Abstract
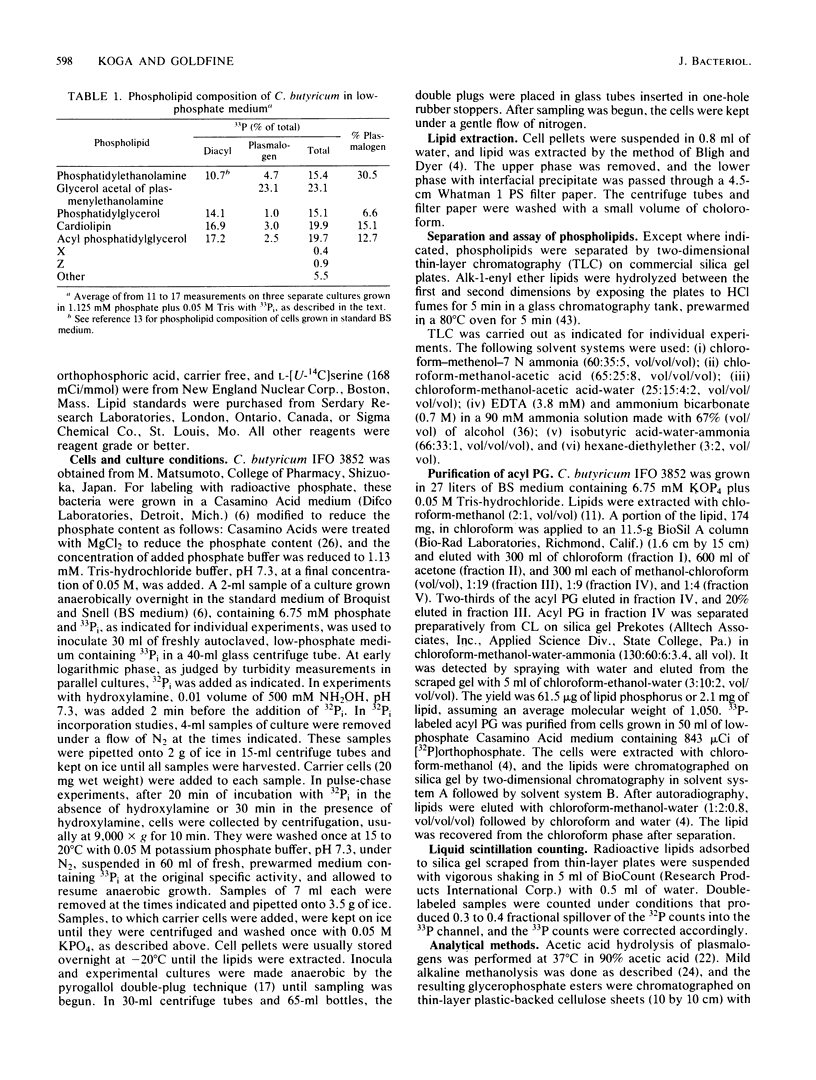
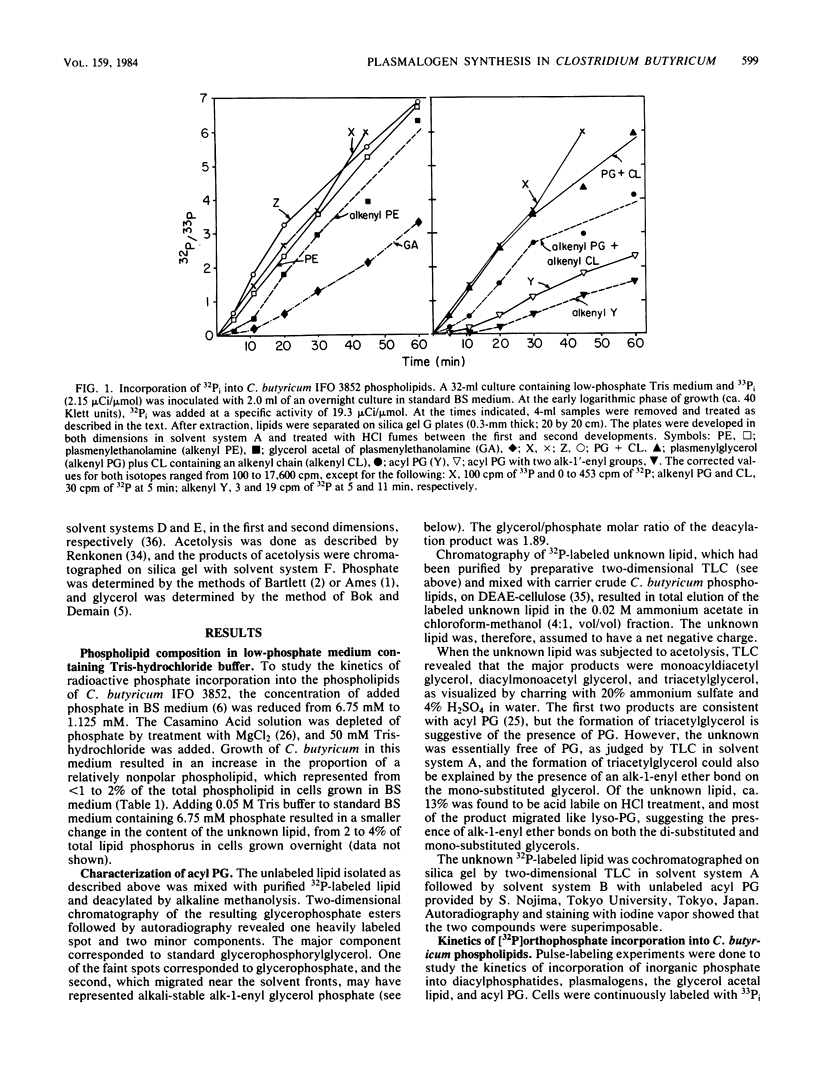
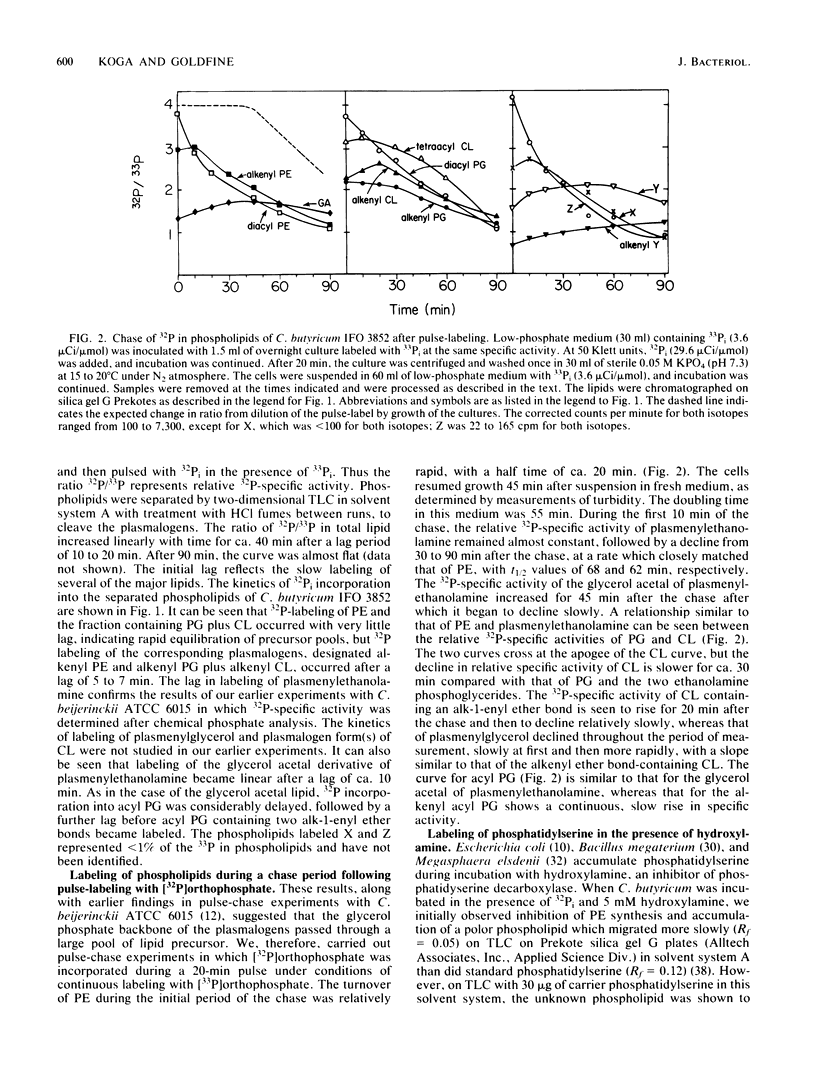
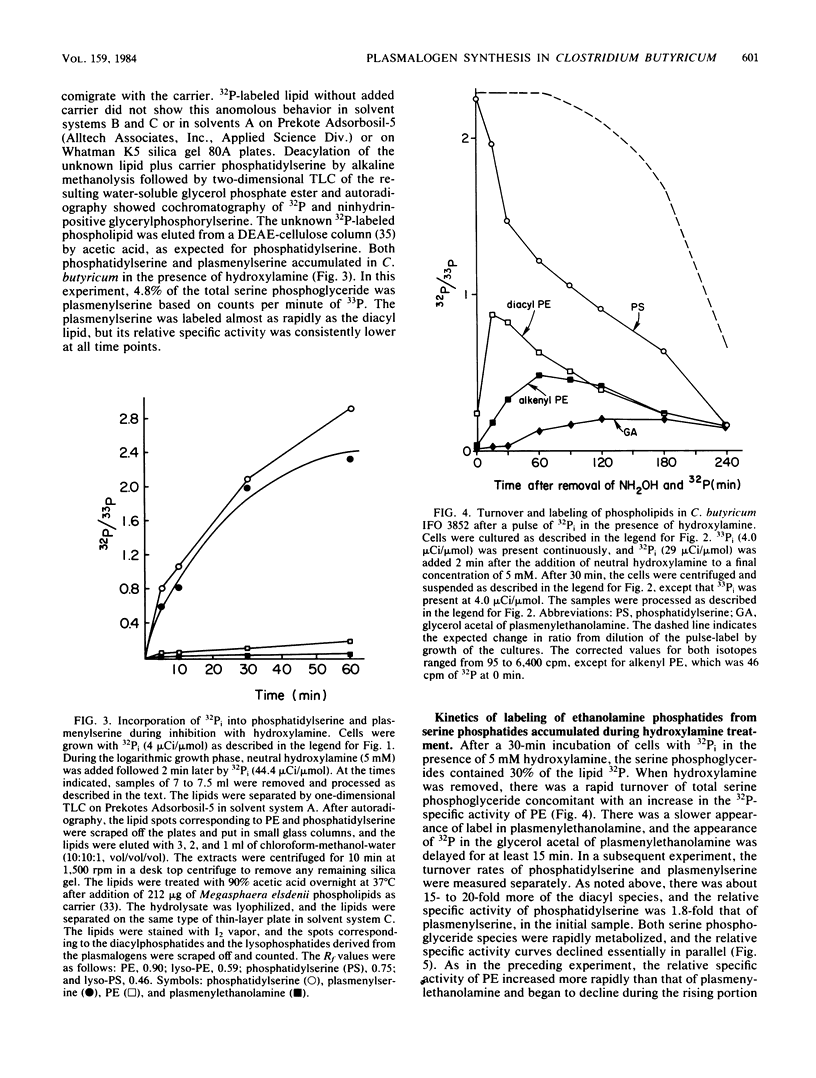
The biosynthesis of the plasmalogen forms of phosphatidylethanolamine (plasmenylethanolamine) and phosphatidylglycerol (plasmenylglycerol) and of the glycerol acetal of plasmenylethanolamine has been studied in cultures of Clostridium butyricum IFO 3852. When growing cells were pulsed with [32P]orthophosphate, there was a lag of 5 to 7 min between the rapid incorporation of label into the acylphosphatides and the rapid incorporation of label into the corresponding plasmalogens. The labeling of the glycerol acetal of plasmenylethanolamine was even slower. In pulse-chase experiments with 32Pi, the kinetics of labeling indicated precursor-product relationships between phosphatidylethanolamine and plasmenylethanolamine and between the latter and its glycerol acetal. A precursor-product relationship was also seen between phosphatidylglycerol and cardiolipin, but the kinetics of labeling of the alkenyl-containing forms of these lipids were not consistent with direct precursor-product relationships with the acyl lipids. In the presence of hydroxylamine and 32Pi, both phosphatidylserine and plasmenylserine accumulated 32P in a ratio of ca. 15:1. Upon release of the inhibition of phosphatidylserine decarboxylase, label appeared in the following sequence: phosphatidylethanolamine, plasmenylethanolamine, and the glycerol acetal of plasmenylethanolamine. Acyl phosphatidylglycerol was identified as a major phospholipid (17% of lipid phosphorus) in C. butyricum grown in low-phosphate (1.13 mM) medium with 50 mM Tris buffer. Of the acyl phosphatidylglycerol, 13% was acid labile. There appear to be two plasmalogen forms of acyl phosphatidylglycerol. One of these has a single alkenyl ether group, and the other has alkenyl ether groups on both glycerols.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BAUMANN N. A., HAGEN P. O., GOLDFINE H. PHOSPHOLIPIDS OF CLOSTRIDIUM BUTYRICUM. STUDIES ON PLASMALOGEN COMPOSITION AND BIOSYNTHESIS. J Biol Chem. 1965 Apr;240:1559–1567. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- BROQUIST H. P., SNELL E. E. Biotin and bacterial growth. I. Relation to aspartate, oleate, and carbon dioxide. J Biol Chem. 1951 Jan;188(1):431–444. [PubMed] [Google Scholar]
- Bok S. H., Demain A. L. An improved colorimetric assay for polyols. Anal Biochem. 1977 Jul;81(1):18–20. doi: 10.1016/0003-2697(77)90593-0. [DOI] [PubMed] [Google Scholar]
- Cain B. D., Donohue T. J., Kaplan S. Kinetic analysis of N-acylphosphatidylserine accumulation and implications for membrane assembly in Rhodopseudomonas sphaeroides. J Bacteriol. 1982 Nov;152(2):607–615. doi: 10.1128/jb.152.2.607-615.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K. S., Hong S. D., Cho J. M., Chang C. S., Lee K. S. Studies on the biosynthesis of acylphosphatidylglycerol in Escherichia coli B and B/r. Biochim Biophys Acta. 1976 Jan 18;486(1):47–54. doi: 10.1016/0005-2760(77)90068-6. [DOI] [PubMed] [Google Scholar]
- Donohue T. J., Cain B. D., Kaplan S. Alterations in the phospholipid composition of Rhodopseudomonas sphaeroides and other bacteria induced by Tris. J Bacteriol. 1982 Nov;152(2):595–606. doi: 10.1128/jb.152.2.595-606.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowhan W., Wickner W. T., Kennedy E. P. Purification and properties of phosphatidylserine decarboxylase from Escherichia coli. J Biol Chem. 1974 May 25;249(10):3079–3084. [PubMed] [Google Scholar]
- GOLDFINE H., BLOCH K. On the origin of unsaturated fatty acids in clostridia. J Biol Chem. 1961 Oct;236:2596–2601. [PubMed] [Google Scholar]
- Goldfine H., Johnston N. C., Bishop D. G. Ether phospholipid asymmetry in Clostridium butyricum. Biochem Biophys Res Commun. 1982 Oct 29;108(4):1502–1507. doi: 10.1016/s0006-291x(82)80077-6. [DOI] [PubMed] [Google Scholar]
- Goldfine H., Khuller G. K., Borie R. P., Silverman B., Selick H., 2nd, Johnston N. C., Vanderkooi J. M., Horwitz A. F. Effects of growth temperature and supplementation with exogenous fatty acids on some physical properties of Clostridium butyricum phospholipids. Biochim Biophys Acta. 1977 Sep 28;488(3):341–352. doi: 10.1016/0005-2760(77)90193-x. [DOI] [PubMed] [Google Scholar]
- HARDMAN J. K., STADTMAN T. C. Metabolism of omega-amino acids. I. Fermentation of gamma-aminobutyric acid by Clostridium aminobutyricum n. sp. J Bacteriol. 1960 Apr;79:544–548. doi: 10.1128/jb.79.4.544-548.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajra A. K. Acyl dihydroxyacetone phosphate: precursor of alkyl ethers. Biochem Biophys Res Commun. 1970;39(6):1037–1044. doi: 10.1016/0006-291x(70)90663-7. [DOI] [PubMed] [Google Scholar]
- Hazlewood G. P., Clarke N. G., Dawson R. M. Complex lipids of a lipolytic and general-fatty-acid-requiring Butyrivibrio sp. isolated from the ovine rumen. Biochem J. 1980 Nov 1;191(2):555–560. doi: 10.1042/bj1910555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill E. E., Lands W. E. Formation of acyl and alkenyl glycerol derivatives in Clostridium butyricum. Biochim Biophys Acta. 1970 Feb 10;202(1):209–211. doi: 10.1016/0005-2760(70)90239-0. [DOI] [PubMed] [Google Scholar]
- Homma H., Nojima S. Synthesis of various phospholipids from 2-acyl lysophospholipids by Escherichia coli extract. J Biochem. 1982 Apr;91(4):1103–1110. doi: 10.1093/oxfordjournals.jbchem.a133792. [DOI] [PubMed] [Google Scholar]
- Johnston N. C., Goldfine H. Lipid composition in the classification of the butyric acid-producing clostridia. J Gen Microbiol. 1983 Apr;129(4):1075–1081. doi: 10.1099/00221287-129-4-1075. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Nishijima M., Tamori Y., Nojima S., Seyama Y., Yamakawa T. Acyl phosphatidylglycerol of Escherichia coli. Biochim Biophys Acta. 1980 Dec 5;620(3):356–363. [PubMed] [Google Scholar]
- Lin S. Y., Riggs A. D. Lac repressor binding to non-operator DNA: detailed studies and a comparison of eequilibrium and rate competition methods. J Mol Biol. 1972 Dec 30;72(3):671–690. doi: 10.1016/0022-2836(72)90184-2. [DOI] [PubMed] [Google Scholar]
- Matsumoto M., Tamiya K., Koizumi K. Studies on neutral lipids and a new type of aldehydogenic ethanolamine phospholipid in Clostridium butyricum. J Biochem. 1971 Mar;69(3):617–620. [PubMed] [Google Scholar]
- Nishijima M., Sa-Eki T., Tamori Y., Doi O., Nojima S. Synthesis of acyl phosphatidylglycerol from phosphatidylglycerol in Escherichia coli K-12. Evidence for the participation of detergent-resistant phospholipase A and heat-labile membrane-bound factor(s). Biochim Biophys Acta. 1978 Jan 27;528(1):107–118. [PubMed] [Google Scholar]
- Olsen R. W., Ballou C. E. Acyl phosphatidylglycerol. A new phospholipid from Salmonella typhimurium. J Biol Chem. 1971 May 25;246(10):3305–3313. [PubMed] [Google Scholar]
- Patterson P. H., Lennarz W. J. Studies on the membranes of bacilli. I. Phospholipid biosynthesis. J Biol Chem. 1971 Feb 25;246(4):1062–1072. [PubMed] [Google Scholar]
- Plackett P., Smith P. F., Mayberry W. R. Lipids of a sterol-nonrequiring Mycoplasma. J Bacteriol. 1970 Nov;104(2):798–807. doi: 10.1128/jb.104.2.798-807.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins R. A., Akkermans-Kruyswijk J., Franklin-Klein W., Lankhorst A., van Golde L. M. Metabolism of serine and ethanolamine plasmalogens in Megasphaera elsdenii. Biochim Biophys Acta. 1974 Jun 26;348(3):361–369. doi: 10.1016/0005-2760(74)90216-1. [DOI] [PubMed] [Google Scholar]
- Prins R. A., Van Golde L. M. Entrance of glycerol into plasmalogens of some strictly anaerobic bacteria and protozoa. FEBS Lett. 1976 Mar 15;63(1):107–111. doi: 10.1016/0014-5793(76)80204-9. [DOI] [PubMed] [Google Scholar]
- RENKONEN O. INDIVIDUAL MOLECULAR SPECIES OF DIFFERENT PHOSPHOLIPID CLASSES. II. A METHOD OF ANALYSIS. J Am Oil Chem Soc. 1965 Apr;42:298–304. doi: 10.1007/BF02540133. [DOI] [PubMed] [Google Scholar]
- Short S. A., White D. C., Aleem M. I. Phospholipid metabolism in Ferrobacillus ferrooxidans. J Bacteriol. 1969 Jul;99(1):142–150. doi: 10.1128/jb.99.1.142-150.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silber P., Borie R. P., Goldfine H. The enzymes of phospholipid synthesis in Clostridium butyricum. J Lipid Res. 1980 Nov;21(8):1022–1031. [PubMed] [Google Scholar]
- Silber P., Borie R. P., Mikowski E. J., Goldfine H. Phospholipid biosynthesis in some anaerobic bacteria. J Bacteriol. 1981 Jul;147(1):57–61. doi: 10.1128/jb.147.1.57-61.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder F., Wykle R. L., Malone B. A new metabolic pathway: biosynthesis of alkyl ether bonds from glyceraldehyde-3-phosphate and fatty alcohols by microsomal enzymes. Biochem Biophys Res Commun. 1969 Feb 7;34(3):315–321. doi: 10.1016/0006-291x(69)90834-1. [DOI] [PubMed] [Google Scholar]
- Tsukagoshi N., Kania M. N., Franklin R. M. Identification of acyl phosphatidylglycerol as a minor phospholipid of Pseudomonas BAL-31. Biochim Biophys Acta. 1976 Nov 19;450(2):131–136. doi: 10.1016/0005-2760(76)90084-9. [DOI] [PubMed] [Google Scholar]
- Van Golde L. M., Akkermans-Kruyswijk J., Franklin-Klein W., Lankhorst A., Prins R. A. Accumulation of phosphatidylserine in strictly anaerobic lactate fermenting bacteria. FEBS Lett. 1975 Apr 15;53(1):57–60. doi: 10.1016/0014-5793(75)80681-8. [DOI] [PubMed] [Google Scholar]
- Viswanathan C. V., Phillips F., Lundberg W. O. Two-dimensional reaction thin-layer chromatography in the analysis of phosphatide plasmalogens. J Chromatogr. 1968 May 21;35(1):66–71. doi: 10.1016/s0021-9673(01)82350-5. [DOI] [PubMed] [Google Scholar]
- van den Bosch H. Phosphoglyceride metabolism. Annu Rev Biochem. 1974;43(0):243–277. doi: 10.1146/annurev.bi.43.070174.001331. [DOI] [PubMed] [Google Scholar]



