Abstract
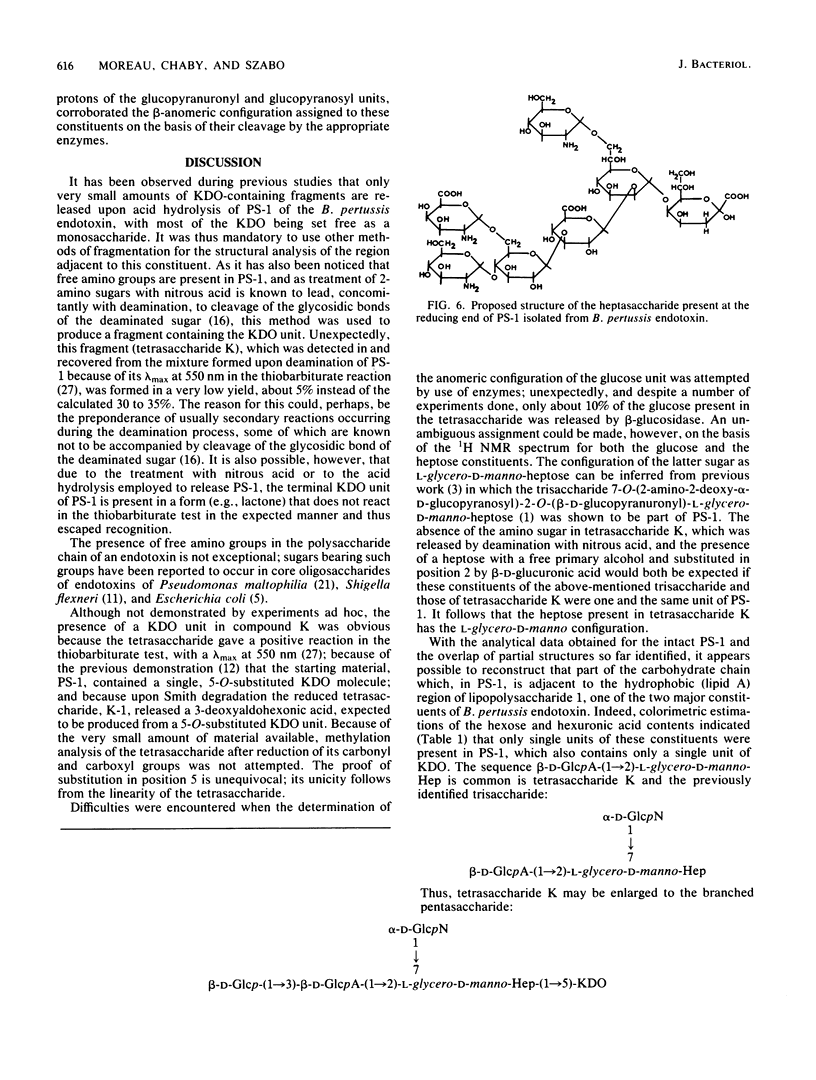
The tetrasaccharide beta-D-glucopyranosyl-(1,3)-beta-D-glucopyranuronyl-(1, 2)-L-glycero-alpha-D-manno-heptopyranosyl-(1,5)-3-deoxy-D-manno-2- octulosonic acid was isolated after treatment of polysaccharide 1 of Bordetella pertussis endotoxin with nitrous acid. Taking into account previously identified di- and trisaccharide fragments and analytical data obtained for the intact polysaccharide 1, we present the structure of a heptasaccharide that is thought to represent the region immediately adjacent to the hydrophobic (lipid A) moiety of lipopolysaccharide 1 of the B. pertussis endotoxin. This heptasaccharide represents 50 to 60% of the complete polysaccharide structure.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chaby R., Moreau M., Szabó L. 2-O-(beta-D-glucuronyl)-7-O-(2-amino-2-deoxy-alpha-D-glucopyranosyl)-L-glycero-D-manno-heptose: a constituent of the Bordetella pertussis endotoxin. Eur J Biochem. 1977 Jun 15;76(2):453–460. doi: 10.1111/j.1432-1033.1977.tb11615.x. [DOI] [PubMed] [Google Scholar]
- Chaby R., Szabó L. 3-Deoxy-2-octulosonic acid 5-phosphate: a component of the endotoxin of Bordetella pertussis. Eur J Biochem. 1975 Nov 1;59(1):277–280. doi: 10.1111/j.1432-1033.1975.tb02452.x. [DOI] [PubMed] [Google Scholar]
- Chaby R., Szabó L. 7-O-(2-Amino-2-deoxy-alpha-D-glucopyranosyl)-L-glycero-D-manno-heptose. A constituent of the endotoxin of Bordetella pertussis. Eur J Biochem. 1976 Nov 1;70(1):115–122. doi: 10.1111/j.1432-1033.1976.tb10962.x. [DOI] [PubMed] [Google Scholar]
- Cohen R. E., Ballou C. E. Linkage and sequence analysis of mannose-rich glycoprotein core oligosaccharides by proton nuclear magnetic resonance spectroscopy. Biochemistry. 1980 Sep 2;19(18):4345–4358. doi: 10.1021/bi00559a031. [DOI] [PubMed] [Google Scholar]
- Fuller N. A., Wu M., Wilkinson R. G., Heath E. C. The biosynthesis of cell wall lipopolysaccharide in Escherichia coli. VII. Characterization of heterogeneous "core" oligosaccharide structures. J Biol Chem. 1973 Nov 25;248(22):7938–7950. [PubMed] [Google Scholar]
- Gahan L. C., Sandford P. A., Conrad H. E. The structure of the serotype 2 capsular polysaccharide of Aerobacter aerogenes. Biochemistry. 1967 Sep;6(9):2755–2767. doi: 10.1021/bi00861a016. [DOI] [PubMed] [Google Scholar]
- Girard R., Chaby R., Bordenave G. Mitogenic response of C3H/HeJ mouse lymphocytes to polyanionic polysaccharides obtained from Bordetella pertussis endotoxin and from other bacterial species. Infect Immun. 1981 Jan;31(1):122–128. doi: 10.1128/iai.31.1.122-128.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- Haeffner-Cavaillon N., Cavaillon J. M., Moreau M., Szabó L. Interleukin 1 secretion by human monocytes stimulated by the isolated polysaccharide region of the Bordetella pertussis endotoxin. Mol Immunol. 1984 May;21(5):389–395. doi: 10.1016/0161-5890(84)90036-1. [DOI] [PubMed] [Google Scholar]
- Haeffner-Cavaillon N., Cavaillon J. M., Szabo L. Macrophage-dependent polyclonal activation of splenocytes by Bordetella pertussis endotoxin and its isolated polysaccharide and Lipid A regions. Cell Immunol. 1982 Nov 15;74(1):1–13. doi: 10.1016/0008-8749(82)90001-6. [DOI] [PubMed] [Google Scholar]
- Haeffner-Cavaillon N., Chaby R., Cavaillon J. M., Szabó L. Lipopolysaccharide receptor on rabbit peritoneal macrophages. I. Binding characteristics. J Immunol. 1982 May;128(5):1950–1954. [PubMed] [Google Scholar]
- Katzenellenbogen E., Romanowska E. Structural studies on Shigella flexneri serotype 6 core region. Eur J Biochem. 1980 Dec;113(1):205–211. doi: 10.1111/j.1432-1033.1980.tb06157.x. [DOI] [PubMed] [Google Scholar]
- Le Dur A., Caroff M., Chaby R., Szabó L. A novel type of endotoxin structure present in Bordetella pertussis. Isolation of two different polysaccharides bound to lipid A. Eur J Biochem. 1978 Mar 15;84(2):579–589. doi: 10.1111/j.1432-1033.1978.tb12201.x. [DOI] [PubMed] [Google Scholar]
- Le Dur A., Chaby R., Szabó L. Isolation of two protein-free and chemically different lipopolysaccharides from Bordetella pertussis phenol-extracted endotoxin. J Bacteriol. 1980 Jul;143(1):78–88. doi: 10.1128/jb.143.1.78-88.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levvy G. A., Hay A. J., Conchie J. Inhibition of glycosidases by aldonolactones of corresponding configuration. 4. Inhibitors of mannosidase and glucosidase. Biochem J. 1964 May;91(2):378–384. doi: 10.1042/bj0910378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACGEE J., DOUDOROFF M. A new phosphorylated intermediate in glucose oxidation. J Biol Chem. 1954 Oct;210(2):617–626. [PubMed] [Google Scholar]
- Mayer H., Schmidt G. The occurrence of three different lipopolysaccharide cores in shigella and their relationship to known enterobacterial core types. Zentralbl Bakteriol Orig A. 1973 Aug;224(3):345–354. [PubMed] [Google Scholar]
- Moreau M., Chaby R., Szabo L. Isolation of a trisaccharide containing 2-amino-2-deoxy-D-galacturonic acid from the Bordetella pertussis endotoxin. J Bacteriol. 1982 Apr;150(1):27–35. doi: 10.1128/jb.150.1.27-35.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal D. J., Wilkinson S. G. Lipopolysaccharides from Pseudomonas maltophilia. Structural studies of the side-chain, core, and lipid-A regions of the lipopolysaccharide from strain NCTC 10257. Eur J Biochem. 1982 Nov;128(1):143–149. [PubMed] [Google Scholar]
- Peppler M. S. Two physically and serologically distinct lipopolysaccharide profiles in strains of Bordetella pertussis and their phenotype variants. Infect Immun. 1984 Jan;43(1):224–232. doi: 10.1128/iai.43.1.224-232.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]
- van Halbeek H., Dorland L., Vliegenthart J. F., Spik G., Cheron A., Mohtreuil J. Structure determination of two oligomannoside-type glycopeptides obtained from bovine lactotransferrin, by 500 MHz 1H-NMR spectroscopy. Biochim Biophys Acta. 1981 Jul;675(2):293–296. doi: 10.1016/0304-4165(81)90240-3. [DOI] [PubMed] [Google Scholar]


