Abstract
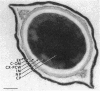
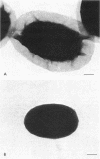
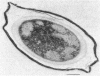
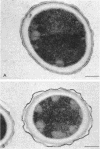
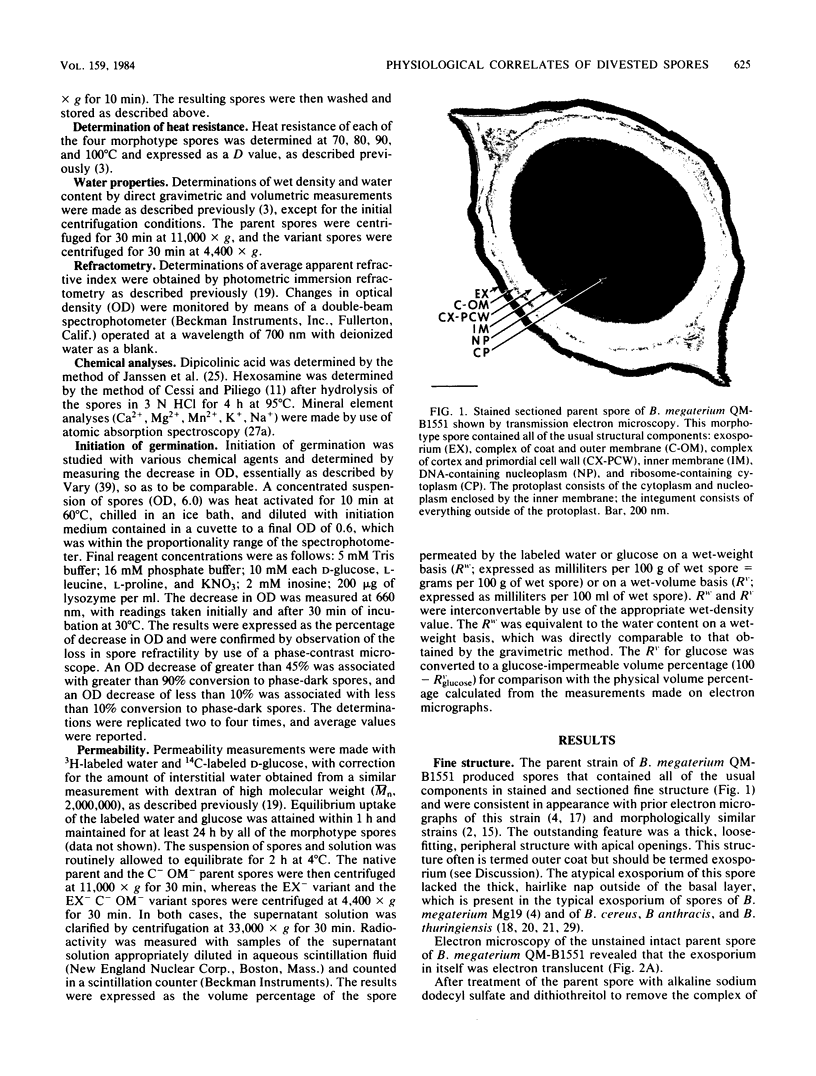
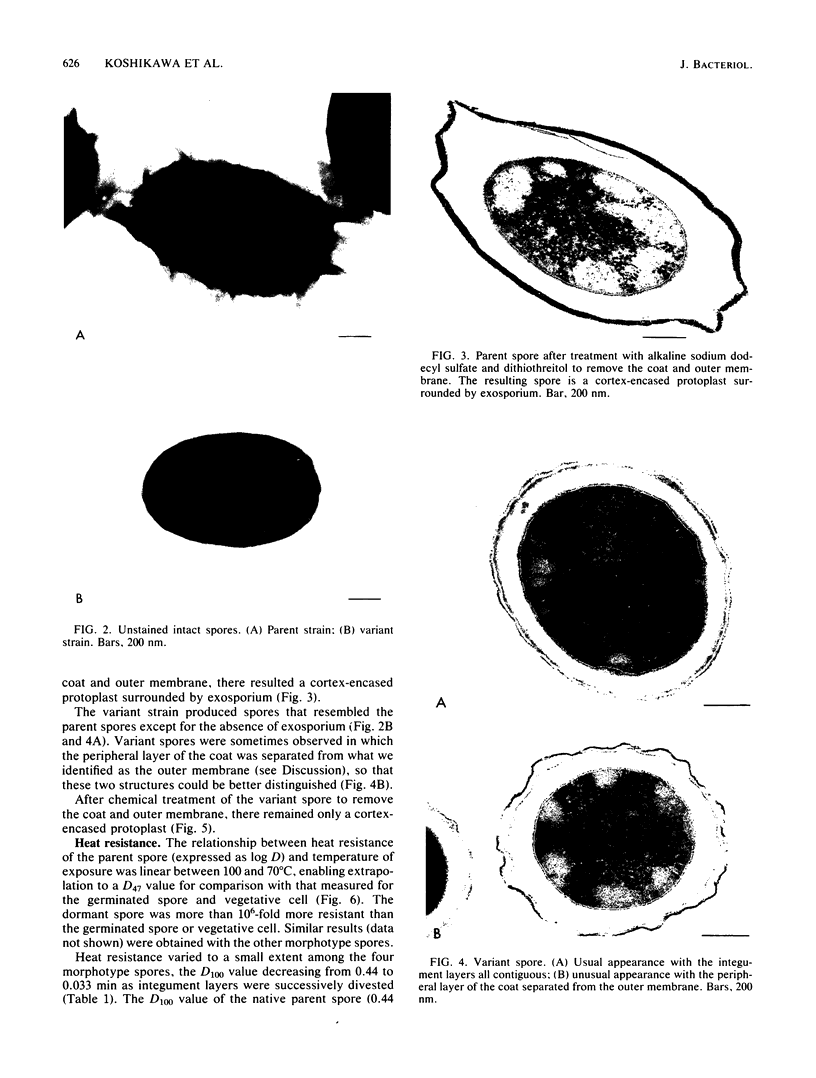
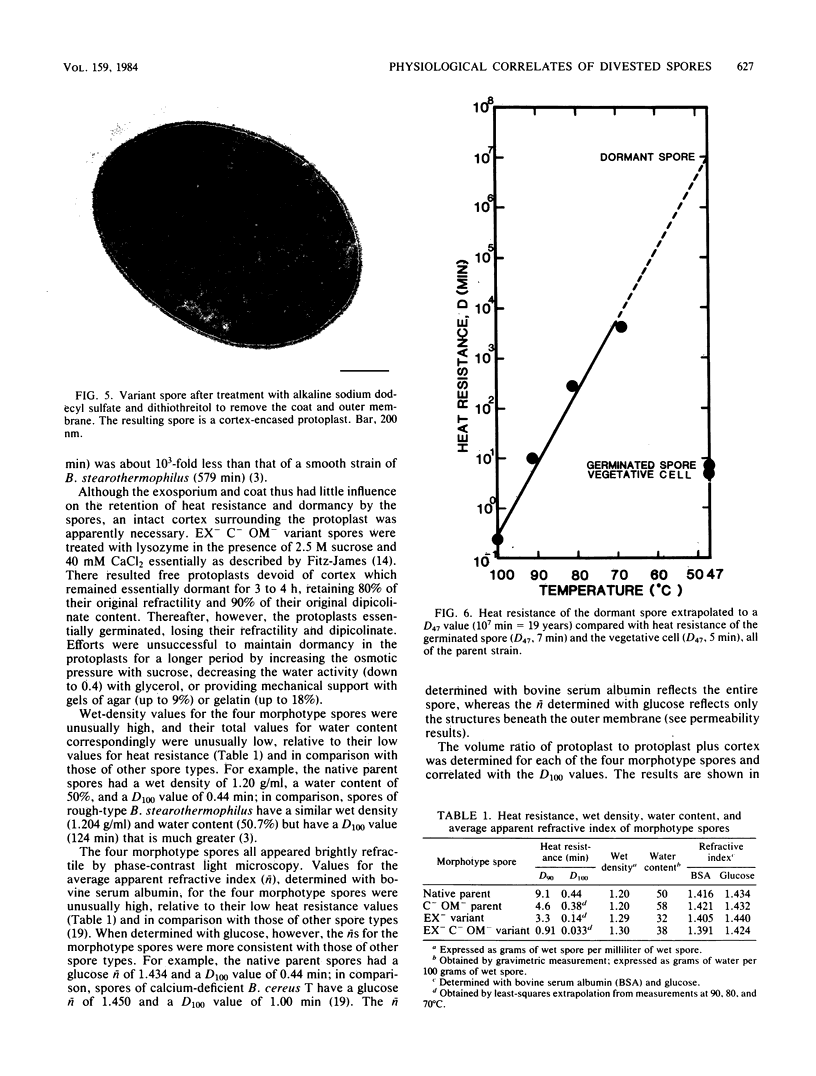
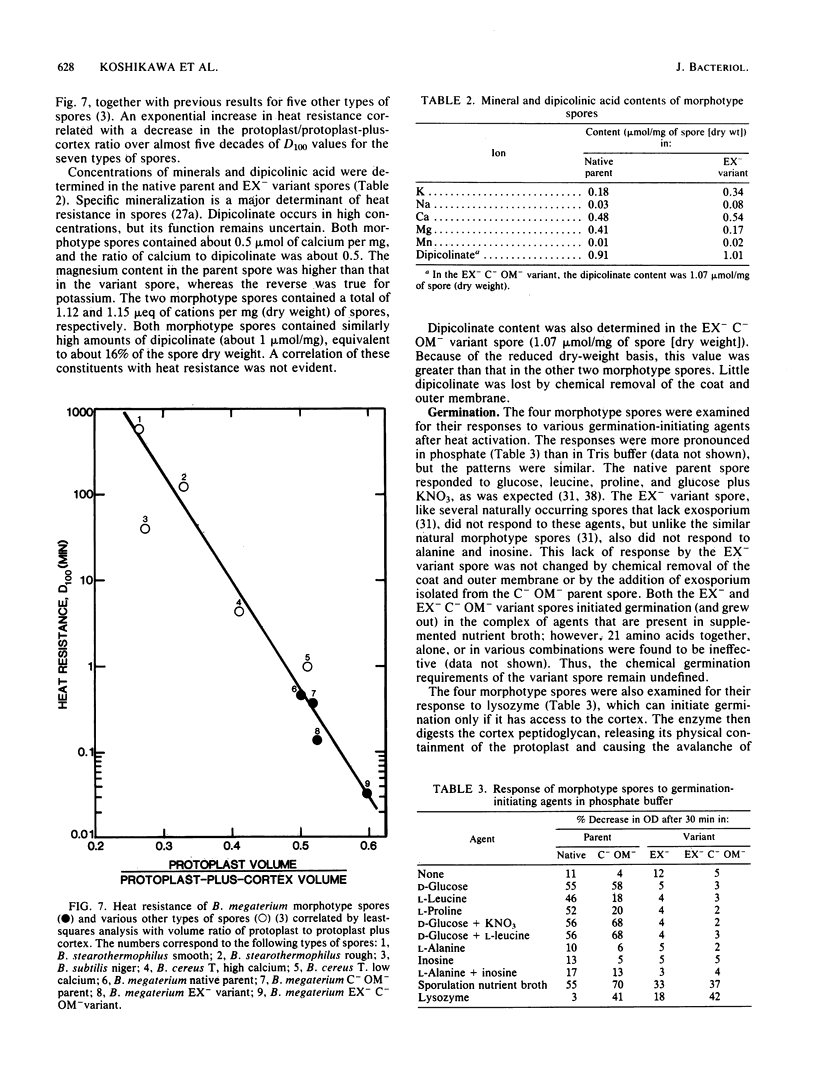
A variant strain that produced spores lacking exosporium was isolated from a culture of Bacillus megaterium QM-B1551. Two additional spore morphotypes were obtained from the parent and variant strains by chemical removal of the complex of coat and outer membrane. Among the four morphotype spores, heat resistance did not correlate with total water content, wet density, refractive index, or dipicolinate or cation content, but did correlate with the volume ratio of protoplast to protoplast plus cortex. The divestment of integument layers exterior to the cortex had little influence on heat resistance. Moreover, the divestment did not change the response of either the parent or the variant spores to various germination-initiating agents, except for making the spores susceptible to germination by lysozyme. The primary permeability barrier to glucose for the intact parent and variant spores was found to be the outer membrane, whereas the barrier for the divested spores was the inner membrane.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson A. I., Fitz-James P. Structure and morphogenesis of the bacterial spore coat. Bacteriol Rev. 1976 Jun;40(2):360–402. doi: 10.1128/br.40.2.360-402.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACK S. H., GERHARDT P. Permeability of bacterial spores. III. Permeation relative to germination. J Bacteriol. 1962 Feb;83:301–308. doi: 10.1128/jb.83.2.301-308.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACK S. H., GERHARDT P. Permeability of bacterial spores. IV. Water content, uptake, and distribution. J Bacteriol. 1962 May;83:960–967. doi: 10.1128/jb.83.5.960-967.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman T. C., Greenamyre J. T., Corner T. R., Pankratz H. S., Gerhardt P. Bacterial spore heat resistance correlated with water content, wet density, and protoplast/sporoplast volume ratio. J Bacteriol. 1982 May;150(2):870–877. doi: 10.1128/jb.150.2.870-877.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman T. C., Pankratz H. S., Gerhardt P. Chemical composition and ultrastructure of native and reaggregated membranes from protoplasts of Bacillus cereus. J Bacteriol. 1974 Mar;117(3):1335–1340. doi: 10.1128/jb.117.3.1335-1340.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman T. C., Pankratz H. S., Gerhardt P. Ultrastructure of the exosporium and underlying inclusions in spores of Bacillus megaterium strains. J Bacteriol. 1972 Mar;109(3):1198–1209. doi: 10.1128/jb.109.3.1198-1209.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertsch L. L., Bonsen P. P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XIV. Phospholipids in Bacillus megaterium. J Bacteriol. 1969 Apr;98(1):75–81. doi: 10.1128/jb.98.1.75-81.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CESSI C., PILIEGO F. The determination of amino sugars in the presence of amino acids and glucose. Biochem J. 1960 Dec;77:508–510. doi: 10.1042/bj0770508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen E. L., Marquis R. E., Child S. Z., Bender G. R. Dielectric properties of native and decoated spores of Bacillus megaterium. J Bacteriol. 1979 Dec;140(3):917–928. doi: 10.1128/jb.140.3.917-928.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassier M., Ryter A. Sur un mutant de Clostridium perfringens donnant des spores sans tuniques à germination lysozyme-dépendante. Ann Inst Pasteur (Paris) 1971 Dec;121(6):717–732. [PubMed] [Google Scholar]
- Crafts-Lighty A., Ellar D. J. The structure and function of the spore outer membrane in dormant and germinating spores of Bacillus megaterium. J Appl Bacteriol. 1980 Feb;48(1):135–145. doi: 10.1111/j.1365-2672.1980.tb05215.x. [DOI] [PubMed] [Google Scholar]
- Fitz-James P. C. Formation of protoplasts from resting spores. J Bacteriol. 1971 Mar;105(3):1119–1136. doi: 10.1128/jb.105.3.1119-1136.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz-James P. C., Young I. E. CYTOLOGICAL COMPARISON OF SPORES OF DIFFERENT STRAINS OF BACILLUS MEGATERIUM. J Bacteriol. 1959 Dec;78(6):755–764. doi: 10.1128/jb.78.6.755-764.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freer J. H., Levinson H. S. Fine structure of Bacillus megaterium during microcycle sporogenesis. J Bacteriol. 1967 Aug;94(2):441–457. doi: 10.1128/jb.94.2.441-457.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERHARDT P., RIBI E. ULTRASTRUCTURE OF THE EXOSPORIUM ENVELOPING SPORES OF BACILLUS CEREUS. J Bacteriol. 1964 Dec;88:1774–1789. doi: 10.1128/jb.88.6.1774-1789.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt P., Beaman T. C., Corner T. R., Greenamyre J. T., Tisa L. S. Photometric immersion refractometry of bacterial spores. J Bacteriol. 1982 May;150(2):643–648. doi: 10.1128/jb.150.2.643-648.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt P. Cytology of Bacillus anthracis. Fed Proc. 1967 Sep;26(5):1504–1517. [PubMed] [Google Scholar]
- Gerhardt P., Pankratz H. S., Scherrer R. Fine structure of the Baccilus thuringiensis spore. Appl Environ Microbiol. 1976 Sep;32(3):438–440. doi: 10.1128/aem.32.3.438-440.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun H. H., Zeikus J. G., Longin R., Millet J., Ryter A. Ultrastructure and extreme heat resistance of spores from thermophilic Clostridium species. J Bacteriol. 1983 Dec;156(3):1332–1337. doi: 10.1128/jb.156.3.1332-1337.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANSSEN F. W., LUND A. J., ANDERSON L. E. Colorimetric assay for dipicolinic acid in bacterial spores. Science. 1958 Jan 3;127(3288):26–27. doi: 10.1126/science.127.3288.26. [DOI] [PubMed] [Google Scholar]
- Kawasaki C., Nishihara T., Kondo M. Ultrastructure and its relation to the fractions isolated from spore coat of Bacillus megaterium. J Bacteriol. 1969 Feb;97(2):944–946. doi: 10.1128/jb.97.2.944-946.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbe R. G., Reich R. R., Duncan C. L. Alteration in ultrastructure and germination of Clostridium perfringens type A spores following extraction of spore coats. Can J Microbiol. 1978 Dec;24(12):1526–1536. doi: 10.1139/m78-244. [DOI] [PubMed] [Google Scholar]
- Matz L. L., Beaman T. C., Gerhardt P. Chemical composition of exosporium from spores of Bacillus cereus. J Bacteriol. 1970 Jan;101(1):196–201. doi: 10.1128/jb.101.1.196-201.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberly B. J., Shafa F., Gerhardt P. Structural details of anthrax spores during stages of transformation into vegetative cells. J Bacteriol. 1966 Jul;92(1):220–228. doi: 10.1128/jb.92.1.220-228.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODE L. J., LEWIS C. W., Jr, FOSTER J. W. Electron microscopy of spores of Bacillus megaterium with special reference to the effects of fixation and thin sectioning. J Cell Biol. 1962 Jun;13:423–435. doi: 10.1083/jcb.13.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode L. J. Correlation between spore structure and spore properties in Bacillus megaterium. J Bacteriol. 1968 Jun;95(6):1979–1986. doi: 10.1128/jb.95.6.1979-1986.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XVII. Sulfhydryl and disulfide levels in dormancy and germination. J Bacteriol. 1969 Dec;100(3):1155–1160. doi: 10.1128/jb.100.3.1155-1160.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay L. K., Vary J. C. Biochemical studies on glucose initiated germination in Bacillus megaterium. Biochim Biophys Acta. 1978 Jan 18;538(2):284–292. doi: 10.1016/0304-4165(78)90356-2. [DOI] [PubMed] [Google Scholar]
- Stelma G. N., Jr, Aronson A. I., Fitz-James P. C. A Bacillus cereus mutant defective in spore coat deposition. J Gen Microbiol. 1980 Jan;116(1):173–185. doi: 10.1099/00221287-116-1-173. [DOI] [PubMed] [Google Scholar]
- THOMAS R. S. ULTRASTRUCTURAL LOCALIZATION OF MINERAL MATTER IN BACTERIAL SPORES BY MICRONINCINERATION. J Cell Biol. 1964 Oct;23:113–133. doi: 10.1083/jcb.23.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vary J. C. Germination of Bacillus megaterium spores after various extraction procedures. J Bacteriol. 1973 Nov;116(2):797–802. doi: 10.1128/jb.116.2.797-802.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vary J. C. Spore germination of Bacillus megaterium QM B1551 mutants. J Bacteriol. 1972 Oct;112(1):640–642. doi: 10.1128/jb.112.1.640-642.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]