J Neurosci. 2006;26:7756–7766. doi: 10.1523/JNEUROSCI.1560-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg M, Terenghi G. Will it be possible to produce peripheral nerves? Surgical Technology International. 2003;11:303–310. [PubMed] [Google Scholar]
- Wright DE, Snider WD. Neurotrophin receptor mRNA expression defines distinct populations of neurons in rat dorsal root ganglia. The Journal of Comparative Neurology. 1995;351:329–338. doi: 10.1002/cne.903510302. [DOI] [PubMed] [Google Scholar]
- Yardley G, Relf B, Lakshmanan J, Reinshagen M, Moore GP. Expression of nerve growth factor mRNA and its translation products in the anagen hair follicle. Experimental Dermatology. 2000;9:283–289. doi: 10.1034/j.1600-0625.2000.009004283.x. [DOI] [PubMed] [Google Scholar]
- Zhou XF, Rush RA, McLachlan EM. Differential expression of the p75 nerve growth factor receptor in glia and neurons of the rat dorsal root ganglia after peripheral nerve transection. J Neurosci. 1996;16:2901–11. doi: 10.1523/JNEUROSCI.16-09-02901.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]




