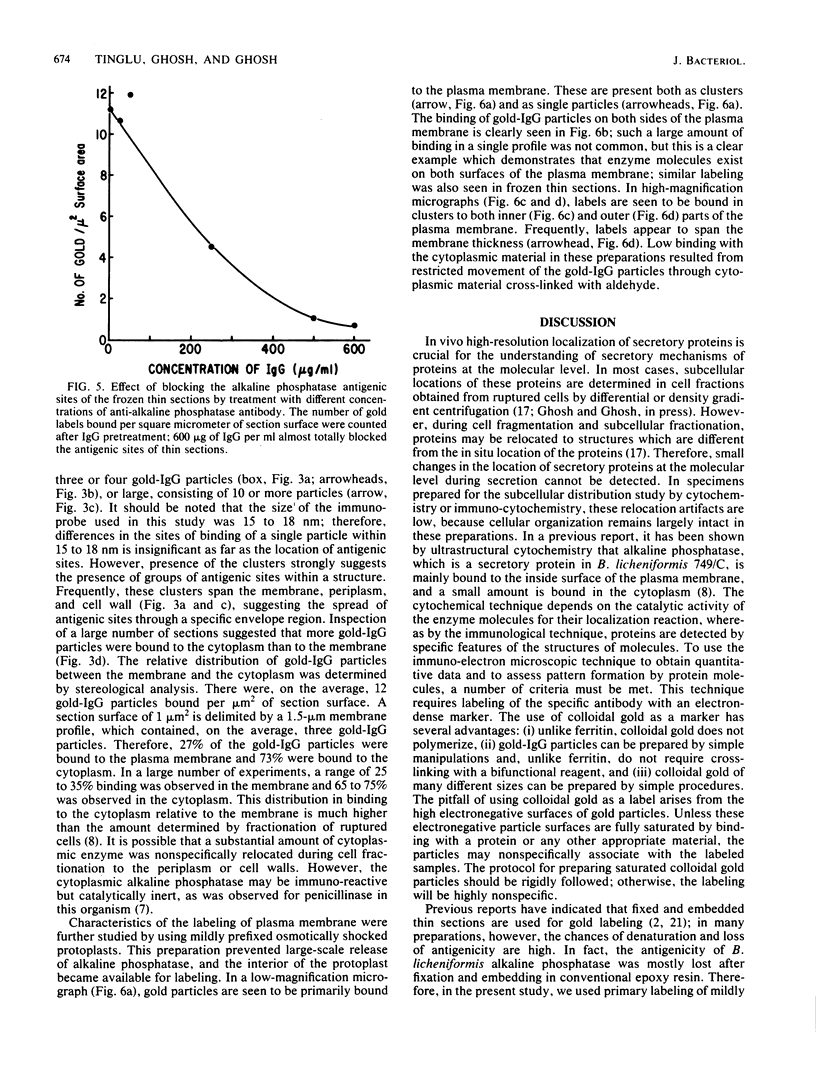
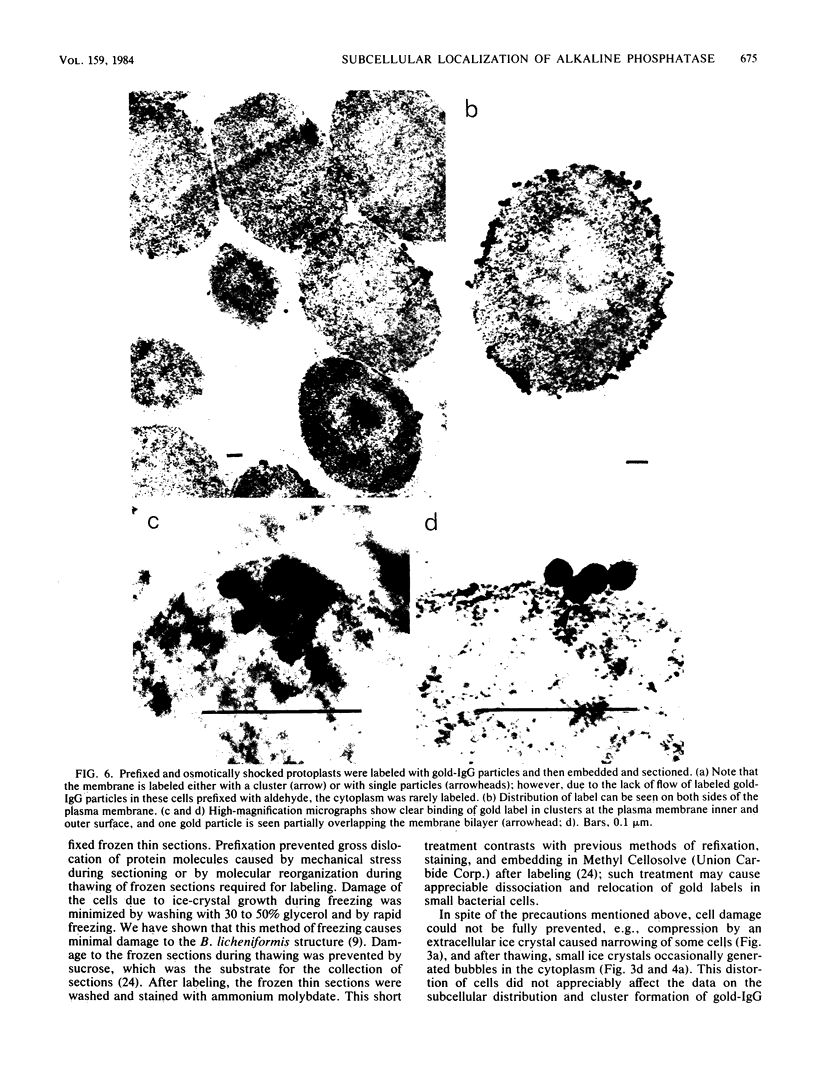
Abstract
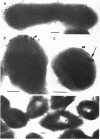
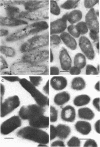
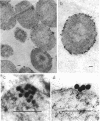
Subcellular distribution of the alkaline phosphatase of Bacillus licheniformis 749/C was determined by an immunoelectron microscopy method. Anti-alkaline phosphatase antibody labeled with 15- to 18-nm colloidal gold particles (gold-immunoglobulin G [IgG] complex) were used for the study. Both the plasma membrane and cytoplasmic material were labeled with the gold-IgG particles. These particles formed clusters in association with the plasma membrane; in contrast, in the cytoplasm the particles were largely dispersed, and only a few clusters were found. The gold-IgG binding was quantitatively estimated by stereological analysis of labeled, frozen thin sections. This estimation of a variety of control samples showed that the labeling was specific for the alkaline phosphatase. Cluster formation of the gold-IgG particles in association with the plasma membrane suggests that existence of specific alkaline phosphatase binding sites (receptors) in the plasma membrane of B. licheniformis 749/C.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer M. E. Structural and functional evidence of cooperativity between membranes and cell wall in bacteria. Int Rev Cytol Suppl. 1981;12:39–70. doi: 10.1016/b978-0-12-364373-5.50012-3. [DOI] [PubMed] [Google Scholar]
- Bendayan M., Zollinger M. Ultrastructural localization of antigenic sites on osmium-fixed tissues applying the protein A-gold technique. J Histochem Cytochem. 1983 Jan;31(1):101–109. doi: 10.1177/31.1.6187796. [DOI] [PubMed] [Google Scholar]
- Christensen A. K. Frozen thin sections of fresh tissue for electron microscopy, with a description of pancreas and liver. J Cell Biol. 1971 Dec;51(3):772–804. doi: 10.1083/jcb.51.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A., Ghosh B. K. Changes in the membrane bound alkaline phosphatase of glucose and lactate grown vegetative cells of Bacillus subtilis SB15. Biochem Biophys Res Commun. 1972 Nov 15;49(4):906–915. doi: 10.1016/0006-291x(72)90298-7. [DOI] [PubMed] [Google Scholar]
- Ghosh A., Ghosh B. K. Immunoelectron microscopic localization of penicillinase in Bacillus licheniformis. J Bacteriol. 1979 Mar;137(3):1374–1385. doi: 10.1128/jb.137.3.1374-1385.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A., Vallespir S., Ghosh B. K. Specificity of subcellular distribution of alkaline phosphatase in Bacillus licheniformis 749/C. Can J Microbiol. 1984 Jan;30(1):113–125. doi: 10.1139/m84-019. [DOI] [PubMed] [Google Scholar]
- Ghosh B. K., Nanninga N. Polymorphism of the mesosome in Bacillus licheniformis (749/C and 749). Influence of chemical fixation monitored by freeze-etching. J Ultrastruct Res. 1976 Jul;56(1):107–120. doi: 10.1016/s0022-5320(76)80144-x. [DOI] [PubMed] [Google Scholar]
- Ghosh B. K., Wouters J. T., Lampen J. O. Distribution of the sites of alkaline phosphatase(s) activity in vegetative cells of Bacillus subtilis. J Bacteriol. 1971 Nov;108(2):928–937. doi: 10.1128/jb.108.2.928-937.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R., Ghosh A., Ghosh B. K. Properties of the membrane-bound alkaline phosphatase from glucose- and lactate-grown cells of Bacillus subtilis SB 15. J Biol Chem. 1977 Oct 10;252(19):6813–6822. [PubMed] [Google Scholar]
- Handley D. A., Arbeeny C. M., Witte L. D., Chien S. Colloidal gold--low density lipoprotein conjugates as membrane receptor probes. Proc Natl Acad Sci U S A. 1981 Jan;78(1):368–371. doi: 10.1073/pnas.78.1.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisberger M., Rosset J., Bauer H. Colloidal gold granules as markers for cell surface receptors in the scanning electron microscope. Experientia. 1975 Oct 15;31(10):1147–1149. doi: 10.1007/BF02326761. [DOI] [PubMed] [Google Scholar]
- Horisberger M., Vonlanthen M. Multiple marking of cell surface receptors by gold granules: simultaneous localization of three lectin receptors on human erythrocytes. J Microsc. 1979 Jan;115(1):97–102. doi: 10.1111/j.1365-2818.1979.tb00155.x. [DOI] [PubMed] [Google Scholar]
- Kumar R., Ghosh A., Ghosh B. K. Alkaline phosphatase secretion-negative mutant of Bacillus licheniformis 749/C. J Bacteriol. 1983 May;154(2):946–954. doi: 10.1128/jb.154.2.946-954.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Michaelis S., Beckwith J. Mechanism of incorporation of cell envelope proteins in Escherichia coli. Annu Rev Microbiol. 1982;36:435–465. doi: 10.1146/annurev.mi.36.100182.002251. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Orci L. Protein A-gold complex for postembedding staining of intracellular antigens. Acta Histochem Suppl. 1980;22:269–273. [PubMed] [Google Scholar]
- Roth J., Binder M. Coloidal gold, ferritin and peroxidase as markers for electron microscopic double labeling lectin techniques. J Histochem Cytochem. 1978 Mar;26(3):163–169. doi: 10.1177/26.3.632554. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T., Singer S. J. Improved procedures for immunoferritin labeling of ultrathin frozen sections. J Cell Biol. 1976 Dec;71(3):894–906. doi: 10.1083/jcb.71.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Ibrahimi I., Blobel G. Translocation of proteins across the endoplasmic reticulum. I. Signal recognition protein (SRP) binds to in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):545–550. doi: 10.1083/jcb.91.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel E. R. Stereological principles for morphometry in electron microscopic cytology. Int Rev Cytol. 1969;26:235–302. doi: 10.1016/s0074-7696(08)61637-x. [DOI] [PubMed] [Google Scholar]
- de Bruijn W. C., Emeis J. J., Vermeer B. J. The application of analytical electron microscopy in the localization of individual LDL-binding sites on cell surfaces. Artery. 1980;8(3):281–287. [PubMed] [Google Scholar]