Abstract
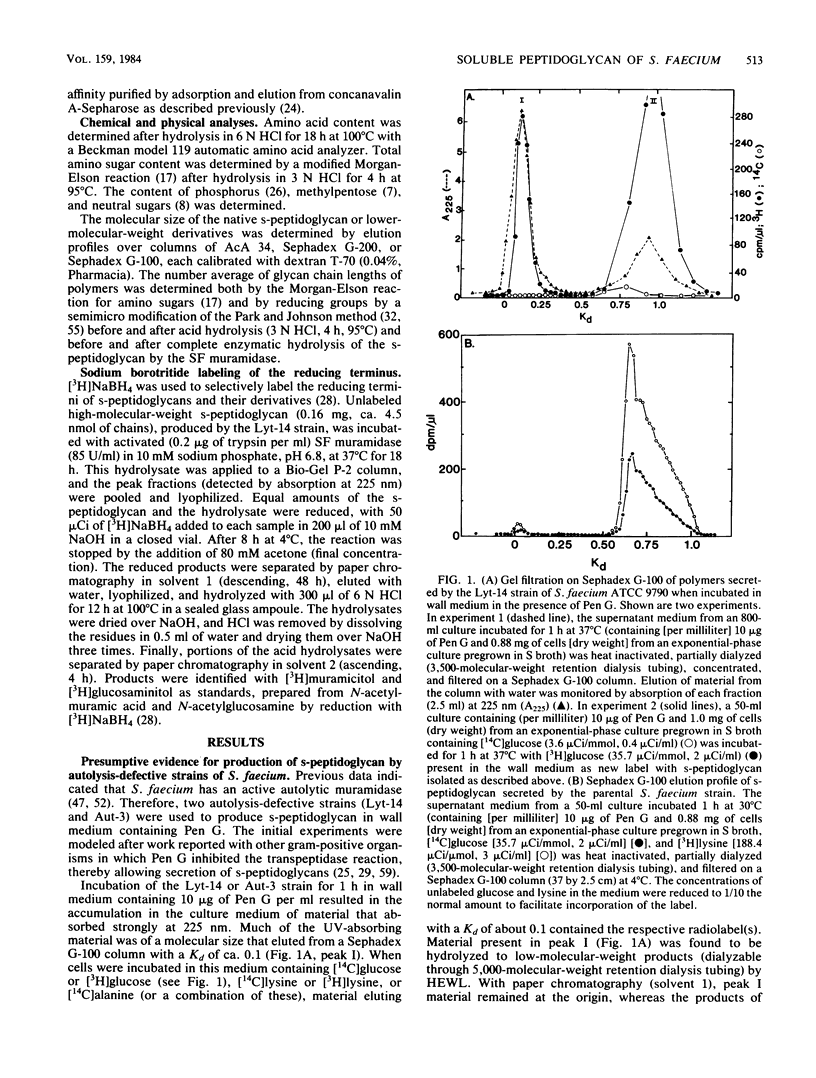
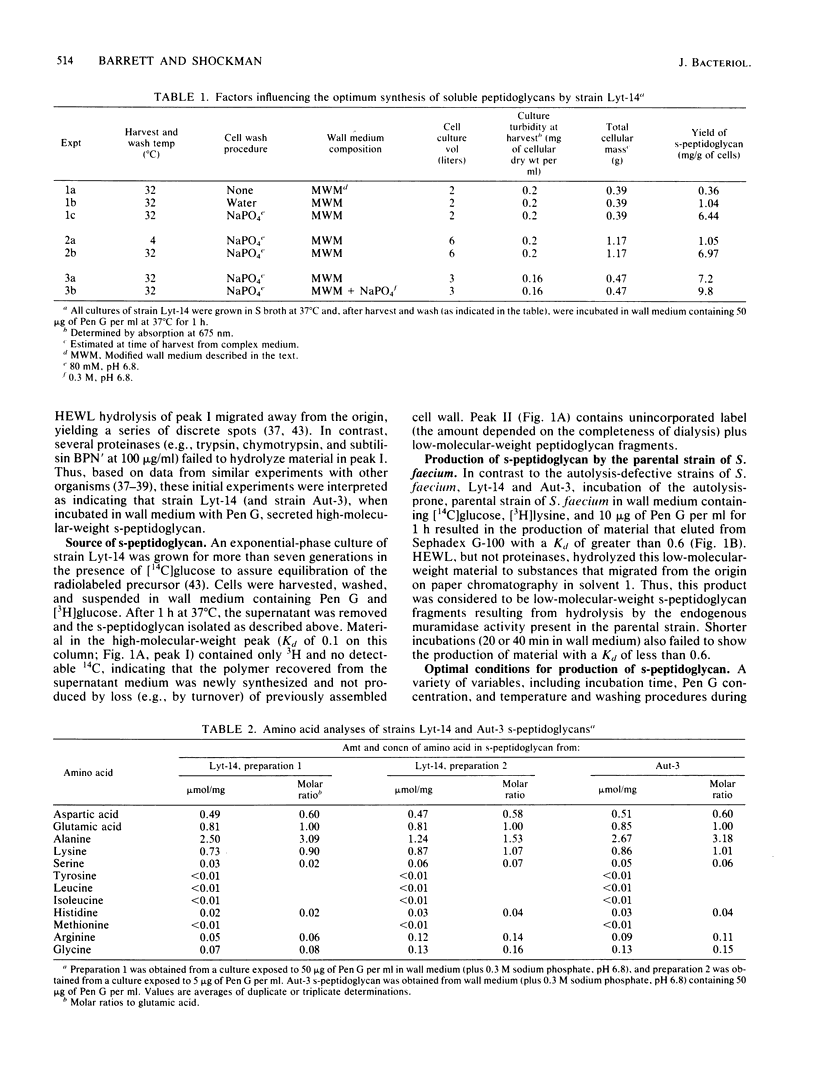
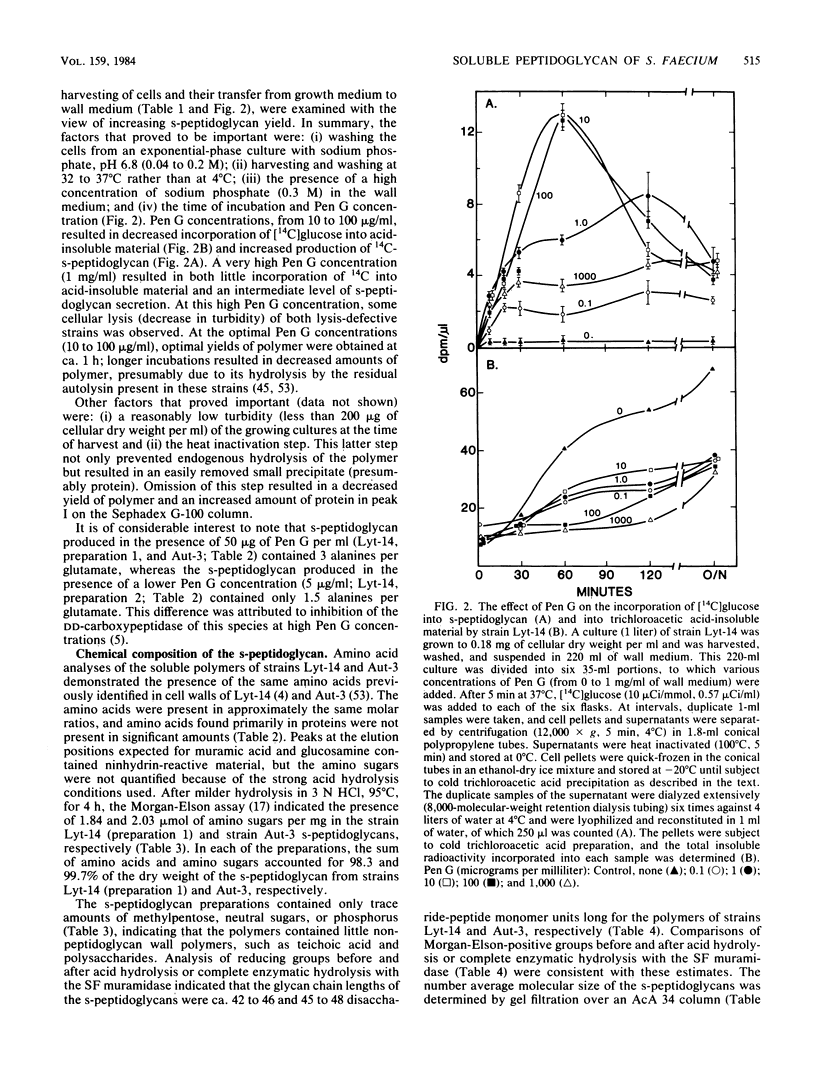
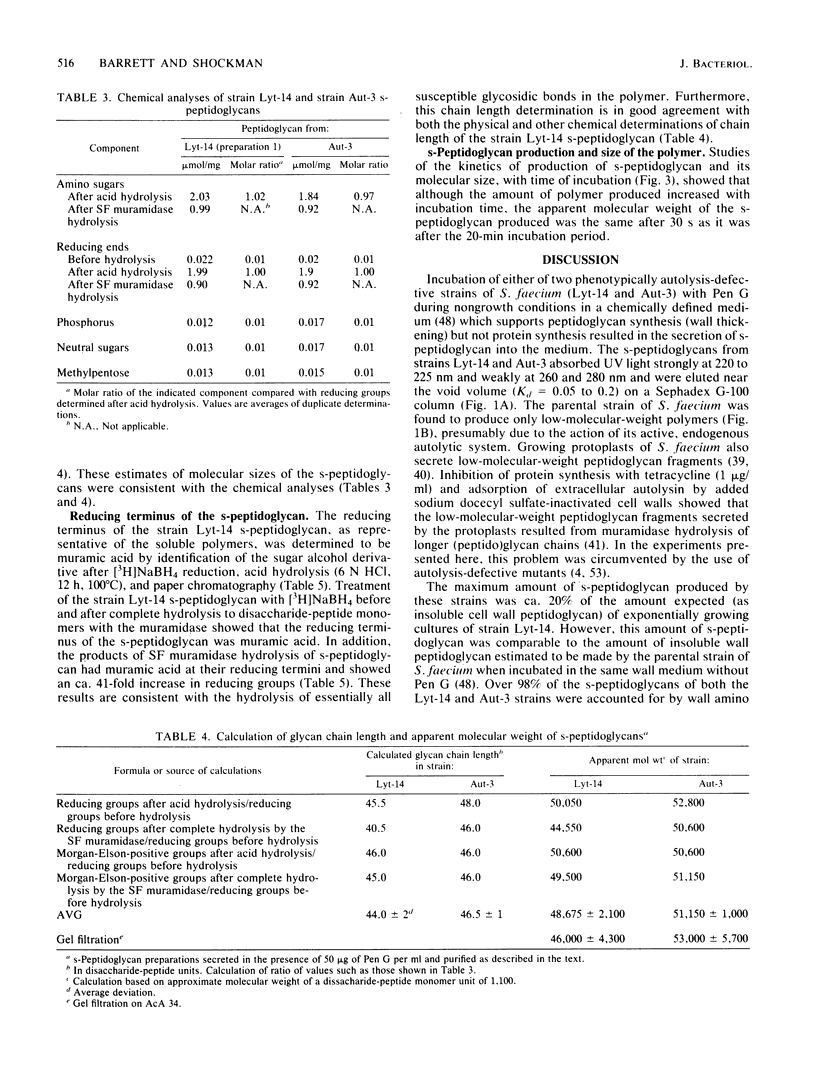
Two phenotypically autolysis-deficient strains of Streptococcus faecium ATCC 9790 were shown to produce high-molecular-weight, soluble, linear, uncross-linked peptidoglycan when incubated with benzylpenicillin in a wall medium which permits cell wall synthesis (wall thickening) but not balanced growth. This high-molecular-weight s-peptidoglycan was shown to have a molecular weight of 46,000 to 54,000, lack peptide cross-links, and be virtually devoid of accessory wall polymers. It was hydrolyzed by hen egg white lysozyme and the endogenous, autolytic N-acetylmuramidase of S. faecium, but was not attacked by proteinases. Chemical analyses of the polymer are consistent with the following structure, where n is the number of repeating disaccharide units: (formula; see text).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akrigg A., Ayad S. R. Studies on the competence-inducing factor of Bacillus subtilis. Biochem J. 1970 Apr;117(2):397–403. doi: 10.1042/bj1170397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. F., Schramm V. L., Shockman G. D. Hydrolysis of soluble, linear, un-cross-linked peptidoglycans by endogenous bacterial N-acetylmuramoylhydrolases. J Bacteriol. 1984 Aug;159(2):520–526. doi: 10.1128/jb.159.2.520-526.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. N., Wong W., Young F. E., Gilpin R. W. Isolation and characterization of a mutant of Staphylococcus aureus deficient in autolytic activity. J Bacteriol. 1976 Mar;125(3):961–967. doi: 10.1128/jb.125.3.961-967.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornett J. B., Redman B. E., Shockman G. D. Autolytic defective mutant of Streptococcus faecalis. J Bacteriol. 1978 Feb;133(2):631–640. doi: 10.1128/jb.133.2.631-640.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyette J., Ghuysen J. M., Binot F., Adriaens P., Meesschaert B., Vanderhaeghe H. Interactions between beta-lactam antibiotics and isolated membranes of Streptococcus faecalis ATCC 9790. Eur J Biochem. 1977 May 2;75(1):231–239. doi: 10.1111/j.1432-1033.1977.tb11522.x. [DOI] [PubMed] [Google Scholar]
- Elliott T. S., Ward J. B., Rogers H. J. Formation of cell wall polymers by reverting protoplasts of Bacillus licheniformis. J Bacteriol. 1975 Nov;124(2):623–632. doi: 10.1128/jb.124.2.623-632.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P. Autolysin(s) of Bacillus subtilis as dechaining enzyme. J Bacteriol. 1970 Aug;103(2):494–499. doi: 10.1128/jb.103.2.494-499.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P., Pelvit M. C., Cunningham W. P. Structural difference between walls from ends and sides of the rod-shaped bacterium Bacillus subtilis. J Bacteriol. 1972 Mar;109(3):1266–1272. doi: 10.1128/jb.109.3.1266-1272.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein J. E. Possible involvement of bacterial autolytic enzymes in flagellar morphogenesis. J Bacteriol. 1979 Feb;137(2):933–946. doi: 10.1128/jb.137.2.933-946.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein J. E., Rogers H. J. Autolytic enzyme-deficient mutants of Bacillus subtilis 168. J Bacteriol. 1976 Sep;127(3):1427–1442. doi: 10.1128/jb.127.3.1427-1442.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W., Rogers H. J. Characterization of Bacillus licheniformis 6346 mutants which have altered lytic enzyme activities. J Bacteriol. 1974 May;118(2):358–368. doi: 10.1128/jb.118.2.358-368.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs-Cleveland E., Gilvarg C. Oligomeric intermediate in peptidoglycan biosynthesis in Bacillus megaterium. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4200–4204. doi: 10.1073/pnas.73.11.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghuysen J. M., Bricas E., Leyh-Bouille M., Lache M., Shockman G. D. The peptide N alpha-(L-alanyl-D-isoglutaminyl)-N epsilon-(D-isoasparaginyl)-L-lysyl-D-alanine and the disaccharide N-acetylglucosaminyl-beta-1,4-N-acetylmuramic acid in cell wall peptidoglycan of Streptococcus faecalis strain ATCC 9790. Biochemistry. 1967 Aug;6(8):2607–2619. doi: 10.1021/bi00860a044. [DOI] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Model for cell wall growth of Streptococcus faecalis. J Bacteriol. 1970 Feb;101(2):643–648. doi: 10.1128/jb.101.2.643-648.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Procaryotic cell division with respect to wall and membranes. CRC Crit Rev Microbiol. 1971 May;1(1):29–72. doi: 10.3109/10408417109104477. [DOI] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Study of cycle of cell wall assembly in Streptococcus faecalis by three-dimensional reconstructions of thin sections of cells. J Bacteriol. 1976 Sep;127(3):1346–1358. doi: 10.1128/jb.127.3.1346-1358.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinks R. P., Daneo-Moore L., Shockman G. D. Relationship between cellular autolytic activity, peptidoglycan synthesis, septation, and the cell cycle in synchronized populations of Streptococcus faecium. J Bacteriol. 1978 Jun;134(3):1074–1080. doi: 10.1128/jb.134.3.1074-1080.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V., Mirelman D., Sharon N., Schwarz U. Novel type of murein transglycosylase in Escherichia coli. J Bacteriol. 1975 Dec;124(3):1067–1076. doi: 10.1128/jb.124.3.1067-1076.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen S. E., Campbell J. N. Amidase activity involved in peptidoglycan biosynthesis in membranes of Micrococcus luteus (sodonensis). J Bacteriol. 1976 Jul;127(1):319–326. doi: 10.1128/jb.127.1.319-326.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura T., Shockman G. D. Purification and some properties of the endogenous, autolytic N-acetylmuramoylhydrolase of Streptococcus faecium, a bacterial glycoenzyme. J Biol Chem. 1983 Aug 10;258(15):9514–9521. [PubMed] [Google Scholar]
- Keglević D., Ladesić B., Hadzija O., Tomasić J., Valinger Z., Pokorny M., Naumski R. Isolation and study of the composition of a peptidoglycan complex excreted by the biotin-requiring mutant of Brevibacterium divaricatum NRRL-2311 in the presence of penicillin. Eur J Biochem. 1974 Mar 1;42(2):389–400. doi: 10.1111/j.1432-1033.1974.tb03351.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- Mauck J., Chan L., Glaser L. Turnover of the cell wall of Gram-positive bacteria. J Biol Chem. 1971 Mar 25;246(6):1820–1827. [PubMed] [Google Scholar]
- McLean C., Werner D. A., Aminoff D. Quantitative determination of reducing sugars, oligosaccharides, and glycoproteins with (3H)borohydride. Anal Biochem. 1973 Sep;55(1):72–84. doi: 10.1016/0003-2697(73)90291-1. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Bracha R., Sharon N. Penicillin-induced secretion of soluble, uncross-linked peptidoglycan by Micrococcus luteus cells. Biochemistry. 1974 Nov 19;13(24):5045–5053. doi: 10.1021/bi00721a028. [DOI] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- PELZER H. MUCOPEPTIDHYDROLASEN IN ESCHERICHIA COLI B. I. NACHWEIS UND WIRKUNGSSPEZIFITAET. Z Naturforsch B. 1963 Nov;18:950–956. [PubMed] [Google Scholar]
- Pooley H. M., Porres-Juan J. M., Shockman G. D. Dissociation of an autolytic enzyme-cell wall complex by treatment with unusually high concentrations of salt. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1134–1140. doi: 10.1016/0006-291x(70)90357-8. [DOI] [PubMed] [Google Scholar]
- RUPLEY J. A. THE HYDROLYSIS OF CHITIN BY CONCENTRATED HYDROCHLORIC ACID, AND THE PREPARATION OF LOW-MOLECULAR-WEIGHT SUBSTRATES FOR LYSOZYME. Biochim Biophys Acta. 1964 Nov 1;83:245–255. doi: 10.1016/0926-6526(64)90001-1. [DOI] [PubMed] [Google Scholar]
- Ranhand J. M., Leonard C. G., Cole R. M. Autolytic activity associated with competent group H streptococci. J Bacteriol. 1971 Apr;106(1):257–268. doi: 10.1128/jb.106.1.257-268.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J. Bacterial growth and the cell envelope. Bacteriol Rev. 1970 Jun;34(2):194–214. doi: 10.1128/br.34.2.194-214.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J., Pooley H. M., Thurman P. F., Taylor C. Wall and membrane growth in bacilli and their mutants. Ann Microbiol (Paris) 1974 Sep;125 B(2):135–147. [PubMed] [Google Scholar]
- Rosenthal R. S., Jungkind D., Daneo-Moore L., Shockman G. D. Evidence for the synthesis of soluble peptidoglycan fragments by protoplasts of Streptococcus faecalis. J Bacteriol. 1975 Oct;124(1):398–409. doi: 10.1128/jb.124.1.398-409.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S., Shockman G. D. Characterization of the presumed peptide cross-links in the soluble peptidoglycan fragments synthesized by protoplasts of Streptococcus faecalis. J Bacteriol. 1975 Oct;124(1):410–418. doi: 10.1128/jb.124.1.410-418.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S., Shockman G. D. Synthesis of peptidoglycan in the form of soluble glycan chains by growing protoplasts (autoplasts) of Streptococcus faecalis. J Bacteriol. 1975 Oct;124(1):419–423. doi: 10.1128/jb.124.1.419-423.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth G. S., Shockman G. D., Daneo-Moore L. Balanced macromolecular biosynthesis in "protoplasts" of Streptococcus faecalis. J Bacteriol. 1971 Mar;105(3):710–717. doi: 10.1128/jb.105.3.710-717.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto H., Tomasz A. Protoplast formation and leakage of intramembrane cell components: induction by the competence activator substance of pneumococci. J Bacteriol. 1975 Jan;121(1):344–353. doi: 10.1128/jb.121.1.344-353.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Barrett J. F. Structure, function, and assembly of cell walls of gram-positive bacteria. Annu Rev Microbiol. 1983;37:501–527. doi: 10.1146/annurev.mi.37.100183.002441. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Cheney M. C. Autolytic enzyme system of Streptococcus faecalis. V. Nature of the autolysin-cell wall complex and its relationship to properties of the autolytic enzyme of Streptococcus faecalis. J Bacteriol. 1969 Jun;98(3):1199–1207. doi: 10.1128/jb.98.3.1199-1207.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Conover M. J., Kolb J. J., Riley L. S., Toennies G. NUTRITIONAL REQUIREMENTS FOR BACTERIAL CELL WALL SYNTHESIS. J Bacteriol. 1961 Jan;81(1):44–50. doi: 10.1128/jb.81.1.44-50.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Daneo-Moore L., Higgins M. L. Problems of cell wall and membrane growth, enlargement, and division. Ann N Y Acad Sci. 1974 May 10;235(0):161–197. doi: 10.1111/j.1749-6632.1974.tb43265.x. [DOI] [PubMed] [Google Scholar]
- Shockman G. D. Symposium on the fine structure and replication of bacteria and their parts. IV. Unbalanced cell-wall synthesis: autolysis and cell-wall thickening. Bacteriol Rev. 1965 Sep;29(3):345–358. doi: 10.1128/br.29.3.345-358.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Thompson J. S., Conover M. J. The autolytic enzyme system of Streptococcus faecalis. II. Partial characterization of the autolysin and its substrate. Biochemistry. 1967 Apr;6(4):1054–1065. doi: 10.1021/bi00856a014. [DOI] [PubMed] [Google Scholar]
- Shungu D. L., Cornett J. B., Shockman G. D. Morphological and physiological study of autolytic-defective Streptococcus faecium strains. J Bacteriol. 1979 May;138(2):598–608. doi: 10.1128/jb.138.2.598-608.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. S., Shockman G. D. A modification of the Park and Johnson reducing sugar determination suitable for the assay of insoluble materials: its application to bacterial cell walls. Anal Biochem. 1968 Feb;22(2):260–268. doi: 10.1016/0003-2697(68)90315-1. [DOI] [PubMed] [Google Scholar]
- Tomasz A. Biological consequences of the replacement of choline by ethanolamine in the cell wall of Pneumococcus: chanin formation, loss of transformability, and loss of autolysis. Proc Natl Acad Sci U S A. 1968 Jan;59(1):86–93. doi: 10.1073/pnas.59.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynecka Z., Ward J. B. Peptidoglycan synthesis in Bacillus licheniformis. The inhibition of cross-linking by benzylpenicillin and cephaloridine in vivo accompanied by the formation of soluble peptidoglycan. Biochem J. 1975 Jan;146(1):253–267. doi: 10.1042/bj1460253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WENZEL M., LENK H. P., SCHUETTE E. [H-3] and its splitting by lysozyme]. Hoppe Seylers Z Physiol Chem. 1961 Dec 29;327:13–20. [PubMed] [Google Scholar]
- Watkinson R. J., Hussey H., Baddiley J. Shared lipid phosphate carrier in the biosynthesis of teichoic acid and peptidoglycan. Nat New Biol. 1971 Jan 13;229(2):57–59. doi: 10.1038/newbio229057a0. [DOI] [PubMed] [Google Scholar]
- Waxman D. J., Yu W., Strominger J. L. Linear, uncross-linked peptidoglycan secreted by penicillin-treated Bacillus subtilis. Isolation and characterization as a substrate for penicillin-sensitive D-alanine carboxypeptidases. J Biol Chem. 1980 Dec 10;255(23):11577–11587. [PubMed] [Google Scholar]
- Wong W., Young F. E., Chatterjee A. N. Regulation of bacterial cell walls: turnover of cell wall in Staphylococcus aureus. J Bacteriol. 1974 Nov;120(2):837–843. doi: 10.1128/jb.120.2.837-843.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG F. E., TIPPER D. J., STROMINGER J. L. AUTOLYSIS OF CELL WALLS OF BACILLUS SUBTILIS. MECHANISM AND POSSIBLE RELATIONSHIP TO COMPETENCE. J Biol Chem. 1964 Oct;239:PC3600–PC3602. [PubMed] [Google Scholar]
- Zeiger A. R., Wong W., Chatterjee A. N., Young F. E., Tuazon C. U. Evidence for the secretion of soluble peptidoglycans by clinical isolates of Staphylococcus aureus. Infect Immun. 1982 Sep;37(3):1112–1118. doi: 10.1128/iai.37.3.1112-1118.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]



