Abstract
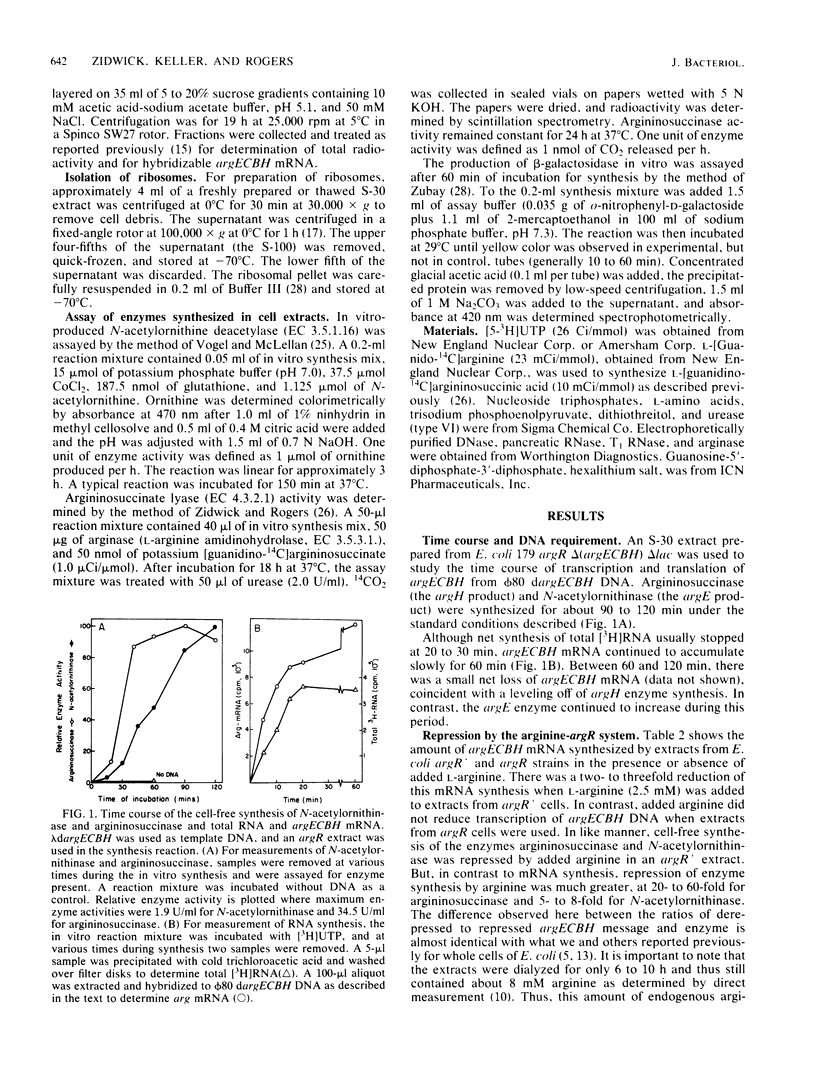
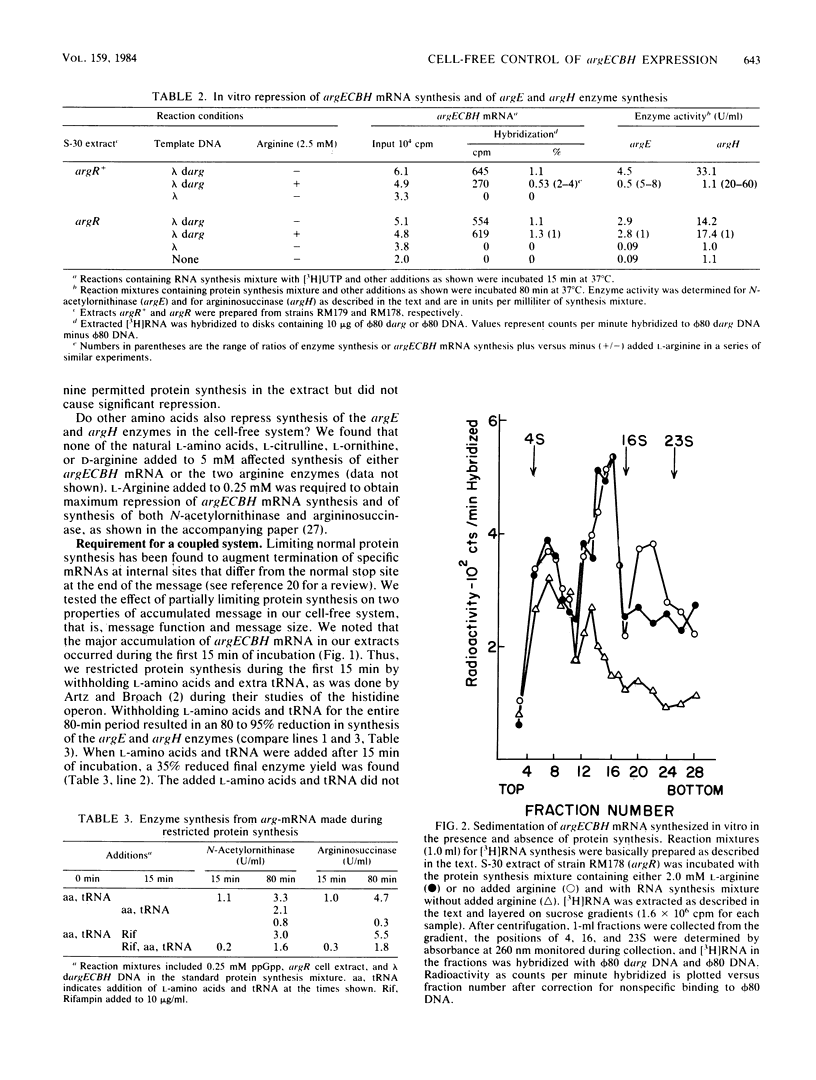
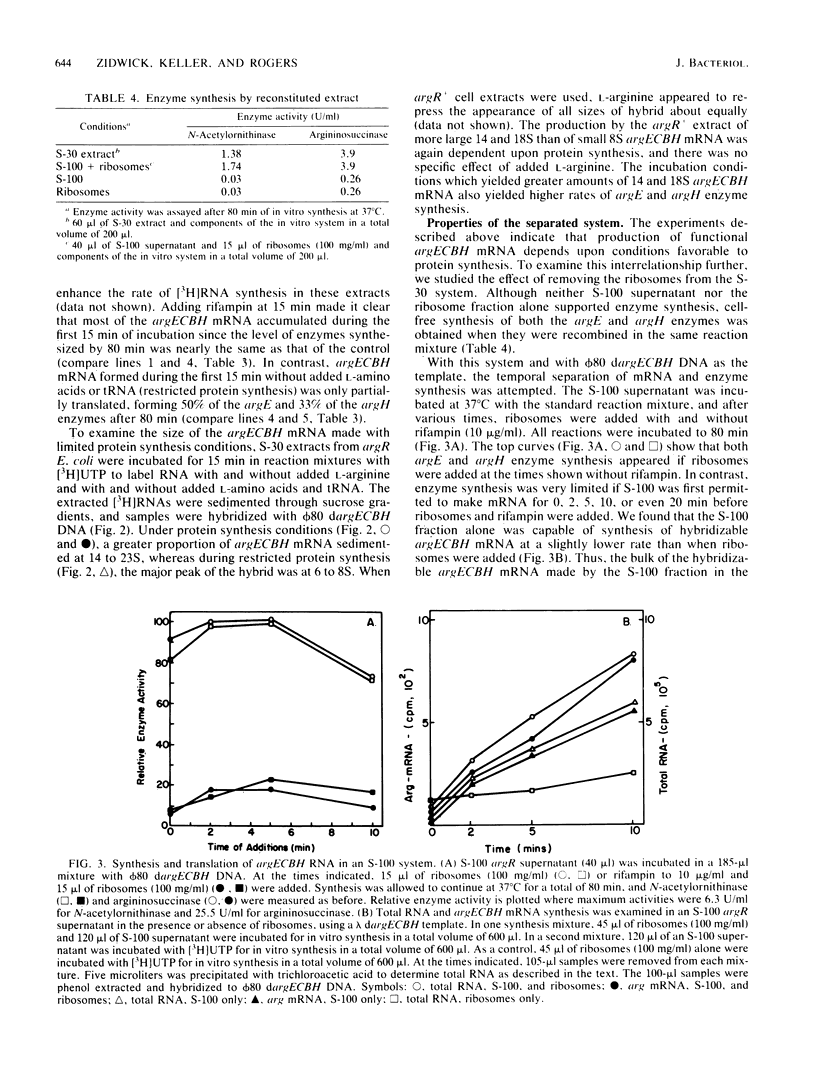
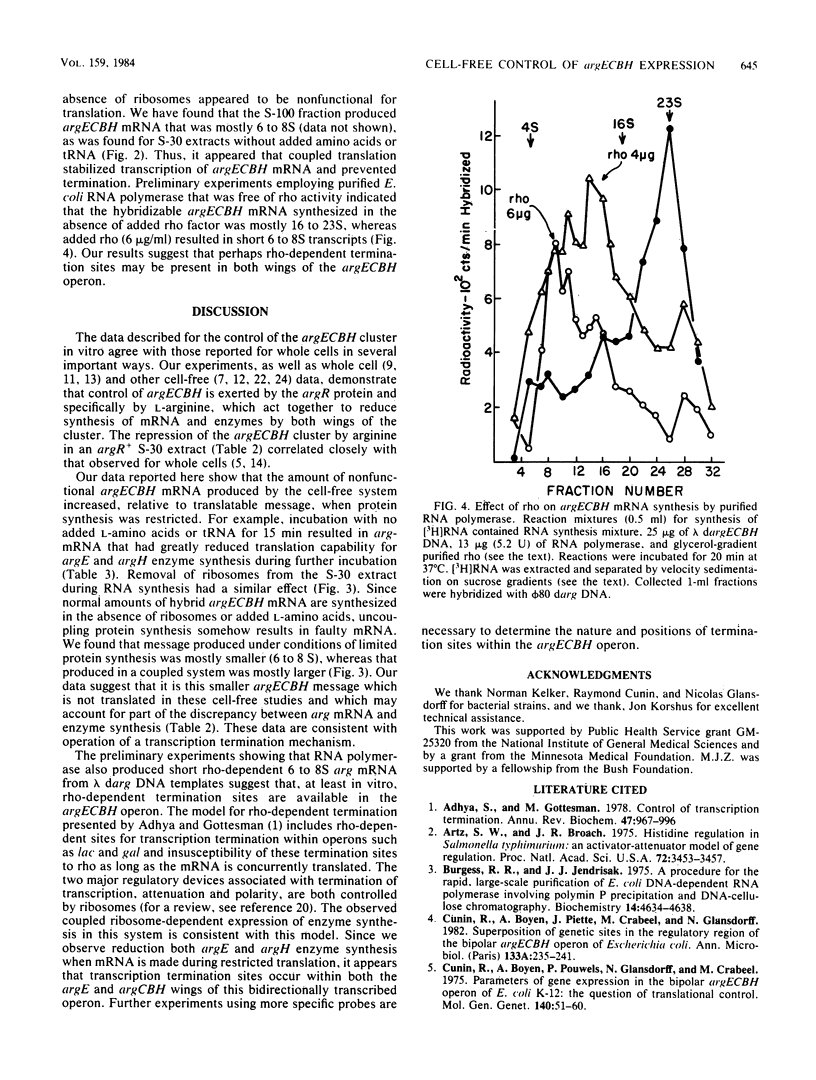
Cell extracts from Escherichia coli were used to study both transcription and coupled translation of the argECBH gene cluster. Argininosuccinase (the argH enzyme) and N-acetylornithinase (the argE enzyme) were synthesized for 90 to 120 min, and hybridizable argECBH mRNA was synthesized for 60 min after the addition of a lambda or phi 80 dargECBH DNA template. L-Arginine (2.5 mM) repressed synthesis by argR+ extracts of argECBH mRNA 2-, to 3-fold, argE enzyme 5- to 8-fold, and argH enzyme 20- to 60-fold. Repression was specific for L-arginine, and argR extracts were insensitive to added L-arginine. The argECBH mRNA made under conditions of restricted protein synthesis had reduced ability to function in the formation of the argE and argH enzymes and was found to be predominantly 6 to 8S in sucrose density gradients. When protein synthesis was allowed, the mRNA formed was functional, and large amounts of 14 to 23S argECBH mRNA appeared on sucrose gradients. An S-100 supernatant freed of ribosomes was capable of producing hybridizable arg mRNA, but significant functional message was only produced when ribosomes were present. When purified RNA polymerase was used, the formation of short 6 to 8S argECBH mRNA was dependent upon added rho protein. The data suggest that rho-dependent sites in the argECBH operon allow early termination of mRNA synthesis when transcription is not coupled to active enzyme synthesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Gottesman M. Control of transcription termination. Annu Rev Biochem. 1978;47:967–996. doi: 10.1146/annurev.bi.47.070178.004535. [DOI] [PubMed] [Google Scholar]
- Artz S. W., Broach J. R. Histidine regulation in Salmonella typhimurium: an activator attenuator model of gene regulation. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3453–3457. doi: 10.1073/pnas.72.9.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Cunin R., Boyen A., Piette J., Crabeel M., Glansdorff N. Superposition of genetic sites in the regulatory region of the bipolar argECBH operon of Escherichia coli. Ann Microbiol (Paris) 1982 Mar-Apr;133(2):235–241. [PubMed] [Google Scholar]
- Cunin R., Boyen A., Pouwels P., Glansdorff N., Crabeel M. Parameters of gene expression in the bipolar argECBH operon of E. coli K12. The question of translational control. Mol Gen Genet. 1975 Sep 15;140(1):51–60. doi: 10.1007/BF00268988. [DOI] [PubMed] [Google Scholar]
- Cunin R., Kelker N., Boyen A., Yang H., Zubay G., Glansdorff N., Maas W. K. Involvement of arginine in in vitro repression of transcription of arginine genes C, B and H in Escherichia coli K 12. Biochem Biophys Res Commun. 1976 Mar 22;69(2):377–382. doi: 10.1016/0006-291x(76)90532-5. [DOI] [PubMed] [Google Scholar]
- Cunin Raymond, Glansdorff Nicolas. Messenger RNA from arginine and phosphoenolpyruvate carboxylase genes in arg R+ and arg R(-) strains of E. coli K-12. FEBS Lett. 1971 Oct 15;18(1):135–137. doi: 10.1016/0014-5793(71)80428-3. [DOI] [PubMed] [Google Scholar]
- Darlix J. L. Stimultaneous purification of Escherichia coli termination factor rho, RNAase III and RNAase H. Eur J Biochem. 1975 Feb 21;51(2):369–376. doi: 10.1111/j.1432-1033.1975.tb03937.x. [DOI] [PubMed] [Google Scholar]
- Elseviers D., Cunin R., Glansdorff N. Control regions within the argECBH gene cluster of Escherichia coli K12. Mol Gen Genet. 1972;117(4):349–366. doi: 10.1007/BF00333028. [DOI] [PubMed] [Google Scholar]
- IZUMI Y. NEW SAKAGUCHI REACTION. Anal Biochem. 1965 Feb;10:218–226. doi: 10.1016/0003-2697(65)90262-9. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A. Control of the argECBH cluster in Escherichia coli. Mol Gen Genet. 1972;117(4):337–348. doi: 10.1007/BF00333027. [DOI] [PubMed] [Google Scholar]
- Kelker N. E., Maas W. K., Yang H. L., Zubay G. In vitro synthesis and repression of argininosuccinase in Escherichia coli K12; partial purification of the arginine repressor. Mol Gen Genet. 1976 Feb 27;144(1):17–20. doi: 10.1007/BF00277298. [DOI] [PubMed] [Google Scholar]
- Kryzek R. A., Rogers P. Dual regulation by arginine of the expression of the Escherichia coli argECBH operon. J Bacteriol. 1976 Apr;126(1):348–364. doi: 10.1128/jb.126.1.348-364.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyzek R. A., Rogers P. Effect of arginine on the stability and size of argECBH messenger ribonucleic acid in Escherichia coli. J Bacteriol. 1976 Apr;126(1):365–376. doi: 10.1128/jb.126.1.365-376.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyzek R., Rogers P. Arginine control of transcription of argECBH messenger ribonucleic acid in Escherichia coli. J Bacteriol. 1972 Jun;110(3):945–954. doi: 10.1128/jb.110.3.945-954.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery-Goldhammer C., Richardson J. P. An RNA-dependent nucleoside triphosphate phosphohydrolase (ATPase) associated with rho termination factor. Proc Natl Acad Sci U S A. 1974 May;71(5):2003–2007. doi: 10.1073/pnas.71.5.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannekoek H., Cunin R., Boyen A., Glansdorff N. In vitro transcription of the bipolar arginine ECBH operon of Escherichia coli K 12. FEBS Lett. 1975 Mar 1;51(1):143–145. doi: 10.1016/0014-5793(75)80872-6. [DOI] [PubMed] [Google Scholar]
- Piette J., Cunin R., Boyen A., Charlier D., Crabeel M., Van Vliet F., Glansdorff N., Squires C., Squires C. L. The regulatory region of the divergent argECBH operon in Escherichia coli K-12. Nucleic Acids Res. 1982 Dec 20;10(24):8031–8048. doi: 10.1093/nar/10.24.8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T. Termination of transcription and its regulation in the tryptophan operon of E. coli. Cell. 1981 Apr;24(1):10–23. doi: 10.1016/0092-8674(81)90496-7. [DOI] [PubMed] [Google Scholar]
- Pouwels P. H., Cunin R., Glansdorff N. Letter: Divergent transcription in the argECBH cluster of genes in Escherichia coli K12. J Mol Biol. 1974 Mar;83(3):421–424. doi: 10.1016/0022-2836(74)90288-5. [DOI] [PubMed] [Google Scholar]
- Rogers P., Kaden T. M., Toth M. Repression of Arg mRNA synthesis by L-arginine in cell-free extracts of Escherichia coli. Biochem Biophys Res Commun. 1975 Aug 18;65(4):1284–1291. doi: 10.1016/s0006-291x(75)80369-x. [DOI] [PubMed] [Google Scholar]
- Rogers P., Krzyzek R., Kaden T. M., Arfman E. Effect of arginine and canavanine on arginine messenger RNA synthesis. Biochem Biophys Res Commun. 1971 Sep;44(5):1220–1226. doi: 10.1016/s0006-291x(71)80216-4. [DOI] [PubMed] [Google Scholar]
- Urm E., Yang H., Zubay G., Kelker N., Maas W. In vitro repression of n- -acetyl-L-ornithinase synthesis in Escherichia coli. Mol Gen Genet. 1973;121(1):1–7. doi: 10.1007/BF00353688. [DOI] [PubMed] [Google Scholar]
- Zidwick M. J., Korshus J., Rogers P. Positive control of expression of the argECBH gene cluster in vitro by guanosine 5'-diphosphate 3'-diphosphate. J Bacteriol. 1984 Aug;159(2):647–651. doi: 10.1128/jb.159.2.647-651.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidwick M. J., Rogers P. The determination by radiochemical assay of argininosuccinase produced in an Escherichia coli system in vitro. Biochem J. 1982 Dec 1;207(3):529–533. doi: 10.1042/bj2070529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubay G. In vitro synthesis of protein in microbial systems. Annu Rev Genet. 1973;7:267–287. doi: 10.1146/annurev.ge.07.120173.001411. [DOI] [PubMed] [Google Scholar]


