Abstract
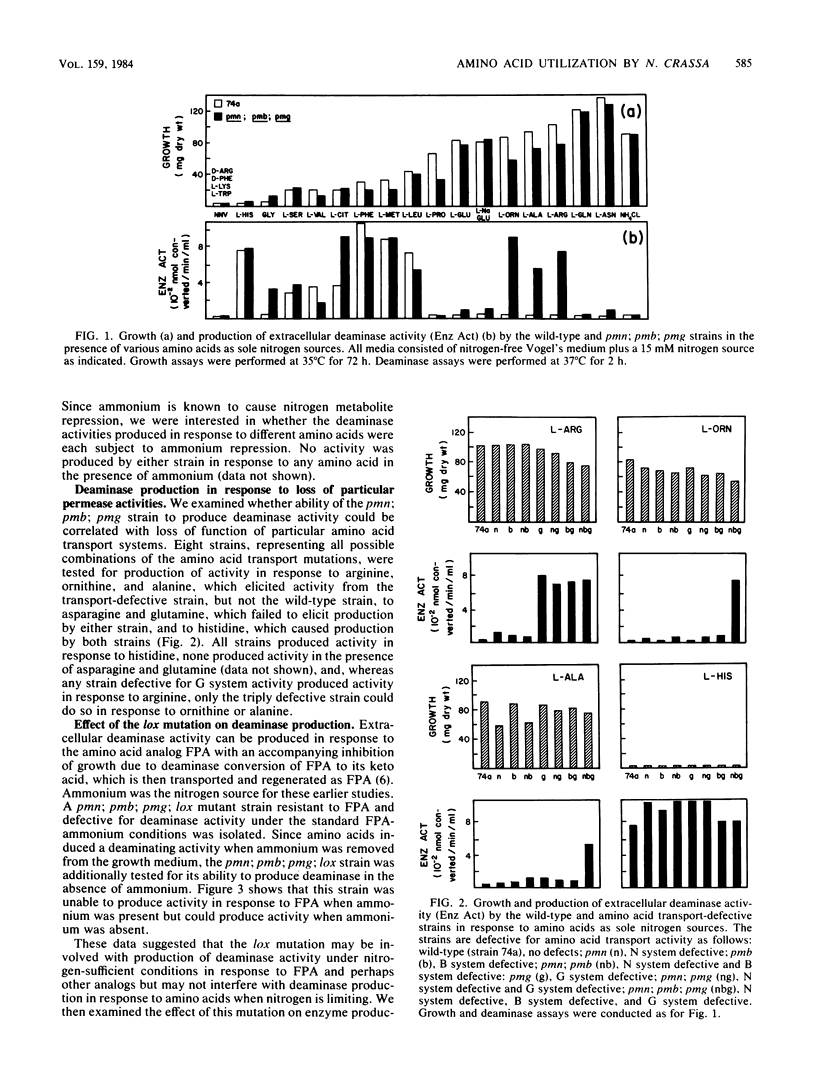
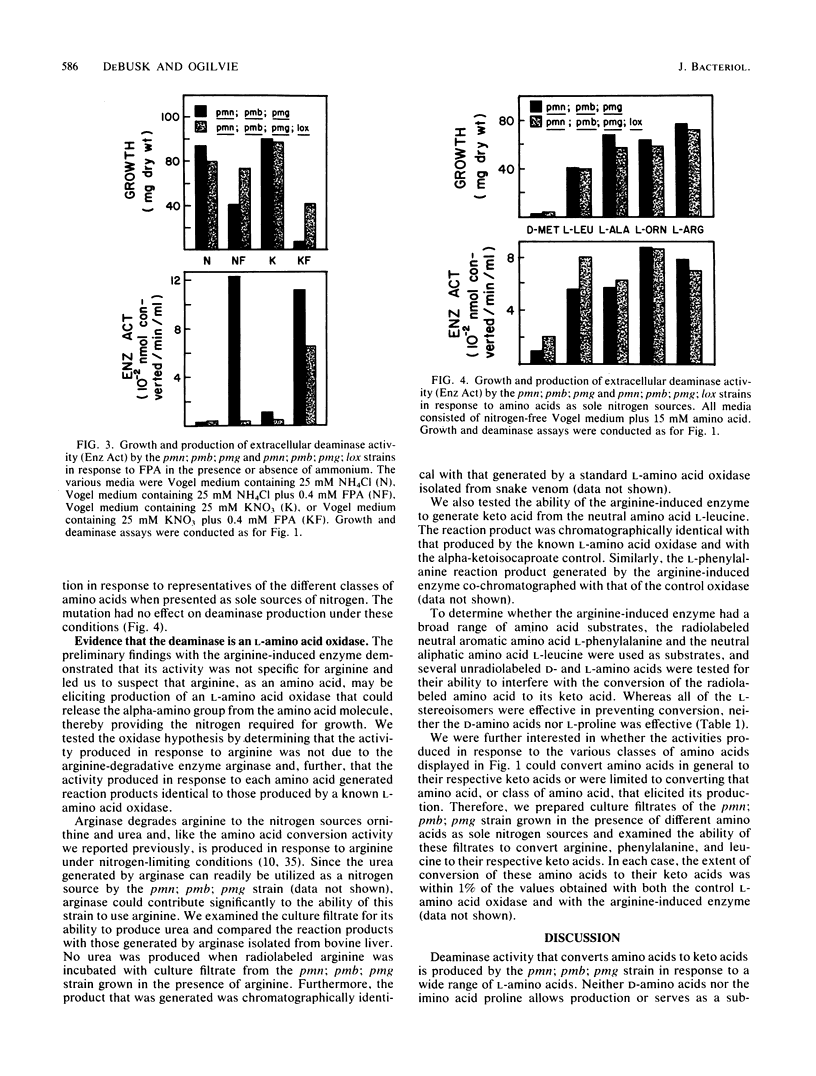
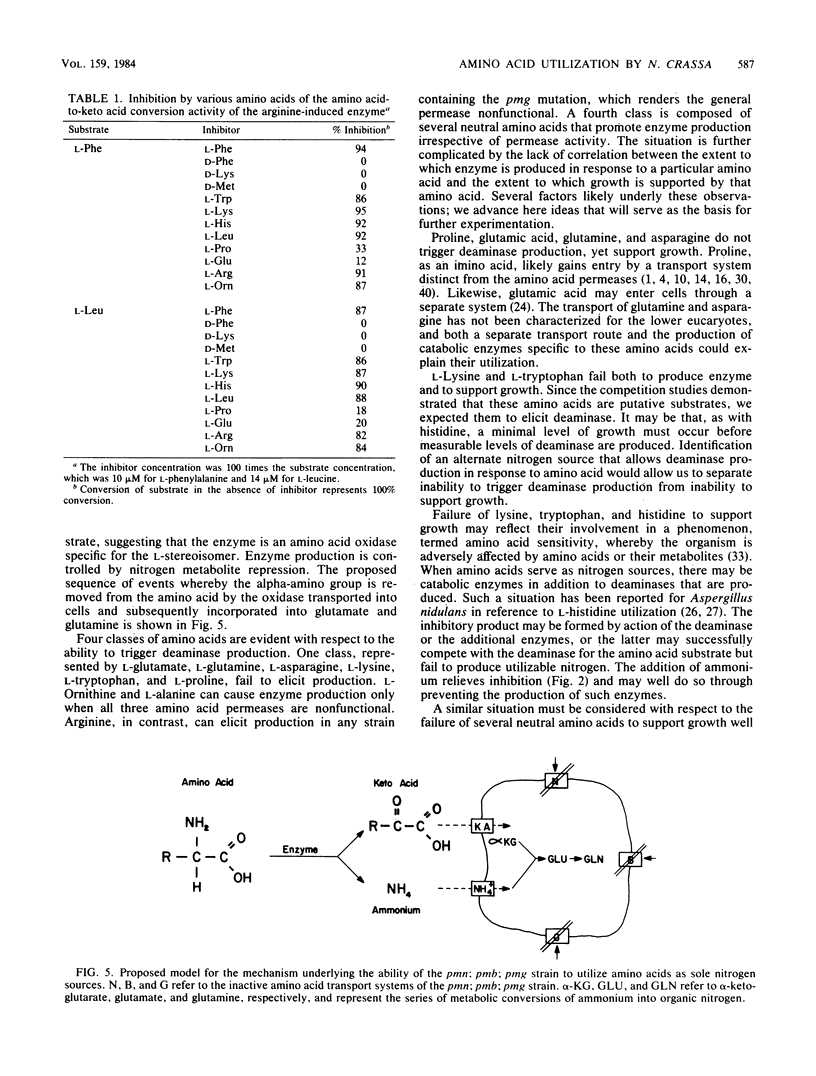
A strain of Neurospora crassa defective in amino acid transport can utilize a variety of amino acids for growth when readily metabolizable nitrogen is limiting. Growth is accompanied by the production of an extracellular deaminase that converts the amino acid to its respective keto acid plus equimolar quantities of utilizable nitrogen in the ammonium ion form. Production of the deaminase is subject to ammonium repression. The relationship between the ability of an amino acid to trigger deaminase production and the presence of particular amino acid permease deficiencies is complex. Four classes of amino acids have been defined with respect to this relationship. The existence of multiple extracellular deaminases is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. The L-amino-acid oxidase of Neurospora. Biochem J. 1951 Dec;50(2):258–268. doi: 10.1042/bj0500258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B. L., Drucker H. Regulation of exocellular protease in Neurospora crassa: induction and repression under conditions of nitrogen starvation. Arch Biochem Biophys. 1977 Aug;182(2):601–613. doi: 10.1016/0003-9861(77)90541-0. [DOI] [PubMed] [Google Scholar]
- Courchesne W. E., Magasanik B. Ammonia regulation of amino acid permeases in Saccharomyces cerevisiae. Mol Cell Biol. 1983 Apr;3(4):672–683. doi: 10.1128/mcb.3.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBusk B. G., DeBusk A. G. Molecular transport in Neurospora crassa. I. Biochemical properties of a phenylalanine permease. Biochim Biophys Acta. 1965 Jun 15;104(1):139–150. doi: 10.1016/0304-4165(65)90229-1. [DOI] [PubMed] [Google Scholar]
- DeBusk R. M., Brown D. T., DeBusk A. G., Penderghast R. D. Alternate mechanism for amino acid entry into Neurospora crassa: extracellular deamination and subsequent keto acid transport. J Bacteriol. 1981 Apr;146(1):163–169. doi: 10.1128/jb.146.1.163-169.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBusk R. M., DeBusk A. G. Physiological and regulatory properties of the general amino acid transport system of Neurospora crassa. J Bacteriol. 1980 Jul;143(1):188–197. doi: 10.1128/jb.143.1.188-197.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBusk R. M., Ogilvie-Villa S. Physiological adaptation to the loss of amino acid transport ability. J Bacteriol. 1982 Oct;152(1):545–548. doi: 10.1128/jb.152.1.545-548.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop P. C., Roon R. J. L-Asparaginase of Saccharomyces cerevisiae: an extracellular Enzyme. J Bacteriol. 1975 Jun;122(3):1017–1024. doi: 10.1128/jb.122.3.1017-1024.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam T. J., Marzluf G. A. Nitrogen regulation of amino acid catabolism in Neurospora crassa. Biochem Genet. 1978 Apr;16(3-4):343–354. doi: 10.1007/BF00484090. [DOI] [PubMed] [Google Scholar]
- Hanson M. A., Marzluf G. A. Control of the synthesis of a single enzyme by multiple regulatory circuits in Neurospora crassa. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1240–1244. doi: 10.1073/pnas.72.4.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder W., Dijkhuizen L. Physiological responses to nutrient limitation. Annu Rev Microbiol. 1983;37:1–23. doi: 10.1146/annurev.mi.37.100183.000245. [DOI] [PubMed] [Google Scholar]
- Jones G. E. Genetic and physiological relationships between L-asparaginase I and asparaginase II in Saccharomyces cerevisiae. J Bacteriol. 1977 Apr;130(1):128–130. doi: 10.1128/jb.130.1.128-130.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasko P. F., Brandriss M. C. Proline transport in Saccharomyces cerevisiae. J Bacteriol. 1981 Oct;148(1):241–247. doi: 10.1128/jb.148.1.241-247.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester G. Genetic control of amino acid permeability in Neurospora crassa. J Bacteriol. 1966 Feb;91(2):677–684. doi: 10.1128/jb.91.2.677-684.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magasanik B. Genetic control of nitrogen assimilation in bacteria. Annu Rev Genet. 1982;16:135–168. doi: 10.1146/annurev.ge.16.120182.001031. [DOI] [PubMed] [Google Scholar]
- Magaña-Schwencke N., Schwencke J. A proline transport system in Saccharomyces chevalieri. Biochim Biophys Acta. 1969 Mar 11;173(2):313–323. doi: 10.1016/0005-2736(69)90114-x. [DOI] [PubMed] [Google Scholar]
- Magill C. W., Nelson S. O., D'Ambrosio S. M., Glover G. I. Histidine uptake in mutant strains of Neurospora crassa via the general transport system for amino acids. J Bacteriol. 1973 Mar;113(3):1320–1325. doi: 10.1128/jb.113.3.1320-1325.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzluf G. A. Regulation of nitrogen metabolism and gene expression in fungi. Microbiol Rev. 1981 Sep;45(3):437–461. doi: 10.1128/mr.45.3.437-461.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHNISHI E., MACLEOD H., HOROWITZ N. H. Mutants of Neurospora deficient in D-amino acid oxidase. J Biol Chem. 1962 Jan;237:138–142. [PubMed] [Google Scholar]
- Ogilvie-Villa S., DeBusk R. M., DeBusk A. G. Characterization of 2-aminoisobutyric acid transport in Neurospora crassa: a general amino acid permease-specific substrate. J Bacteriol. 1981 Sep;147(3):944–948. doi: 10.1128/jb.147.3.944-948.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pall M. L. Amino acid transport in Neurospora crassa. 3. Acidic amino acid transport. Biochim Biophys Acta. 1970 Sep 15;211(3):513–520. doi: 10.1016/0005-2736(70)90256-7. [DOI] [PubMed] [Google Scholar]
- Pall M. L. Amino acid transport in Neurospora crassa. I. Properties of two amino acid transport systems. Biochim Biophys Acta. 1969 Jan 28;173(1):113–127. doi: 10.1016/0005-2736(69)90042-x. [DOI] [PubMed] [Google Scholar]
- Pall M. L. Amino acid transport in Neurospora crassa. II. Properties of a basic amino acid transport system. Biochim Biophys Acta. 1970 Mar 17;203(1):139–149. doi: 10.1016/0005-2736(70)90044-1. [DOI] [PubMed] [Google Scholar]
- Pall M. L. Amino acid transport in Neurospora crassa. IV. Properties and regulation of a methionine transport system. Biochim Biophys Acta. 1971 Mar 9;233(1):201–214. doi: 10.1016/0005-2736(71)90372-5. [DOI] [PubMed] [Google Scholar]
- Polkinghorne M. A., Hynes M. J. L-histidine utilization in Aspergillus nidulans. J Bacteriol. 1982 Mar;149(3):931–940. doi: 10.1128/jb.149.3.931-940.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polkinghorne M., Hynes M. J. Effect of L-histidine on the catabolism of nitrogenous compounds in Aspergillus nidulans. J Gen Microbiol. 1975 Mar;87(1):185–187. doi: 10.1099/00221287-87-1-185. [DOI] [PubMed] [Google Scholar]
- Rao E. Y., Rao T. K., Debusk A. G. Isolation and characterization of a mutant of Neurospora crassa deficient in general amino acid permease activity. Biochim Biophys Acta. 1975 Nov 17;413(1):45–51. doi: 10.1016/0005-2736(75)90057-7. [DOI] [PubMed] [Google Scholar]
- Schwencke J., Magaña-Schwencke N. Derepression of a proline transport system in Saccharomyces chevalieri by nitrogen starvation. Biochim Biophys Acta. 1969 Mar 11;173(2):302–312. doi: 10.1016/0005-2736(69)90113-8. [DOI] [PubMed] [Google Scholar]
- Sikora L. A., Marzluf G. A. Regulation of L-phenylalanine ammonia-lyase by L-phenylalanine and nitrogen in Neurospora crassa. J Bacteriol. 1982 Jun;150(3):1287–1291. doi: 10.1128/jb.150.3.1287-1291.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikora L., Marzluf G. A. Regulation of L-amino acid oxidase and of D-amino acid oxidase in Neurospora crassa. Mol Gen Genet. 1982;186(1):33–39. doi: 10.1007/BF00422908. [DOI] [PubMed] [Google Scholar]
- Sumrada R., Cooper T. Basic amino acid inhibition of growth in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1976 Jan 26;68(2):598–602. doi: 10.1016/0006-291x(76)91187-6. [DOI] [PubMed] [Google Scholar]
- THAYER P. S., HOROWITZ N. H. The L-amino acid oxidase of Neurospora. J Biol Chem. 1951 Oct;192(2):755–767. [PubMed] [Google Scholar]
- Vaca G., Mora J. Nitrogen regulation of arginase in Neurospora crassa. J Bacteriol. 1977 Sep;131(3):719–725. doi: 10.1128/jb.131.3.719-725.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfinbarger L., Jr, Debusk A. G. Molecular transport. I. In vivo studies of transport mutants of Neurospora crassa with altered amino acid competition patterns. Arch Biochem Biophys. 1971 Jun;144(2):503–511. doi: 10.1016/0003-9861(71)90355-9. [DOI] [PubMed] [Google Scholar]
- Wolfinbarger L., Jr, Jervis H. H., DeBusk A. G. Active transport of L-aspartic acid in Neurospora crassa. Biochim Biophys Acta. 1971 Oct 12;249(1):63–68. doi: 10.1016/0005-2736(71)90083-6. [DOI] [PubMed] [Google Scholar]
- Woodward J. R., Cirillo V. P. Amino acid transport and metabolism in nitrogen-starved cells of Saccharomyces cerevisiae. J Bacteriol. 1977 May;130(2):714–723. doi: 10.1128/jb.130.2.714-723.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZALOKAR M. Kinetics of amino acid uptake and protein synthesis in Neurospora. Biochim Biophys Acta. 1961 Jan 29;46:423–432. doi: 10.1016/0006-3002(61)90573-x. [DOI] [PubMed] [Google Scholar]



