Abstract
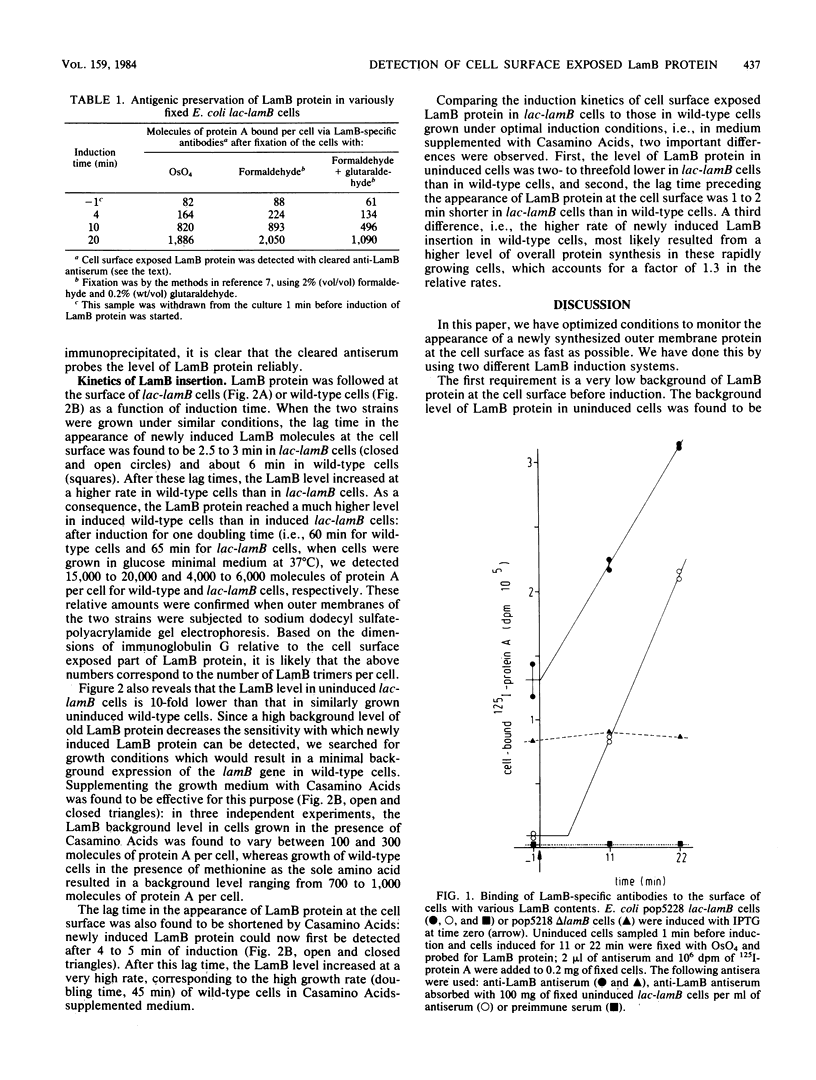
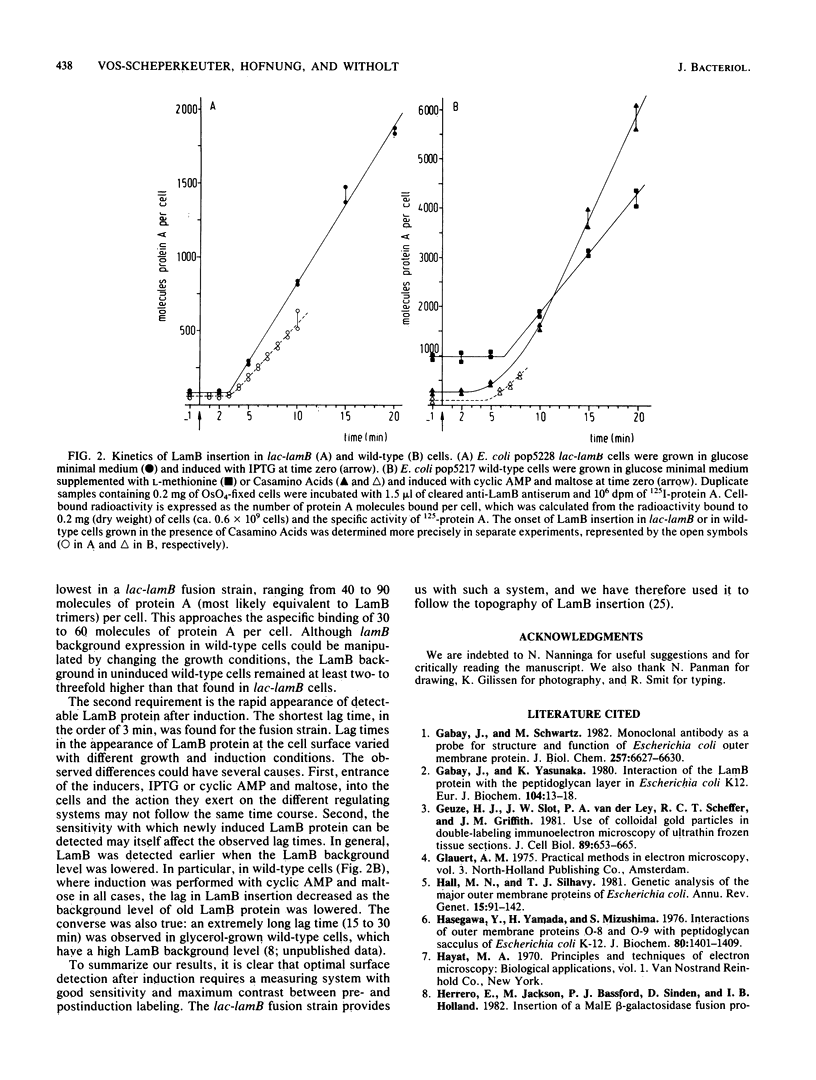
The kinetics of the appearance at the cell surface of the outer membrane LamB protein after induction were determined by using specific antibodies and radioiodinated protein A as a probe. This was done in two different induction systems. First, LamB protein was induced in a wild-type strain by the simultaneous addition of cyclic AMP and maltose. Second, an operon fusion strain in which the lamB gene is expressed under lac promoter control was used; in this system, LamB protein can be induced by isopropyl-beta-D-thiogalactopyranoside. When uninduced cells were grown in glucose minimal medium, background expression of the lamB gene was found to be ca. 10-fold lower in lac-lamB cells than in wild-type cells. The level of LamB protein present in uninduced wild-type cells could, however, be reduced by supplementing the growth medium with Casamino Acids. After induction, the LamB protein appeared at the cell surface of both strains within a few minutes, and then the LamB level per cell increased linearly. The time lag in cell surface exposure of LamB protein differed slightly under both induction conditions: the LamB protein appeared at the surface of lac-lamB cells within 3 min of induction, whereas in wild-type cells it could not be detected earlier than after 4 to 5 min of induction.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gabay J., Schwartz M. Monoclonal antibody as a probe for structure and function of an Escherichia coli outer membrane protein. J Biol Chem. 1982 Jun 25;257(12):6627–6630. [PubMed] [Google Scholar]
- Gabay J., Yasunaka K. Interaction of the lamB protein with the peptidoglycan layer in Escherichia coli K12. Eur J Biochem. 1980 Feb;104(1):13–18. doi: 10.1111/j.1432-1033.1980.tb04393.x. [DOI] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., van der Ley P. A., Scheffer R. C. Use of colloidal gold particles in double-labeling immunoelectron microscopy of ultrathin frozen tissue sections. J Cell Biol. 1981 Jun;89(3):653–665. doi: 10.1083/jcb.89.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. Genetic analysis of the major outer membrane proteins of Escherichia coli. Annu Rev Genet. 1981;15:91–142. doi: 10.1146/annurev.ge.15.120181.000515. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y., Yamada H., Mizushima S. Interactions of outer membrane proteins O-8 and O-9 with peptidoglycan sacculus of Escherichia coli K-12. J Biochem. 1976 Dec;80(6):1401–1409. doi: 10.1093/oxfordjournals.jbchem.a131413. [DOI] [PubMed] [Google Scholar]
- Herrero E., Jackson M., Bassford P. J., Sinden D., Holland I. B. Insertion of a MalE beta-galactosidase fusion protein into the envelope of Escherichia coli disrupts biogenesis of outer membrane proteins and processing of inner membrane proteins. J Bacteriol. 1982 Oct;152(1):133–139. doi: 10.1128/jb.152.1.133-139.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofnung M., Lepouce E., Braun-Breton C. General method for fine mapping of the Escherichia coli K-12 lamB gene: localization of missense mutations affecting bacteriophage lambda adsorption. J Bacteriol. 1981 Dec;148(3):853–860. doi: 10.1128/jb.148.3.853-860.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstra H., Dankert J. Preparation and quantitative determination of antibodies against major outer mambranes proteins of Escherichia coli O26 K60. J Gen Microbiol. 1980 Apr;117(2):437–447. doi: 10.1099/00221287-117-2-437. [DOI] [PubMed] [Google Scholar]
- Marchal C., Greenblatt J., Hofnung M. malB region in Escherichia coli K-12: specialized transducing bacteriophages and first restriction map. J Bacteriol. 1978 Dec;136(3):1109–1119. doi: 10.1128/jb.136.3.1109-1119.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchal C., Perrin D., Hedgpeth J., Hofnung M. Synthesis and maturation of lambda receptor in Escherichia coli K-12: in vivo and in vitro expression of gene lamB under lac promoter control. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1491–1495. doi: 10.1073/pnas.77.3.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis S., Beckwith J. Mechanism of incorporation of cell envelope proteins in Escherichia coli. Annu Rev Microbiol. 1982;36:435–465. doi: 10.1146/annurev.mi.36.100182.002251. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Wu H. C. Proteins of the outer membrane of gram-negative bacteria. Annu Rev Microbiol. 1980;34:369–422. doi: 10.1146/annurev.mi.34.100180.002101. [DOI] [PubMed] [Google Scholar]
- Overbeeke N., Van Scharrenburg G., Lugtenberg B. Antigenic relationships between pore proteins of Escherichia coli K12. Eur J Biochem. 1980 Sep;110(1):247–254. doi: 10.1111/j.1432-1033.1980.tb04862.x. [DOI] [PubMed] [Google Scholar]
- Palva E. T. Major outer membrane protein in Salmonella typhimurium induced by maltose. J Bacteriol. 1978 Oct;136(1):286–294. doi: 10.1128/jb.136.1.286-294.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Ryter A., Shuman H., Schwartz M. Intergration of the receptor for bacteriophage lambda in the outer membrane of Escherichia coli: coupling with cell division. J Bacteriol. 1975 Apr;122(1):295–301. doi: 10.1128/jb.122.1.295-301.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. The adsorption of coliphage lambda to its host: effect of variations in the surface density of receptor and in phage-receptor affinity. J Mol Biol. 1976 May 25;103(3):521–536. doi: 10.1016/0022-2836(76)90215-1. [DOI] [PubMed] [Google Scholar]
- Simpson R. B. Contacts between Escherichia coli RNA polymerase and thymines in the lac UV5 promoter. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3233–3237. doi: 10.1073/pnas.76.7.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueba F. J., Woldringh C. L. Changes in cell diameter during the division cycle of Escherichia coli. J Bacteriol. 1980 Jun;142(3):869–878. doi: 10.1128/jb.142.3.869-878.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos-Scheperkeuter G. H., Pas E., Brakenhoff G. J., Nanninga N., Witholt B. Topography of the insertion of LamB protein into the outer membrane of Escherichia coli wild-type and lac-lamB cells. J Bacteriol. 1984 Aug;159(2):440–447. doi: 10.1128/jb.159.2.440-447.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witholt B., Boekhout M., Brock M., Kingma J., Heerikhuizen H. V., Leij L. D. An efficient and reproducible procedure for the formation of spheroplasts from variously grown Escherichia coli. Anal Biochem. 1976 Jul;74(1):160–170. doi: 10.1016/0003-2697(76)90320-1. [DOI] [PubMed] [Google Scholar]



