Abstract
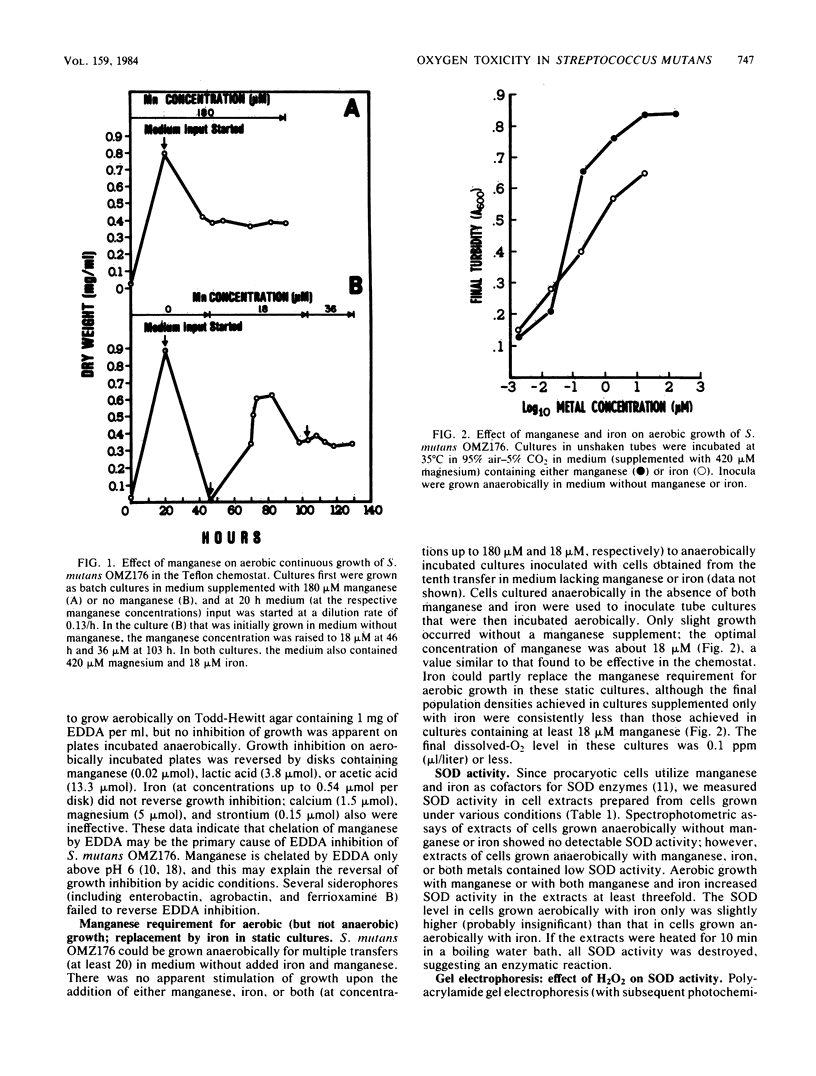
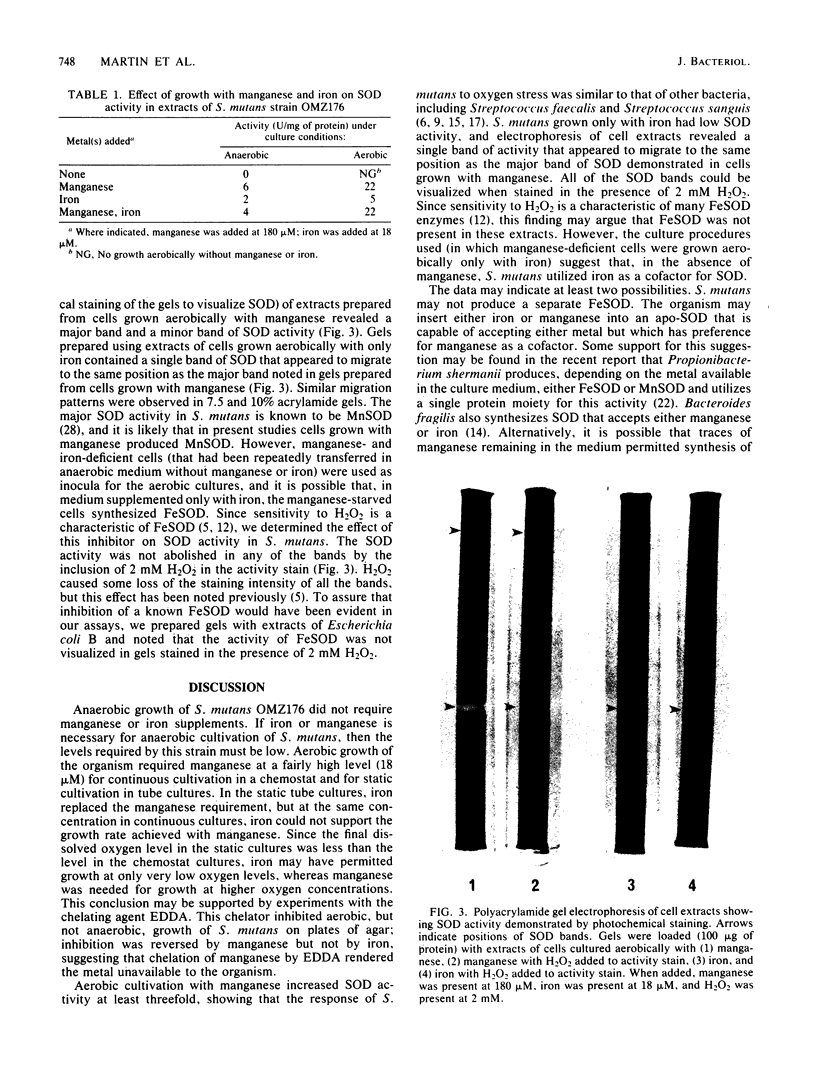
When cultured anaerobically in a chemically defined medium that was treated with Chelex-100 to lower its trace metal content, Streptococcus mutans OMZ176 had no apparent requirement for manganese or iron. Manganese or iron was necessary for aerobic cultivation in deep static cultures. During continuous aerobic cultivation in a stirred chemostat, iron did not support the growth rate achieved with manganese. Since the dissolved oxygen level in the chemostat cultures was higher than the final level in the static cultures, manganese may be required for growth at elevated oxygen levels. In medium supplemented with manganese, cells grown anaerobically contained a low level of superoxide dismutase (SOD) activity; aerobic cultivation increased SOD activity at least threefold. In iron-supplemented medium, cells grown anaerobically also had low SOD activity; aerobic incubation resulted in little increase in SOD activity. Polyacrylamide gel electrophoresis of the cell extracts revealed a major band and a minor band of SOD activity in the cells grown with manganese; however, cells grown with iron contained a single band of SOD activity with an Rf value similar to that of the major band found in cells grown with manganese. None of the SOD activity bands were abolished by the inclusion of 2 mM hydrogen peroxide in the SOD activity strain. S. mutans may not produce a separate iron-containing SOD but may insert either iron or manganese into an apo-SOD protein. Alternatively, iron may function in another activity (not SOD) that augments the defense against oxygen toxicity at low SOD levels.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTONI F., KELETI T. Immunobiological studies on crystalline alcohol dehydrogenases from closely related yeast species. Nature. 1957 May 18;179(4568):1020–1020. doi: 10.1038/1791020a0. [DOI] [PubMed] [Google Scholar]
- Adkins B. L., Losee F. L. A study of the covariation of dental caries prevalence and multiple trace element content of water supplies. N Y State Dent J. 1970 Dec;36(10):618–622. [PubMed] [Google Scholar]
- Aranha H., Strachan R. C., Arceneaux J. E., Byers B. R. Effect of trace metals on growth of Streptococcus mutans in a teflon chemostat. Infect Immun. 1982 Feb;35(2):456–460. doi: 10.1128/iai.35.2.456-460.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Beighton D. The influence of manganese on carbohydrate metabolism and caries induction by Streptococcus mutans strain Ingbritt. Caries Res. 1982;16(2):189–192. doi: 10.1159/000260596. [DOI] [PubMed] [Google Scholar]
- Bridges S. M., Salin M. L. Distribution of iron-containing superoxide dismutase in vascular plants. Plant Physiol. 1981 Aug;68(2):275–278. doi: 10.1104/pp.68.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton L., Malinowski D. P., Fridovich I. Superoxide dismutase and oxygen metabolism in Streptococcus faecalis and comparisons with other organisms. J Bacteriol. 1978 Apr;134(1):229–236. doi: 10.1128/jb.134.1.229-236.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J. A. A biochemical approach to the control of dental caries. Biochem Soc Trans. 1977;5(4):1232–1239. doi: 10.1042/bst0051232. [DOI] [PubMed] [Google Scholar]
- Curzon M. E., Crocker D. C. Relationships of trace elements in human tooth enamel to dental caries. Arch Oral Biol. 1978;23(8):647–653. doi: 10.1016/0003-9969(78)90189-9. [DOI] [PubMed] [Google Scholar]
- DiGuiseppi J., Fridovich I. Oxygen toxicity in Streptococcus sanguis. The relative importance of superoxide and hydroxyl radicals. J Biol Chem. 1982 Apr 25;257(8):4046–4051. [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Glass R. L., Rothman K. J., Espinal F., Vélez H., Smith N. J. The prevalence of human dental caries and water-borne trace metals. Arch Oral Biol. 1973 Sep;18(9):1099–1104. doi: 10.1016/0003-9969(73)90083-6. [DOI] [PubMed] [Google Scholar]
- Gregory E. M., Dapper C. H. Isolation of iron-containing superoxide dismutase from Bacteroides fragilis: reconstitution as a Mn-containing enzyme. Arch Biochem Biophys. 1983 Jan;220(1):293–300. doi: 10.1016/0003-9861(83)90413-7. [DOI] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Induction of superoxide dismutase by molecular oxygen. J Bacteriol. 1973 May;114(2):543–548. doi: 10.1128/jb.114.2.543-548.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Enzymatic defenses against the toxicity of oxygen and of streptonigrin in Escherichia coli. J Bacteriol. 1977 Mar;129(3):1574–1583. doi: 10.1128/jb.129.3.1574-1583.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leverett D. H. Fluorides and the changing prevalence of dental caries. Science. 1982 Jul 2;217(4554):26–30. doi: 10.1126/science.7089534. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Newbrun E. Sugar and dental caries: a review of human studies. Science. 1982 Jul 30;217(4558):418–423. doi: 10.1126/science.7046052. [DOI] [PubMed] [Google Scholar]
- Ong S. A., Peterson T., Neilands J. B. Agrobactin, a siderophore from Agrobacterium tumefaciens. J Biol Chem. 1979 Mar 25;254(6):1860–1865. [PubMed] [Google Scholar]
- Rogers H. J. Iron-Binding Catechols and Virulence in Escherichia coli. Infect Immun. 1973 Mar;7(3):445–456. doi: 10.1128/iai.7.3.445-456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan R. C., Aranha H., Lodge J. S., Arceneaux J. E., Byers B. R. Teflon chemostat for studies of trace metal metabolism in Streptococcus mutans and other bacteria. Appl Environ Microbiol. 1982 Jan;43(1):257–260. doi: 10.1128/aem.43.1.257-260.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L., Pera K. A. Oxygen metabolism of Streptococcus mutans: uptake of oxygen and release of superoxide and hydrogen peroxide. J Bacteriol. 1983 Jun;154(3):1236–1244. doi: 10.1128/jb.154.3.1236-1244.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance P. G., Keele B. B., Jr, Rajagopalan K. V. Superoxide dismutase from Streptococcus mutans. Isolation and characterization of two forms of the enzyme. J Biol Chem. 1972 Aug 10;247(15):4782–4786. [PubMed] [Google Scholar]




