Abstract
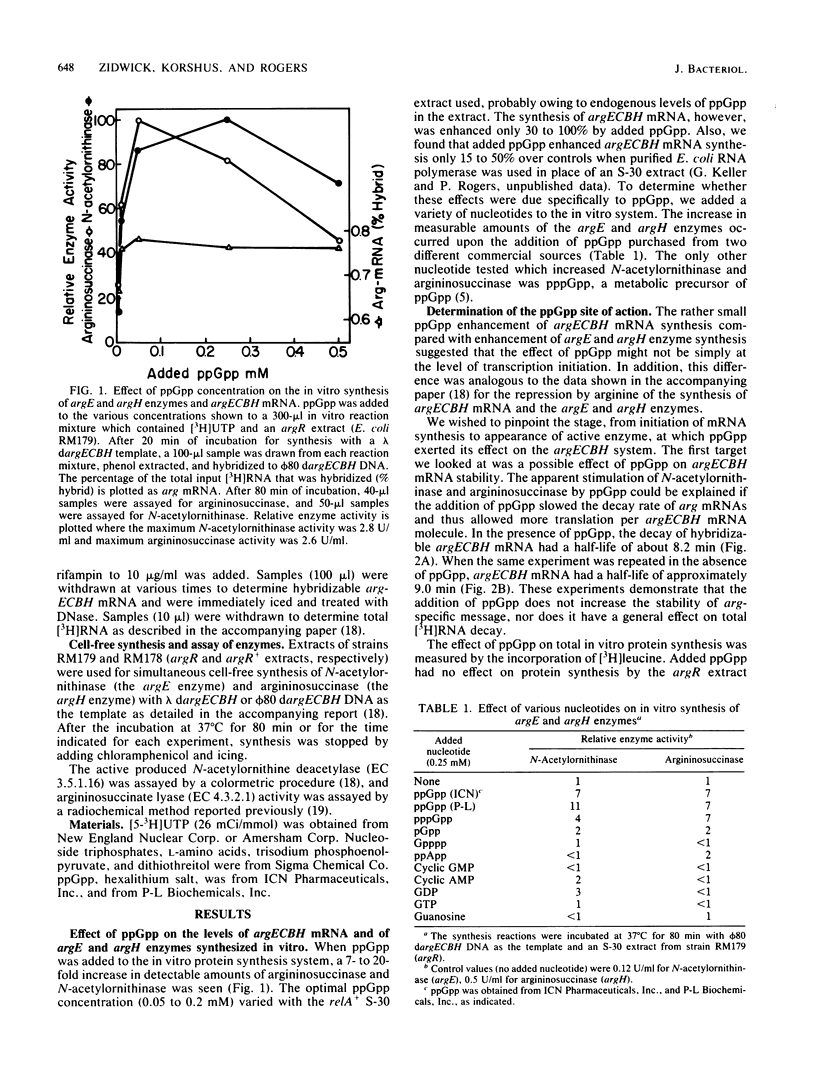
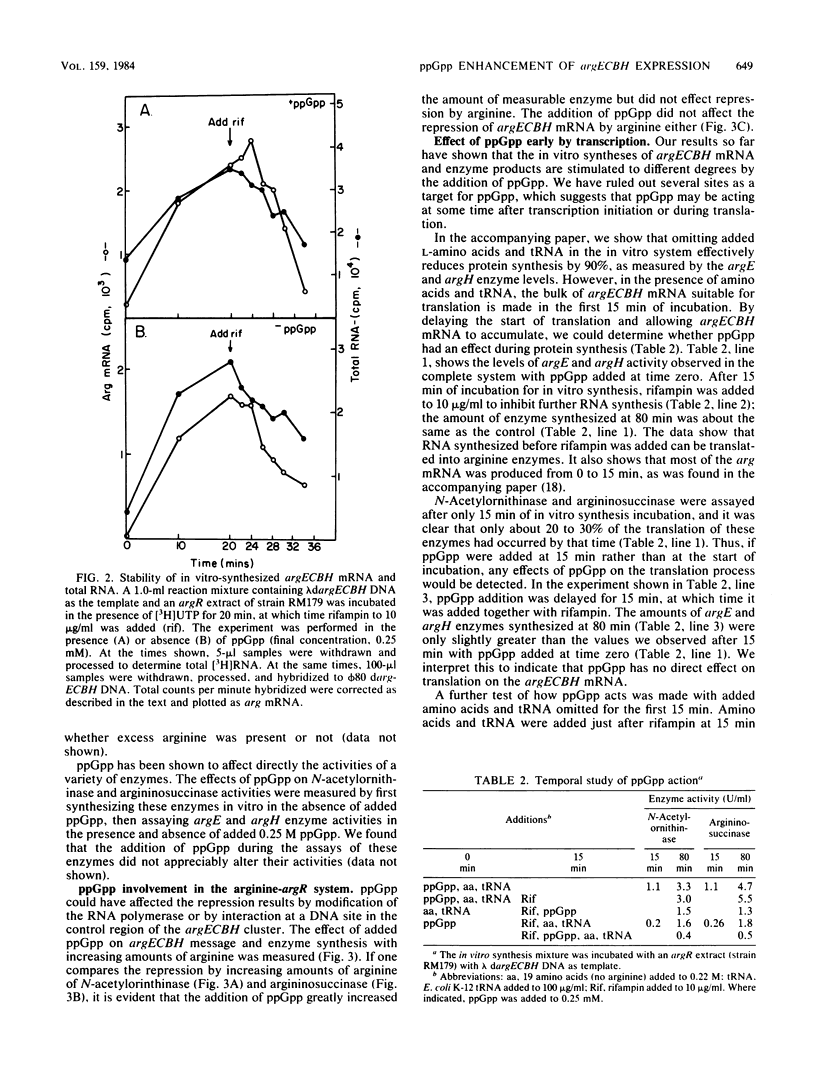
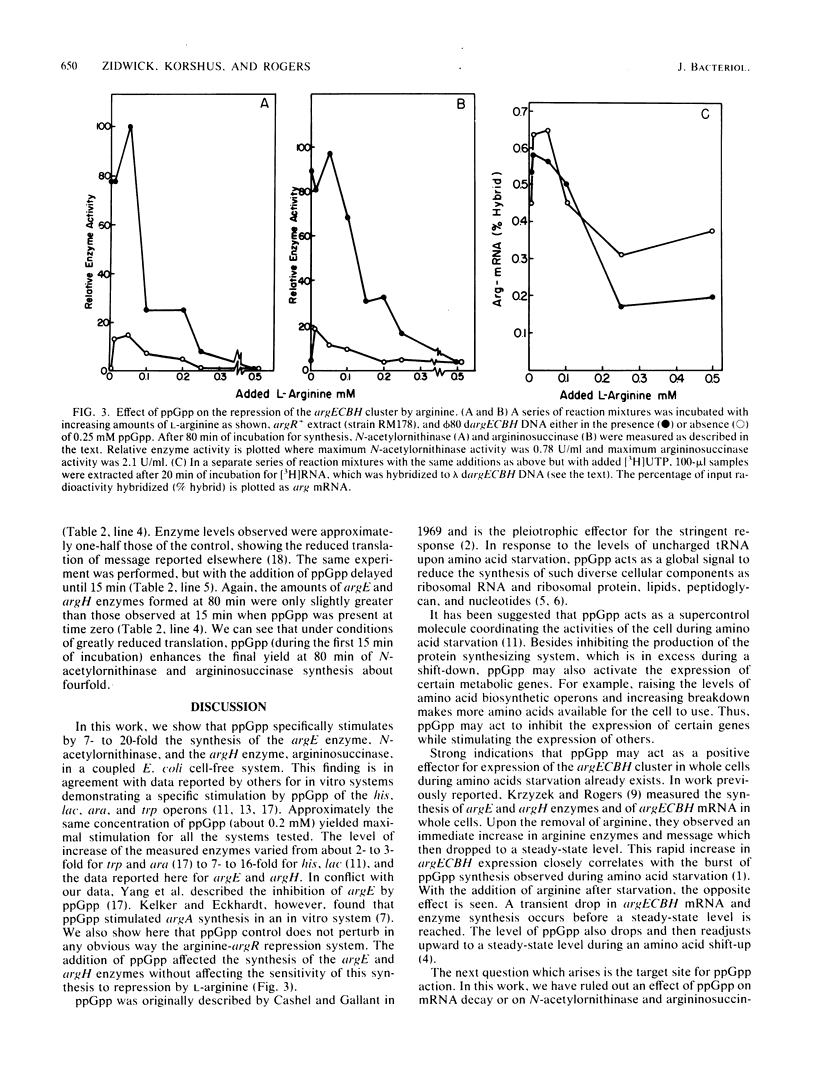
By using a cell-free system derived from Escherichia coli, it was found that guanosine 5'-diphosphate 3'-diphosphate (ppGpp) was a positive effector for expression of both wings of bidirectionally transcribed argECBH gene cluster. A 7- to 20-fold increase in the synthesis of both argininosuccinase (the argH enzyme) and N-acetylornithinase (the argE enzyme) resulted with added ppGpp (0.2 mM optimum). Synthesis of hybridizable argECBH mRNA was enhanced only 30 to 100% by added ppGpp. Of the various guanosine nucleotides tested, only pppGpp mimicked ppGpp. Added ppGpp had no important effect upon (i) measurable argE or argH enzyme activity, (ii) total protein synthesis in the cell-free system, or (iii) the rate of decay of hybridizable argECBH mRNA. With extracts of an argR+ strain, added ppGpp had no effect on the repression of enzyme or mRNA synthesis by L-arginine. By using a two-stage system in which the bulk of argECBH mRNA was synthesized while protein synthesis was delayed, we showed that ppGpp acted at some point during transcription.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cashel M., Gallant J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature. 1969 Mar 1;221(5183):838–841. doi: 10.1038/221838a0. [DOI] [PubMed] [Google Scholar]
- Cashel M. The control of ribonucleic acid synthesis in Escherichia coli. IV. Relevance of unusual phosphorylated compounds from amino acid-starved stringent strains. J Biol Chem. 1969 Jun 25;244(12):3133–3141. [PubMed] [Google Scholar]
- Elseviers D., Cunin R., Glansdorff N. Control regions within the argECBH gene cluster of Escherichia coli K12. Mol Gen Genet. 1972;117(4):349–366. doi: 10.1007/BF00333028. [DOI] [PubMed] [Google Scholar]
- Friesen J. D., Fiil N. P., von Meyenburg K. Synthesis and turnover of basal level guanosine tetraphosphate in Escherichia coli. J Biol Chem. 1975 Jan 10;250(1):304–309. [PubMed] [Google Scholar]
- Gallant J. A. Stringent control in E. coli. Annu Rev Genet. 1979;13:393–415. doi: 10.1146/annurev.ge.13.120179.002141. [DOI] [PubMed] [Google Scholar]
- Ishiguro E. E., Ramey W. D. Inhibition of in vitro peptidoglycan biosynthesis in Escherichia coli by guanosine 5'-diphosphate 3'-diphosphate. Can J Microbiol. 1980 Dec;26(12):1514–1518. doi: 10.1139/m80-253. [DOI] [PubMed] [Google Scholar]
- Kelker N., Eckhardt T. Regulation of argA operon expression in Escherichia coli K-12: cell-free synthesis of beta-galactosidase under argA control. J Bacteriol. 1977 Oct;132(1):67–72. doi: 10.1128/jb.132.1.67-72.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston R. E., Chamberlin M. J. Pausing and attenuation of in vitro transcription in the rrnB operon of E. coli. Cell. 1981 Dec;27(3 Pt 2):523–531. doi: 10.1016/0092-8674(81)90394-9. [DOI] [PubMed] [Google Scholar]
- Kryzek R. A., Rogers P. Dual regulation by arginine of the expression of the Escherichia coli argECBH operon. J Bacteriol. 1976 Apr;126(1):348–364. doi: 10.1128/jb.126.1.348-364.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyzek R. A., Rogers P. Effect of arginine on the stability and size of argECBH messenger ribonucleic acid in Escherichia coli. J Bacteriol. 1976 Apr;126(1):365–376. doi: 10.1128/jb.126.1.365-376.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primakoff P., Artz S. W. Positive control of lac operon expression in vitro by guanosine 5'-diphosphate 3'-diphosphate. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1726–1730. doi: 10.1073/pnas.76.4.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers P., Krzyzek R., Kaden T. M., Arfman E. Effect of arginine and canavanine on arginine messenger RNA synthesis. Biochem Biophys Res Commun. 1971 Sep;44(5):1220–1226. doi: 10.1016/s0006-291x(71)80216-4. [DOI] [PubMed] [Google Scholar]
- Stephens J. C., Artz S. W., Ames B. N. Guanosine 5'-diphosphate 3'-diphosphate (ppGpp): positive effector for histidine operon transcription and general signal for amino-acid deficiency. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4389–4393. doi: 10.1073/pnas.72.11.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers A. Modulation of RNA polymerase specificity by ppGpp. Mol Gen Genet. 1976 Aug 19;147(2):225–232. doi: 10.1007/BF00267575. [DOI] [PubMed] [Google Scholar]
- Wagner E. G., Ehrenberg M., Kurland C. G. Kinetic suppression of translational errors by (p)ppGpp. Mol Gen Genet. 1982;185(2):269–274. doi: 10.1007/BF00330797. [DOI] [PubMed] [Google Scholar]
- Winkler M. E., Yanofsky C. Pausing of RNA polymerase during in vitro transcription of the tryptophan operon leader region. Biochemistry. 1981 Jun 23;20(13):3738–3744. doi: 10.1021/bi00516a011. [DOI] [PubMed] [Google Scholar]
- Yang H. L., Zubay G., Urm E., Heiness G., Cashel M. Effects of guanosine tetraphosphate, guanosine pentaphosphate, and beta-gamma methylenyl-guanosine pentaphosphate on gene expression of Escherichia coli in vitro. Proc Natl Acad Sci U S A. 1974 Jan;71(1):63–67. doi: 10.1073/pnas.71.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidwick M. J., Keller G., Rogers P. Regulation and coupling of argECBH mRNA and enzyme synthesis in cell extracts of Escherichia coli. J Bacteriol. 1984 Aug;159(2):640–646. doi: 10.1128/jb.159.2.640-646.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidwick M. J., Rogers P. The determination by radiochemical assay of argininosuccinase produced in an Escherichia coli system in vitro. Biochem J. 1982 Dec 1;207(3):529–533. doi: 10.1042/bj2070529. [DOI] [PMC free article] [PubMed] [Google Scholar]


