Abstract
By using EPR measurements of radiation-induced radicals it is possible to utilize human fingernails to estimate radiation dose after-the-fact. One of the potentially limiting factors in this approach is the presence of artifacts due to mechanically induced EPR signals (MIS) caused by mechanical stress during the collection and preparation of the samples and the so-called background (non-radiation) signal (BKS). The MIS and BKS have spectral parameters (shape, g-factor and linewidth) that overlap with the radiation-induced signal (RIS) and therefore, if not taken into account properly, could result in a considerable overestimation of the dose. We have investigated the use of different treatments of fingernails with chemical reagents to reduce the MIS and BKS. The most promising chemical treatment (20 min with 0.1 M dithiothreitol aqueous solution) reduced the contribution of MIS and BKS to the total intensity of EPR signal of irradiated fingernails by a factor of 10. This makes it potentially feasible to measure doses as low as 1 Gy almost immediately after irradiation. However, the chemical treatment reduces the intensity of the RIS and modifies dose dependence. This can be compensated by use of an appropriate calibration curve for assessment of dose. On the basis of obtained results it appears feasible to develop a field-deployable protocol that could use EPR measurements of samples of fingernails to assist in the triage of individuals with potential exposure to clinically significant doses of radiation.
1. Introduction
Experience has demonstrated that despite all precautions, radiation accidents occur. According to the Radiation Emergency Assistance Center/Training Site Radiation Accident Registries, in the period of 1944–2004 there were 421 major radiation accidents worldwide. There also is a high possibility of a terrorist act or the use of a strategic nuclear weapon that could result in the radiation exposure of civilians and military personnel. Therefore, development of a non-invasive, rapid and reliable method for measuring radiation dose that is able to provide results immediately after the radiation event is highly desirable. The use of fingernails as an EPR radiation dosimeter has a number of potential advantages including high sensitivity (Symons et al., 1995; Trompier et al., 2007a, b estimated low dose limits as 1–2 Gy); sampling is much more facile compared to hematologically based biodosimetry sampling (does not require drawing blood); and the measurement can be made at the site of the incident (does not require transport of the sample to a different site, avoiding the considerable logistical problems of linking back the individual with the sample under disaster conditions). The radiation-induced EPR signal persists for many hours and is dose proportional. If needed, the signal can be preserved indefinitely by storage at low temperatures.
But there is a problem—it is known from Symons et al. (1995) and Chandra and Symons (1987) that cutting fingernails generates mechanically induced radicals (MIS). In 1995, Symons et al. presented evidence that the dominant MIS species is a sulfur centered radical. The MIS has spectral parameters (shape, g-factor and linewidth) quite similar to the radiation-induced signal (RIS). Calculations that do not take this into account obviously overestimate the absorbed dose, e.g. the MIS could give a dose offset up to 10 Gy.
There are several possible approaches to try to solve this problem: annealing of the sample to accelerate fading of the MIS: numerical deconvolution of the fingernail spectrum to determine the radiation response from the complex spectrum; chemical treatment of the cut fingernails to oxidize the radical further; or a chemical reduction of the mechanically induced radicals.
It was reported by Chandra and Symons in 1987 that treatment with sodium thioglycolate reduces the MIS in cut fingernails. However, in the later paper by Symons et al. (1995), the authors, without reporting the details on which their decision was based, stated that they discontinued the use of this treatment because it “significantly modified the radiation response in fingernails”. In the present communication we report the results of the use of seven different chemicals (acetone, hydroxylamine, d,l-dithiothreitol, urea, sodium thioglycolate and hydrogen peroxide) to reduce the MIS in fingernails. Special attention was paid to the dose dependence of EPR radiation response in fingernails treated with d,l-dithiothreitol, a commonly used chemically reducing agent for proteins and peptides, which we found to be the most effective chemical treatment.
2. Experimental
Fingernails were collected from four different donors and were used in these experiments. Sharp surgical scissors were used to cut the fingernails. The pieces of fingernails all were about 1–2 mm wide and about 7–10 mm long. The typical sample mass was 15 mg. All chemicals were obtained from Aldrich and were 99 + % pure. The water used for the rinsing steps and for solutions was 18.2 MΩ and was provided by Millipore (Billerica, MA). A 137Cs radiation source with a dose rate of 0.7 Gy/min was used for irradiation. EPR measurements were carried out on a X-band (9–10 GHz) EPR spectrometer Bruker ELEXSYS 500 (Bruker BioSpin) equipped with a super-high-Q resonator ER 4123SHQE at room temperature, using the following conditions: HF modulation: 100 kHz; amplitude of HF modulation: 5 G; receiver gain: 60 db; time constant: 81.92 ms; number of points: 1024; sweep time: 41.96 s; number of scans: 10; total recording time: 7 min; central field: 3510 G; sweep width: 150 G; incident mw power: 2 mW.
Two experimental designs were used in this study:
Experimental design 1. Freshly cut fingernails from the right/left hands of one person (total of 100 mg of fingernails) were pooled and split into seven (7) portions (~ 15 mg each), and then each portion was treated for 5 min with 500 μL of the aqueous solutions of the six different reagents and one sample (control) was not treated (Table 1). Following treatment, all samples were rinsed first with 500 μL DI water (18.2 MΩ) for 5 min, then separated by microfiltration, then treated with 500 μL acetone for 3 min and separated again by microfiltration. The samples were then dried in vacuum over for 50 min at room temperature. The EPR peak-to-peak amplitude of the MIS was used as a measure of the treatment efficacy.
Table 1.
Samples’ treatment description
| Sample code | Treatment | Sample mass (mg) |
|---|---|---|
| Co | None | 15.1 |
| C × 0 | Acetone | 14.5 |
| C × 1 | 0.1 hydroxylamine | 15.5 |
| C × 2 | 0.1 d,l-dithiothreitol | 17.6 |
| C × 3 | 0.6M urea | 14.7 |
| C × 4 | 0.1M sodium thioglycolate | 14.7 |
| C × 5 | 5% H2O2 | 14.7 |
| C × 6 | 10% H2O2 | 13.7 |
Experiment 2. Freshly cut fingernails from right/left hands of one person (totally 100 mg of fingernails) were pooled and split into six (6) portions (~ 15 mg each). Approximately 20 min after trimming, five samples were irradiated with a 137Cs source to a dose of 1, 2, 3, 4 or 8 Gy. One sample was not irradiated and used as a control. Following irradiation the samples were treated with 0.1 M dithiothreitol (the reagent determined as a best treatment) for 5 min, washed with DI water (18.2 MΩ), acetone, and dried in vacuum at room temperature for 50 min. The procedure for the chemical treatment of the samples was then repeated for 5 min, and the EPR measurements were repeated and then the chemical treatment of the samples was repeated for 10 min and the EPR measurements were repeated again.
3. Results and discussion
Fig. 1 shows typical results of the experiments using the various treatments on unirradiated samples from one donor. Similar results were obtained with repetitions from the same donor and from measurements with three other donors, but because the experimental conditions had some variation, at this time the additional data should be viewed as supportive but cannot contribute to a statement of statistical significance. The best result (minimum MIS) was with the treatment with 0.1 M dithiothreitol for 5 min. This treatment reduced the MIS by a factor of 4 compared to the control sample. It therefore was selected to measure the dose response in irradiated fingernails (Fig. 2). According to Symons et al. (1995), the EPR spectrum of irradiated samples consists of three principal components:
Mechanically induced signal (MIS)
Native or background signal (BKS)
Radiation-induced signal (RIS)
Fig. 1.
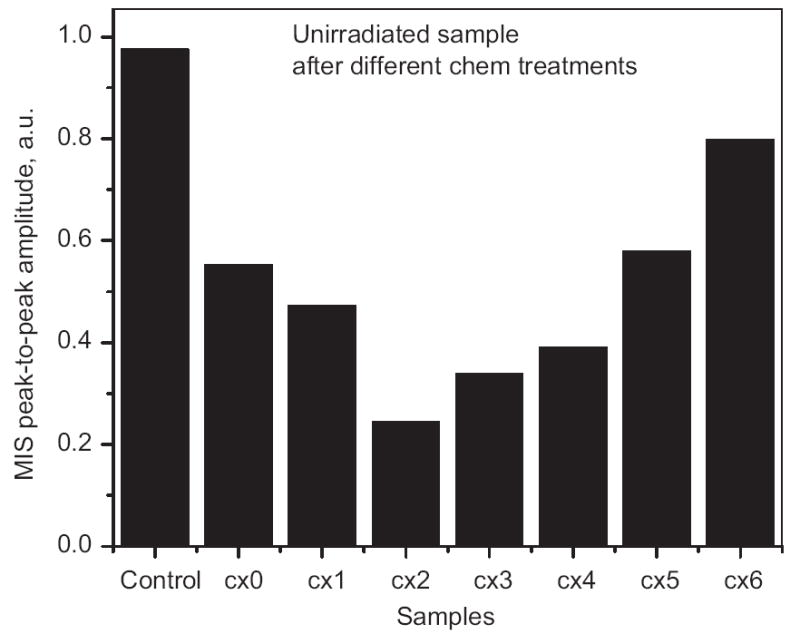
Effect of different chemical treatments (see Table 1 for the abbreviations) on the peak-to-peak amplitude of the MIS. All samples were 15 mg. Sample C × 2 (treated for 5 min with 0.1 M dithiothreitol) was found to have the minimal MIS.
Fig. 2.
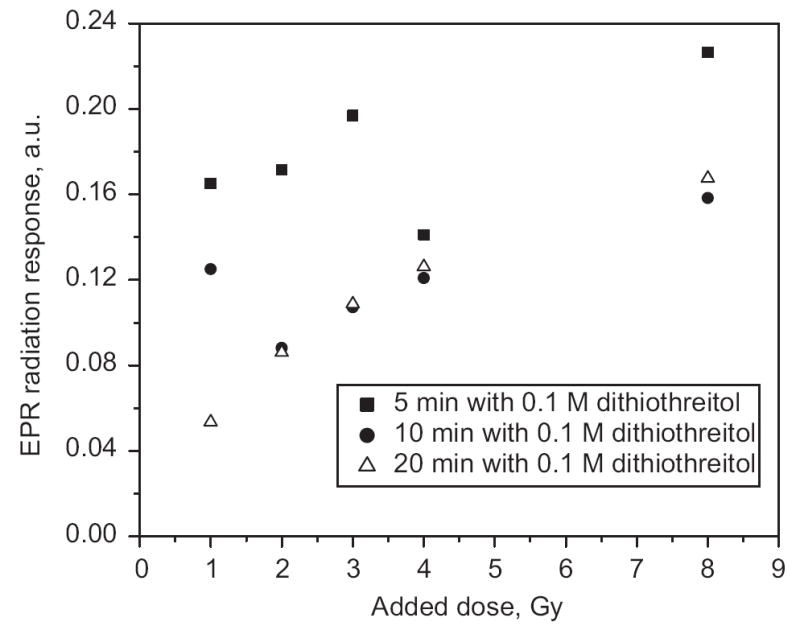
The dependence of the magnitude of EPR signal on radiation dose with different lengths of time of treatment with dithiothreitol. Uncertainty of the EPR radiation response measurements was determined as standard deviation of the three repeated measurements. The point sizes reflect uncertainties of the measurements.
Fig. 2 shows that a 5-min treatment did not completely consistently reduce the MIS. After a repeated 5-min treatment (total time is 10 min), almost all samples (excluding the 1-Gy sample) had a significant reduction in peak-to-peak amplitude, which did not change with an additional 10-min treatment (except for the 1-Gy sample in this set of results, which was further reduced by the additional 10-min treatment). Fig. 3 illustrates the appearance of the EPR signal at different doses. Although the data presented here are too sparse to draw firm conclusions, the results suggest that a dose as low as 1 Gy could be measured in fingernails using a treatment procedure similar to that described in this paper.
Fig. 3.
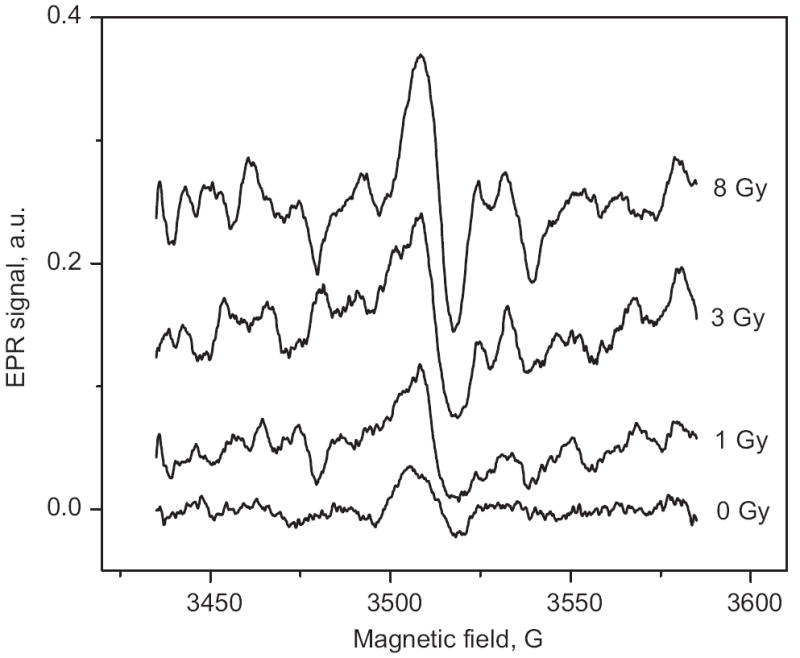
EPR spectra of fingernails treated with dithiothreitol for 20 min after receiving different radiation doses. The spectrum of the sample irradiated with 1 Gy is clearly distinguishable from the unirradiated sample (0 Gy).
Fig. 2 indicates the dose dependences of the EPR radiation response in fingernails treated with dithiothreitol at the different treatment time. While the chemical treatment could affect all the components of the EPR signal, e.g. MIS, BKS and RIS, the results obtained for unirradiated samples (Fig. 1) indicate that the treatment principally reduced the non-radiation components, although some reduction of RIS may also have occurred.
The dose dependence in untreated fingernails (see, for example, Dalgarno and McClymont, 1989; Symons et al., 1995; Trompier et al., 2007a, b) is reported to have a linear relationship for doses below 60 Gy. However, after chemical treatment the dose dependence in the fingernails became non-linear. In the case of tooth enamel, Grun (1996) suggested using the following equation to describe the non-linear dose dependence behavior:
| (1) |
where A is EPR dose response, Imax is maximal EPR dose response (saturation level), DE is the dose to be determined, D0 is characteristic saturation dose. A least-square fit of the experimental data (Fig. 4) gives D0 = (3.9 ± 0.1) Gy and DE = (0.30 ± 0.05) Gy. Thus chemical treatment procedure that was used reduced the dose offset from MIS and BKS from 5–10 to 0.3 Gy. This makes us optimistic that we will be able to develop a method of dose measurement that will be applicable immediately after an unexpected exposure event.
Fig. 4.
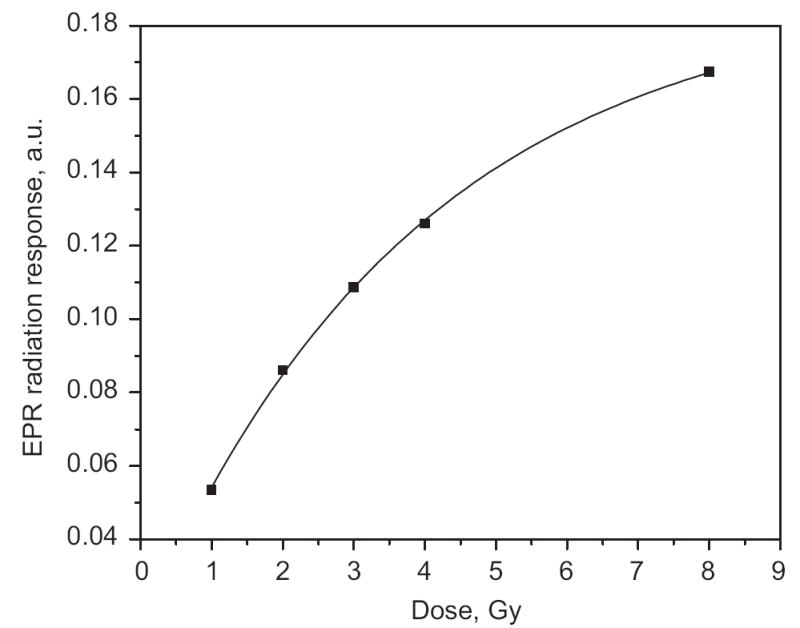
Modeling of the dose–response curve after treatment for 20 min with dithiothreitol. The curve is a least-square fit of dose dependence by the Grun model A = Imax(1 − exp(−(D + DE)/D0)), where A is EPR dose response, Imax is maximal EPR dose response (saturation level), D is the dose to be determined, D0 is the characteristic saturation dose, and DE is the “background dose” (DE was determined to be 0.3 Gy).
4. Conclusions
The chemical treatment procedure (10–20 min with an aqueous solution of 0.1 M dithiothreitol) reduced the MIS and BKS to 0.3 Gy from 10 Gy. These results suggest that it should be feasible to measure radiation doses in fingernails to below 1 Gy almost immediately after irradiation. Further experiments with replications and extension to using fingernails in which the MIS is generated after the irradiation (this could be done by irradiating “aged” fingernail clippings whose original MIS has decayed). The chemical treatment also reduced the intensity of the RIS and modified its dose dependence and therefore a calibration based on the altered response needs to be utilized (instead of a linear relationship). On the basis of these results it appears feasible to develop a field-deployable protocol that could use EPR measurements of samples of fingernails to assist in the triage of individuals with potential exposure to clinically significant doses of radiation. We are actively working on establishing the validity of this attractive possibility.
Acknowledgments
This study was supported in part by NIH Grant U19 AI067733 and used the facilities of the EPR Center for the Study of Viable Systems (P41 EB002032).
References
- Chandra H, Symons MCR. Sulphur radicals formed by cutting alpha-keratin. Nature. 1987;328:833–834. doi: 10.1038/328833a0. [DOI] [PubMed] [Google Scholar]
- Dalgarno BG, McClymont JD. Evaluation of ESR as a radiation accident dosimetry technique. Appl Radiat Isot. 1989;40:1013–1020. [Google Scholar]
- Grun R. Errors in dose assessment introduced by the use of the linear part of a saturating dose–response curve. Radiat Meas. 1996;26:297–305. [Google Scholar]
- Symons MCR, Chandra H, Wyatt JI. Electron paramagnetic resonance spectra of irradiated fingernails: a possible measure of accidental exposure. Radiat Prot Dosimetry. 1995;58:11–15. [Google Scholar]
- Trompier F, Romanyukha A, Kornak L, Calas C, LeBlanc B, Clairand I, Mitchell CA, Swartz H. Electron paramagnetic resonance radiation dosimetry with fingernails. 2007a doi: 10.1016/j.radmeas.2007.05.024. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trompier F, Kornak L, Calas C, Romanyukha A, LeBlanc B, Clairand I, Mitchell CA, Swartz H. Protocols for emergency dosimetry with fingernails using EPR spectrometry. 2007b doi: 10.1016/j.radmeas.2007.05.024. submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]


