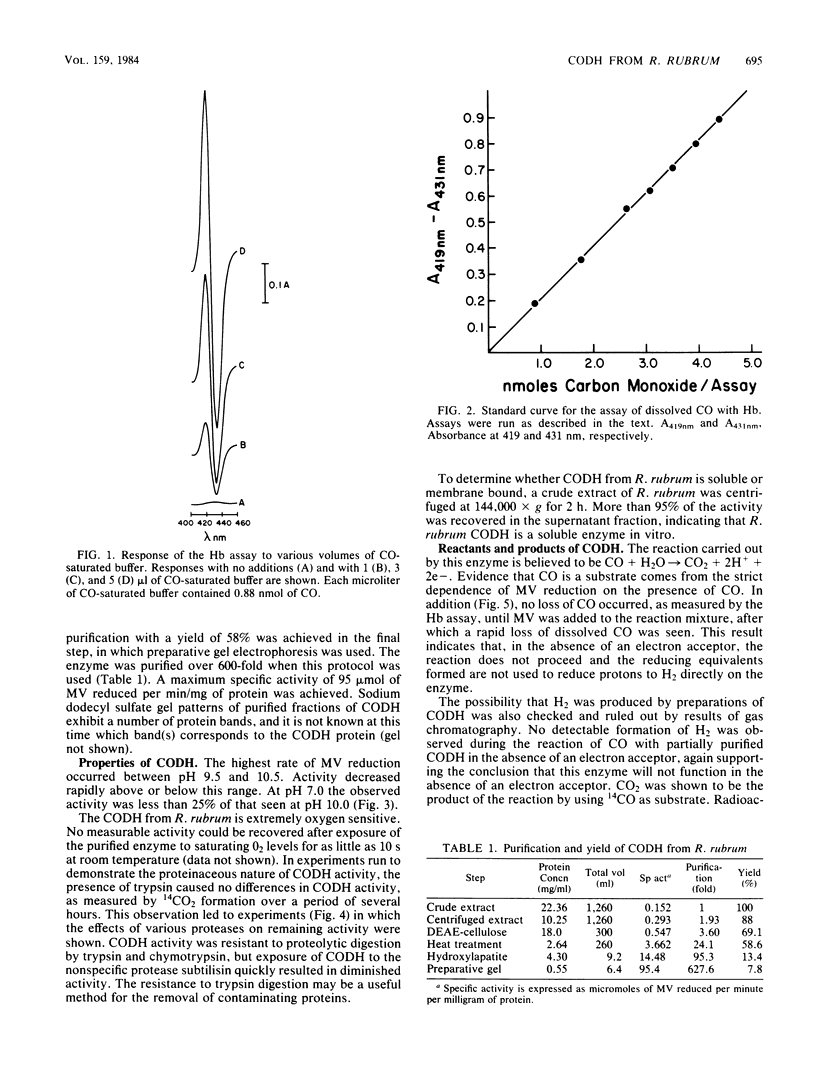
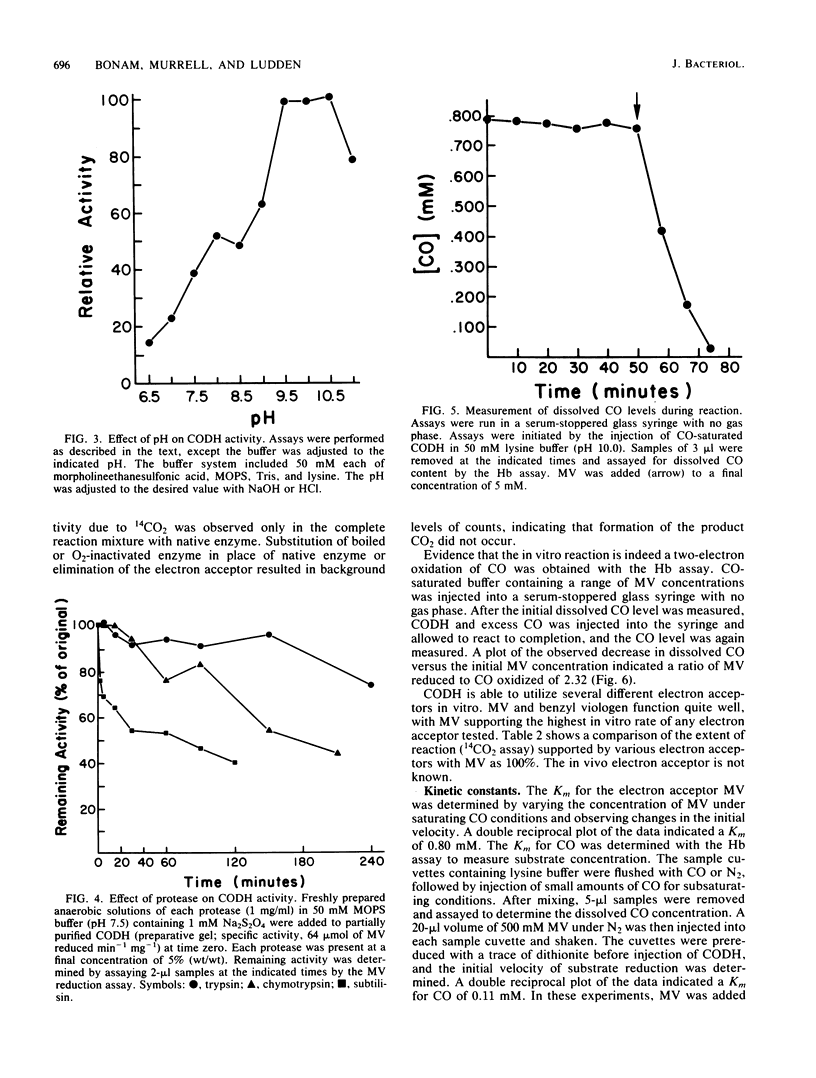
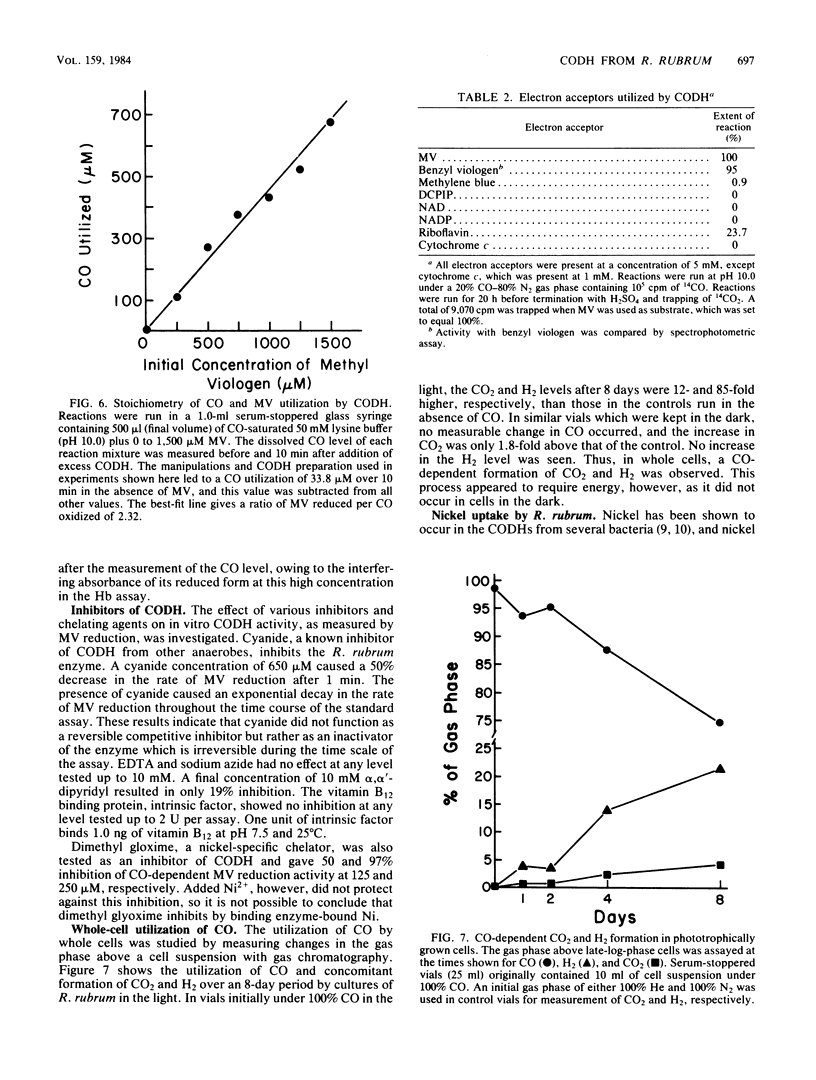
Abstract
The carbon monoxide dehydrogenase from the photosynthetic bacterium Rhodospirillum rubrum was purified over 600-fold by DEAE-cellulose chromatography, heat treatment, hydroxylapatite chromatography, and preparative scale gel electrophoresis. In vitro, this enzyme catalyzed a two-electron oxidation of CO to form CO2 as the product. The reaction was dependent on the addition of an electron acceptor. The enzyme was oxygen labile, heat stable, and resistant to tryptic and chymotryptic digestion. Optimum in vitro activity occurred at pH 10.0. A sensitive, hemoglobin-based assay for measuring dissolved CO levels is presented. The in vitro Km for CO was determined to be 110 microM. CO, through an unknown mechanism, stimulated hydrogen evolution in whole cells, suggesting the presence of a reversible hydrogenase in R. rubrum which is CO insensitive in vivo.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. W., Hall D. O. Isolation of the membrane-bound hydrogenase from Rhodospirillum rubrum. Biochem Biophys Res Commun. 1977 Jul 25;77(2):730–737. doi: 10.1016/s0006-291x(77)80039-9. [DOI] [PubMed] [Google Scholar]
- Anderson L., Fuller R. C. Photosynthesis in Rhodospirillum rubrum. 3. Metabolic control of reductive pentose phosphate and tricarboxylic acid cycle enzymes. Plant Physiol. 1967 Apr;42(4):497–509. doi: 10.1104/pp.42.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels L., Fuchs G., Thauer R. K., Zeikus J. G. Carbon monoxide oxidation by methanogenic bacteria. J Bacteriol. 1977 Oct;132(1):118–126. doi: 10.1128/jb.132.1.118-126.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekert G. B., Thauer R. K. Carbon monoxide oxidation by Clostridium thermoaceticum and Clostridium formicoaceticum. J Bacteriol. 1978 Nov;136(2):597–606. doi: 10.1128/jb.136.2.597-606.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekert G., Ritter M. Nickel requirement of Acetobacterium woodii. J Bacteriol. 1982 Aug;151(2):1043–1045. doi: 10.1128/jb.151.2.1043-1045.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake H. L., Hu S. I., Wood H. G. Purification of carbon monoxide dehydrogenase, a nickel enzyme from Clostridium thermocaceticum. J Biol Chem. 1980 Aug 10;255(15):7174–7180. [PubMed] [Google Scholar]
- Drake H. L., Hu S. I., Wood H. G. Purification of five components from Clostridium thermoaceticum which catalyze synthesis of acetate from pyruvate and methyltetrahydrofolate. Properties of phosphotransacetylase. J Biol Chem. 1981 Nov 10;256(21):11137–11144. [PubMed] [Google Scholar]
- Drake H. L. Occurrence of nickel in carbon monoxide dehydrogenase from Clostridium pasteurianum and Clostridium thermoaceticum. J Bacteriol. 1982 Feb;149(2):561–566. doi: 10.1128/jb.149.2.561-566.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genthner B. R., Bryant M. P. Growth of Eubacterium limosum with Carbon Monoxide as the Energy Source. Appl Environ Microbiol. 1982 Jan;43(1):70–74. doi: 10.1128/aem.43.1.70-74.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch P. Photosynthetic bacterium growing under carbon monoxide. Nature. 1968 Feb 10;217(5128):555–556. doi: 10.1038/217555a0. [DOI] [PubMed] [Google Scholar]
- Hu S. I., Drake H. L., Wood H. G. Synthesis of acetyl coenzyme A from carbon monoxide, methyltetrahydrofolate, and coenzyme A by enzymes from Clostridium thermoaceticum. J Bacteriol. 1982 Feb;149(2):440–448. doi: 10.1128/jb.149.2.440-448.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. M., Hegeman G. D. Purification and some properties of carbon monoxide dehydrogenase from Pseudomonas carboxydohydrogena. J Bacteriol. 1981 Dec;148(3):904–911. doi: 10.1128/jb.148.3.904-911.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang W., Wolf H. U., Zander R. A sensitive continuous and discontinuous photometric determination of oxygen, carbon dioxide, and carbon monoxide in gases and fluids. Anal Biochem. 1979 Jan 15;92(2):255–264. doi: 10.1016/0003-2697(79)90656-0. [DOI] [PubMed] [Google Scholar]
- Ludden P. W., Burris R. H. Purification and properties of nitrogenase from Rhodospirillum rubrum, and evidence for phosphate, ribose and an adenine-like unit covalently bound to the iron protein. Biochem J. 1978 Oct 1;175(1):251–259. doi: 10.1042/bj1750251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynd L., Kerby R., Zeikus J. G. Carbon monoxide metabolism of the methylotrophic acidogen Butyribacterium methylotrophicum. J Bacteriol. 1982 Jan;149(1):255–263. doi: 10.1128/jb.149.1.255-263.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer O. Chemical and spectral properties of carbon monoxide: methylene blue oxidoreductase. The molybdenum-containing iron-sulfur flavoprotein from Pseudomonas carboxydovorans. J Biol Chem. 1982 Feb 10;257(3):1333–1341. [PubMed] [Google Scholar]
- Meyer O., Schlegel H. G. Biology of aerobic carbon monoxide-oxidizing bacteria. Annu Rev Microbiol. 1983;37:277–310. doi: 10.1146/annurev.mi.37.100183.001425. [DOI] [PubMed] [Google Scholar]
- Meyer O., Schlegel H. G. Carbon monoxide:methylene blue oxidoreductase from Pseudomonas carboxydovorans. J Bacteriol. 1980 Jan;141(1):74–80. doi: 10.1128/jb.141.1.74-80.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORMEROD J. G., ORMEROD K. S., GEST H. Light-dependent utilization of organic compounds and photoproduction of molecular hydrogen by photosynthetic bacteria; relationships with nitrogen metabolism. Arch Biochem Biophys. 1961 Sep;94:449–463. doi: 10.1016/0003-9861(61)90073-x. [DOI] [PubMed] [Google Scholar]
- Ragsdale S. W., Clark J. E., Ljungdahl L. G., Lundie L. L., Drake H. L. Properties of purified carbon monoxide dehydrogenase from Clostridium thermoaceticum, a nickel, iron-sulfur protein. J Biol Chem. 1983 Feb 25;258(4):2364–2369. [PubMed] [Google Scholar]
- Ragsdale S. W., Ljungdahl L. G., DerVartanian D. V. EPR evidence for nickel-substrate interaction in carbon monoxide dehydrogenase from Clostridium thermoaceticum. Biochem Biophys Res Commun. 1982 Sep 30;108(2):658–663. doi: 10.1016/0006-291x(82)90880-4. [DOI] [PubMed] [Google Scholar]
- Sawicki C. A., Gibson Q. H. A photochemical method for rapid and precise determination of carbon monoxide levels in blood. Anal Biochem. 1979 Apr 15;94(2):440–449. doi: 10.1016/0003-2697(79)90387-7. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Fuchs G., Käufer B., Schnitker U. Carbon-monoxide oxidation in cell-free extracts of Clostridium pasteurianum. Eur J Biochem. 1974 Jun 15;45(2):343–349. doi: 10.1111/j.1432-1033.1974.tb03559.x. [DOI] [PubMed] [Google Scholar]
- Uffen R. L. Anaerobic growth of a Rhodopseudomonas species in the dark with carbon monoxide as sole carbon and energy substrate. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3298–3302. doi: 10.1073/pnas.73.9.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uffen R. L. Metabolism of carbon monoxide by Rhodopseudomonas gelatinosa: cell growth and properties of the oxidation system. J Bacteriol. 1983 Sep;155(3):956–965. doi: 10.1128/jb.155.3.956-965.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakim B. T., Uffen R. L. Membrane association of the carbon monoxide oxidation system in Rhodopseudomonas gelatinosa. J Bacteriol. 1983 Jan;153(1):571–573. doi: 10.1128/jb.153.1.571-573.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall J. D., Weaver P. F., Gest H. Genetic transfer of nitrogenase-hydrogenase activity in Rhodopseudomonas capsulata. Nature. 1975 Dec 18;258(5536):630–631. doi: 10.1038/258630a0. [DOI] [PubMed] [Google Scholar]
- YAGI T. Enzymic oxidation of carbon monoxide. Biochim Biophys Acta. 1958 Oct;30(1):194–195. doi: 10.1016/0006-3002(58)90263-4. [DOI] [PubMed] [Google Scholar]



