Abstract
Fas is expressed constitutively in colonic epithelial cells and is also expressed in colon carcinomas and in cultured colon carcinoma cell lines. However, the potential role of Fas signaling in mediating apoptosis in cells of this type remains unknown. We have developed human colon carcinoma cell models deficient in thymidylate synthase that demonstrate acute (TS− cells) or delayed (Thy4 cells) apoptosis following DNA damage induced by thymineless stress. Complete protection of cells from acute apoptosis and prolongation of delayed apoptosis was obtained following exposure to the NOK-1 monoclonal antibody (inhibitory to Fas signaling) during the period of dThd deprivation. These results suggested that apoptosis induced by thymineless stress was regulated by autocrine signaling via Fas–FasL interactions. Fas expression was high in both TS− and Thy4 cells. However, FasL, undetectable in synchronous cultures, was up-regulated in TS− cells at 48 hr, when cells were undergoing acute apoptosis, and in Thy4 cells at 96 hr, correlating with the delayed onset of thymineless death. FasL expression also correlated with acute apoptosis induced in parental GC3/cl cells, commencing at 48 hr, following thymidylate synthase inhibition by 5-fluorouracil/leucovorin exposure. Fas-mediated apoptosis induced by the cytotoxic anti-Fas monoclonal antibody CH-11 was inhibited following adenoviral delivery of a Bcl-2 cDNA, and Bcl-2 also protected cells from acute apoptosis induced by dThd deprivation. Taken together, these data demonstrate a functional Fas system in these cultured colon carcinoma cell models, and they demonstrate that Fas–FasL interactions can link DNA damage induced by thymineless stress to the apoptotic machinery of colon carcinoma cells.
The cell surface receptor Fas (Apo-1; CD95) and its ligand (FasL) are known regulators of apoptosis in cells of the immune system. The Fas system is involved in the peripheral deletion of autoimmune cells (1–3), in activation-induced apoptosis of T cells (4, 5), and in cytotoxic T cell-mediated killing (6). However, the role of Fas-induced apoptosis in cells other than those of the lymphoid system remains unknown. Fas is a type I integral membrane protein characterized by cysteine-rich residues, it belongs to the tumor necrosis factor receptor superfamily, and it expresses an intracellular death domain required for rapid signaling from the receptor (7). Fas ligand is a type II transmembrane protein, is homologous with tumor necrosis factor and related cytokines, and is expressed on activated T and natural killer cells (8). Following the ligation of FasL to Fas, apoptosis is initiated (9).
Analyses of tissues from embryonic and adult mice demonstrated expression of Fas and FasL in several immune-privileged and adult tissues (10). Fas mRNA was detected in distinct cell types of the developing sinus, thymus, lung, and liver, and FasL was detected in submaxillary gland epithelial cells and in the developing nervous system. In the adult mouse, coexpression of Fas and FasL was found in the thymus, lung, spleen, small intestine, large intestine, seminal vesicle, prostate, and uterus, tissues largely characterized by high rates of cell turnover and apoptotic cell death.
Studies in human colonic epithelium demonstrated constitutive expression of Fas in the normal colon within the cytoplasm and at the basolateral surface of every epithelial cell, irrespective of its localization in the crypt or on the mucosal surface (11), suggesting that the Fas system may be involved in the regulation of normal cell turnover and colonic tissue homeostasis. Fas expression was reduced in carcinomas and demonstrated variable expression in cultured colon carcinoma cell lines (11). Deregulated control of mechanisms governing apoptosis are involved in oncogenesis and progression of colon carcinomas. A high frequency of missense mutations in the p53 tumor suppressor gene (≥75%) linked to disrupted G1 checkpoint function are associated with progression from adenoma to carcinoma (12), and up-regulated expression of the survival factor Bcl-2 has been identified (13). Fas has been induced in p53-transfected cells of this histiotype (14), and decreased anti-Fas sensitivity in colorectal carcinoma may be associated with increased expression of Bcl-2 (15) in addition to decreased Fas expression.
Thymineless death is the mechanism of cell killing associated with 5-fluorouracil (FUra) in colon cancer and remains the most effective agent for therapy of this disease. We have examined whether the Fas system can mediate apoptosis in malignant colonic epithelial cells following DNA damage induced by this mechanism and have developed human neoplastic cell models deficient in thymidylate synthase that address events downstream of dTTP depletion in an unambiguous manner. These models express either acute or delayed apoptosis following the induction of DNA damage by thymineless stress (16, 17), and TS− cells simulate, both temporally and biochemically, the thymineless state induced in parental GC3/cl cells following inhibition of thymidylate synthase by FUra combined with leucovorin (LV; ref. 18). The current study has established that thymineless stress can induce apoptosis via Fas signaling in colon carcinoma cells, and it identifies a fundamental mechanism that may be important in the regulation of apoptosis in malignant epithelial cells treated with agents that target thymidylate synthase.
MATERIALS AND METHODS
Cell Lines.
A thymidylate synthase-deficient mutant clone (TS−) selected from parental GC3/c1 human colon carcinoma cells, which is deficient in thymidylate synthase mRNA and protein and auxotrophic for dThd, has been well characterized (16–19). In addition, a clone of TS−, Thy4, was selected following repeated exposure to dThd depletion for its ability to withstand prolonged periods of dThd deprivation (16, 17).
Clonogenic Assays.
Cells were plated in six-well plates at a density of 3,000 cells/well in 2 ml of folate-free RPMI 1640 medium containing 10% dialyzed fetal bovine serum, physiologic folate (80 nM [6R,S]5-methyltetrahydrofolate), 712 μM Ca2+, and 20 μM dThd. Cells were also treated with the NOK-1 mAb (PharMingen; 100 ng/ml) or a mouse IgG1 isotype-matched control mAb (PharMingen; 100 ng/ml) at the time of plating. Following overnight attachment, cells were washed with 2 ml of Hanks’ balanced salt solution (37°C) and subsequently deprived of dThd by refeeding with dThd-free medium containing the respective antibodies. Alternatively, cells were synchronized in G0 by leucine deprivation (Leu− dThd+) for 3 days before dThd withdrawal (Leu+ dThd−) in the presence of the appropriate antibodies. At various times for up to 7 days, cells were rescued with dThd (20 μM) added to individual wells, and clonogenic survival was determined 11 days after dThd restoration (16, 17). Parental GC3/cl cells were treated with 1 μM FUra combined with 1 μM LV and rescued with dThd (20 μM) at various times for up to 96 hr. Clonogenic survival was determined 7 days after dThd restoration (18).
The ability of Bcl-2 to protect from apoptosis induced by dThd deprivation was examined in asynchronous cultures of Thy4 cells following adenoviral delivery of a Bcl-2 cDNA. The cDNA (20) was subcloned into the BamHI site of pSP64Cla (modified from pSP64; Promega) and subsequently subcloned into the ClaI site of the pAVS6.DNA adenoviral vector (Genetic Therapy, Gaithersburg, MD). Following recombination with Addl327 DNA, replication-incompetent adenovirus containing the basic vector backbone sequences (Ad-VC) or a Bcl-2 cDNA (Ad-Bcl-2) was amplified in the embryonic kidney cell line 293, purified, and titered as described (21). Thy4 cells were plated as described and subsequently transduced with Ad-VC or Ad-Bcl-2 at multiplicities of infection (mois) of 10, 50, or 100 for 24 hr before dThd withdrawal. Cells were deprived of dThd for 72 hr before dThd (20 μM) rescue, and clonogenic survival was determined after a further 11 days.
The ability of Bcl-2 to protect cells from Fas-mediated apoptosis induced by treatment with the cytotoxic anti-Fas mAb CH-11 was examined. TS− cells were plated as described in the presence of 20 μM dThd and transduced with Ad-VC or Ad-Bcl-2 at an moi of 50 for 24 hr before treatment with various concentrations of CH-11 (20–100 ng/ml; MBL International, Nagoya, Japan). Following 72 hr of exposure to CH-11, antibody was removed, and clonogenic survival was determined after a further 11 days.
Expression of Fas and FasL.
Fas expression in cell lines was determined by ELISA. Cell extracts were prepared in NETN buffer (100 mM NaCl/1 mM EDTA/20 mM Tris, pH 8.0/0.2% Nonidet P-40 with 10 μg/ml each leupeptin, aprotinin, antipain/1 mM sodium orthovanadate; 20 × 106 cells/ml); 96-well assay plates (Maxisorp; Nunc) were coated with capture antibody (0.1 ml/well, 2 μg/ml purified anti-human Fas mAb; PharMingen catalog no. 65311A) in 0.1 M Na2HPO4 (pH 9.0) overnight at 4°C. Wells were washed three times with PBS/0.5% Tween 20 (PBST) and blocked with 1% BSA (in PBST) at room temperature for 30 min, followed by three PBST washings. Samples and standard (recombinant soluble human Fas; PharMingen, 25–2500 pg/0.1 ml) were diluted in 1% BSA-PBST; 100 μl was added to each well and incubated overnight at 4°C. Plates were washed four times in PBST, and biotin-labeled anti-human Fas mAb DX2 (PharMingen) at 0.5 μg/ml in 1% BSA-PBST was added and incubated at room temperature for 3 hr. After six washes, avidin-horseradish peroxidase (PharMingen), diluted 1:1,000 in 1% BSA-PBST, was added, and the solution was incubated at room temperature for 50 min. Following eight washings, the color was developed in 100 μl/well ABTS substrate solution {3.3 mg of 2,2′-azino-bis[3-ethylbenzthiazoline-6-sulfonic acid] in 11 ml of 0.1 M anhydrous citric acid (pH 4.35) and 10 μl of 30% H2O2} for 30–60 min. OD was read at 405 nm.
Expression of FasL was determined by RT-PCR in asynchronous or G0-synchronized cultures of TS− and Thy4 cells exposed to dThd-depleted or -replete conditions. Cells were grown in T75 or T175 flasks; 20 × 106 cells were washed with 10 ml of Hanks’ balanced salt solution, and total RNA was extracted in RNAzol B using standard procedures. The first strand cDNA was synthesized in 20 μl of reaction mixture from 1 μg total RNA using an oligo(dT) primer and a cDNA cycle kit (InVitrogen). PCR for FasL from 4 μl of the RNA:DNA template was conducted as described (21) using 35 cycles to yield a 223- or 344-bp product. B-Actin control PCR (540 bp) was performed to monitor reverse transcriptase–PCR (RT-PCR) amplification efficiency. RT-PCR products were separated by electrophoresis in a 2% agarose gel and visualized by ethidium bromide staining and UV light illumination. Forward and reverse primers used to amplify FasL (22, 23) and B-actin (23) were: FasLF1, 5′-CACCCCAGTCCACCCCCTGA-3′; FasLR1, 5′-AGGGGCAGGTTGTTGCAAGA-3′; FasLF2, 5′-GGATTGGGCCTGGGGATGTTTCA-3′; FasLR2, 5′-TTGTGGCTCAGGGGCAGGTTGTTG-3′; B-actF, 5′-GTGGGGCGCCCCAGGCACCA-3′; and B-actR, 5′-CTCCTTAATGTCACGCACGATTTC-3′.
RESULTS
Fas-Mediated Apoptosis in the Regulation of Thymineless Death.
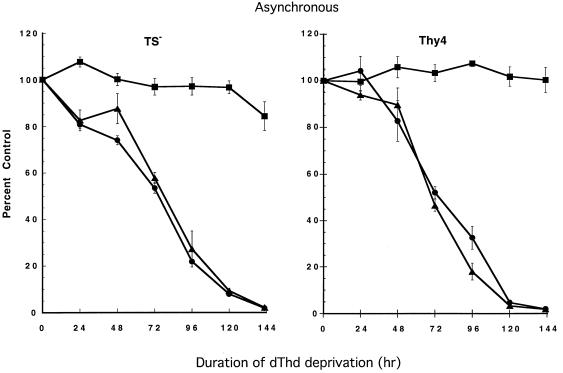
To determine whether autocrine signaling via Fas was involved in linking the thymineless state to the apoptotic machinery of the cell, cells were incubated with the NOK-1 mAb that prevents the Fas–FasL interaction and is inhibitory to Fas signaling. In asynchronously growing TS− and Thy4 cells, which both undergo acute apoptosis following induction of the thymineless state, complete protection from apoptosis occurred when cells were simultaneously incubated with NOK-1 (Fig. 1). Cells incubated with an IgG1 isotype-matched control in the absence of dThd demonstrated the same kinetics of cell kill as cells exposed to dThd deprivation alone. As determined by clonogenic assay, cells lost 50% clonogenic potential in 72 hr, and only 5% of cells formed colonies at 5 days. Coincubation with NOK-1 yielded complete protection from the induction of apoptosis, even at 5 days, when cell survival would be anticipated to be minimal.
Figure 1.
Protection from apoptosis induced by dThd deprivation in asynchronously growing cultures of TS− and Thy4 by a Fas-inhibiting antibody that binds to FasL. For clonogenic assays, cells were plated as described and treated with the NOK-1 mAb (100 ng/ml; ▪), a mouse IgG1 isotype-matched control mAb (100 ng/ml; ▴), or no antibody (•) at the time of plating. Following overnight attachment, cells were washed with 2 ml of Hanks’ balanced salt solution (37°C) and subsequently deprived of dThd by refeeding with dThd-free medium containing the respective antibodies. At various times for up to 6 days, cells were rescued with dThd (20 μM) added to individual wells, and clonogenic survival was determined after a further 11 days. Data represent the mean ± SD of triplicate determinations.
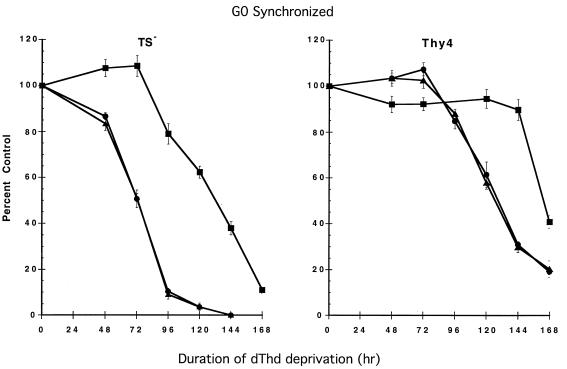
To confirm that NOK-1 could also protect from acute apoptosis in TS− and delayed apoptosis in Thy4 following release from G0 in the absence of dThd, cells were coincubated with NOK-1 during dThd withdrawal (Fig. 2). Clonogenic survival was reduced by 50% in 72 hr in TS− in IgG1 isotype-matched controls, in contrast to NOK-1-treated cells, in which 50% survival was evident after a further 3 days, indicating substantial protection from thymineless death. Induction of apoptosis at 6 days in Thy4 was also further delayed following NOK-1 treatment.
Figure 2.
Enhanced survival of TS− (acute apoptosis) and Thy4 (delayed apoptosis) in the presence of NOK-1 antibody following G0 synchronization and release in the absence of dThd. Following plating and overnight attachment, cells were synchronized in G0 by leucine deprivation before dThd withdrawal in the presence of the appropriate antibodies. Cells were rescued with 20 μM dThd as described, and clonogenic survival was determined 11 days after dThd rescue. •, No antibody; •, NOK-1; and ▴, IgG1 isotype-matched control. Results are the means ± SD of three determinations per time point.
Expression of Fas and FasL.
Subsequent analysis of Fas and FasL expression by ELISA and by RT-PCR, respectively (Table 1), demonstrated high levels of expression of Fas in TS− and Thy4 in comparison to CaCo2, a human colon carcinoma cell line insensitive to Fas-mediated apoptosis and expressing low endogenous levels of the receptor. However, FasL expression was undetectable in asynchronous cultures of TS− and Thy4, as measured by RT-PCR, in contrast to CaCo2, which expressed higher levels.
Table 1.
Expression of Fas and FasL in asynchronously growing cell lines
| Cell line | Fas, pg/106 cells | FasL (relative OD) |
|---|---|---|
| TS− | 1,210 | — |
| Thy4 | 727 | — |
| CaCo2 | 0 | 10.9 |
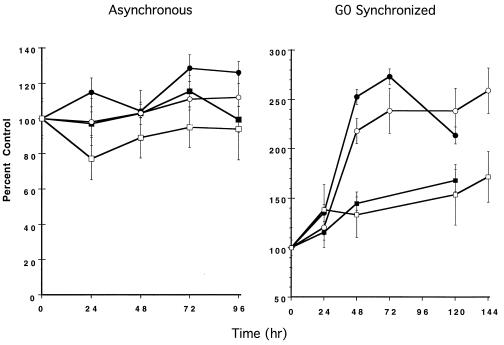
The expression of Fas was also examined under conditions of dThd deprivation in asynchronous populations and following release from G0 arrest in the absence of dThd, and it was also examined under dThd-replete conditions. Fas demonstrated minimal elevation in asynchronously growing cells under dThd-starved or -replete conditions or in cells released from G0 in the presence of dThd (Fig. 3). In contrast, when cells were released from G0 in the absence of dThd, Fas expression was up-regulated 2- to 3-fold, although there was no correlation with either acute or delayed apoptosis (Fig. 3).
Figure 3.
Fas expression in asynchronous or G0-synchronized cultures of TS− and Thy4 exposed to dThd-depleted or -replete conditions, determined by ELISA, as described. OD was read at 405 nm. For TS−: ▪, dThd−; and •, dThd+. For Thy4: □, dThd−; and ○, dThd+. Data represent the mean ± SD of triplicate determinations and protein extracted from equivalent cell numbers.
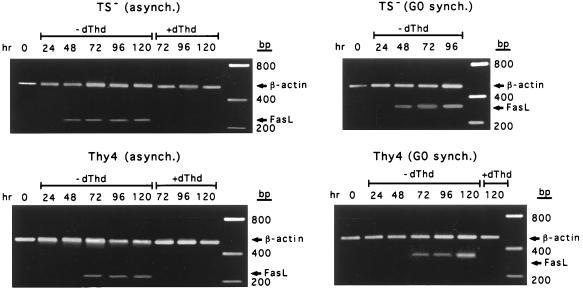
Of particular interest, however, was the strong correlation between the up-regulated expression of FasL determined by RT-PCR and the induction of apoptosis under the various conditions, which was independent of the cellular proliferation state (Fig. 4). Levels of FasL were significantly elevated during commitment to cell death but remained low in Thy4 cells during the period of cytostasis when cells were released from G0 in the absence of dThd. This was not due to the inability to proliferate because FasL expression was also undetectable in cells actively cycling in the presence of dThd (Fig. 4). Thus, in asynchronously growing TS− and Thy4 cells and in TS− released from G0 in the absence of dThd, elevated expression of FasL was detectable from 48 hr, when cells were undergoing acute apoptosis, as determined from clonogenic survival. In Thy4 released from G0, up-regulated expression of FasL was detected initially at 96 hr, correlating with the delayed onset of thymineless death.
Figure 4.
Expression of FasL determined by RT-PCR in asynchronous or G0-synchronized cultures of TS− and Thy4 exposed to dThd-depleted or -replete conditions. Data are representative experiments conducted at least three times.
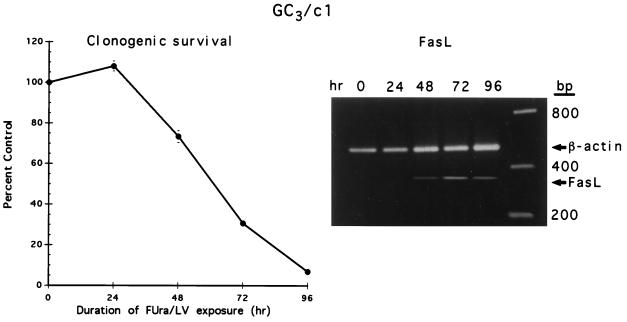
To determine whether up-regulated expression of FasL was involved in the response to treatment with FUra/LV that targets FUra to the thymidylate synthase locus, parental GC3/cl cells were treated with 1 μM FUra combined with 1 μM LV, known to induce apoptosis via thymineless stress (18). Clonogenic survival was reduced to 73, 31, and 7% at 48, 72, and 96 hr, respectively (Fig. 5). Under these conditions of dTTP depletion, FasL expression was determined by RT-PCR (Fig. 5). Similar to data derived in TS− and Thy4, expression of FasL was not detected in asynchronously growing, untreated cultures. However, following FUra/LV exposure, FasL was detected at 48 hr, and expression was elevated at 72 hr and 96 hr as cells committed to apoptosis following induction of the thymineless state.
Figure 5.
Clonogenic survival of GC3/cl cells treated with FUra (1 μM) and LV (1 μM). At various times, dThd (20 μM) was restored, and clonogenic survival determined as described. Data represent to mean ± SD of three determinations at each time point. (Inset) Expression of FasL, determined by RT-PCR, during FUra/LV treatment of GC3/cl cells. The data are from a representative experiment conducted twice.
Protection from Apoptosis by Bcl-2.
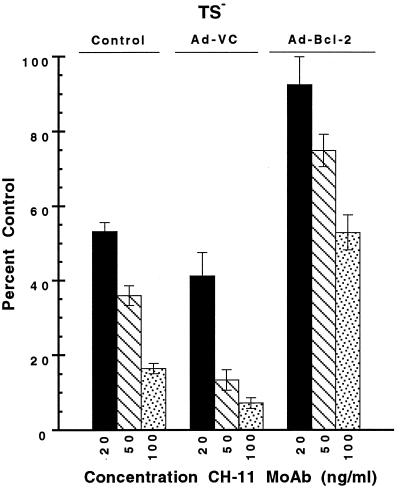
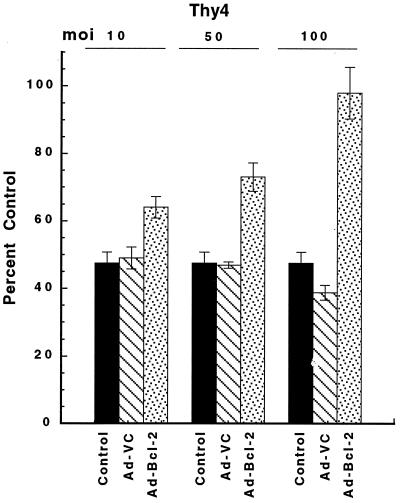
To determine whether Bcl-2 could protect cells from Fas-mediated apoptosis, asynchronously growing TS− cells were treated for 72 hr with the cytotoxic mAb CH-11, in either the absence or presence of Bcl-2 following adenoviral transduction at an moi of 50 (Ad-Bcl-2; Fig. 6). At all concentrations of CH-11 examined (20–100 ng/ml), Bcl-2 protected TS− cells from Fas-dependent apoptosis. Because up-regulated expression of Bcl-2 due to lack of functional p53 activity has been associated with cytostasis and, hence, delayed apoptosis in Thy4 (17), the ability of Bcl-2 to protect from apoptosis induced by thymineless stress was investigated in asynchronous Thy4 cultures following dThd withdrawal and adenoviral delivery of Bcl-2 (Ad-Bcl-2) at mois of 10, 50, and 100 (Fig. 7). A survival advantage was obtained in the presence of Bcl-2, increasing as the moi increased, and together with the data of Fig. 6, results suggest a protective effect of Bcl-2 in thymineless death, mediated downstream of Fas.
Figure 6.
Protection from CH-11-induced apoptosis by Bcl-2 in asynchronous cultures of TS− cells. Cells were plated as described in the presence of dThd (20 μM) and were transduced with Ad-VC or Ad-Bcl-2 at an moi of 50 for 24 hr before dThd withdrawal and treatment with various concentrations of CH-11 (20–100 ng/ml). Following 72 hr of exposure to CH-11, antibody was removed, and clonogenic survival was determined after a further 11 days. Data represent the mean ± SD of three determinations at each CH-11 concentration.
Figure 7.
Protection from apoptosis by Ad-Bcl-2 in asynchronous cultures of Thy4. Thy4 cells were plated as described and transduced with Ad-VC or Ad-Bcl-2 at mois of 10, 50, or 100 for 24 hr before dThd withdrawal. Cells were deprived of dThd for 72 hr before dThd (20 μM) rescue. Clonogenic survival was determined after a further 11 days. Data are the mean ± SD of triplicate determinations at each moi.
DISCUSSION
Thymidylate synthase-deficient colon carcinoma cells (TS−), previously selected from a cultured colon carcinoma cell line (GC3/c1), undergo acute apoptosis following dThd deprivation of asynchronously growing cultures or of cells released from G0 arrest and simulate the thymineless state induced by treatment of GC3/cl cells with FUra/LV in combinations (18). In TS− cells, dThd withdrawal initiates an imbalance in the dATP/dTTP pools followed by inhibition of DNA synthesis (18); subsequent DNA damage comprises the formation of single- and double-stranded DNA breaks (19) and nucleosomal DNA ladders (17, 18). Apoptosis commences at 24 hr and requires proliferation for the damage to be expressed (17). It is known that apoptosis in TS− is regulated by functional wild-type p53 and does not require the induction of p21Waf1 (17). The Thy4 cell line, selected from TS−, undergoes acute apoptosis when dThd is withdrawn from asynchronously growing cultures but demonstrates delayed apoptosis following release from G0 arrest in the absence of dThd (16, 17). Delayed apoptosis of Thy4 cells is characterized by arrest close to the G1–S boundary, an event independent of p21Waf1 but associated with up-regulated expression of Bcl-2 (17). Apoptosis subsequently occurs between 5 and 6 days after dThd deprivation with endonucleosomal DNA cleavage and is also dependent upon functional wild-type p53. Little is understood, however, concerning subsequent signals involved in DNA damage recognition in TS− and Thy4 and the ultimate signaling pathway that links these signals to the apoptotic machinery of the cell.
Taken together, the data presented demonstrate an intact Fas system in the cultured colon carcinoma cell models used for this analysis, and they demonstrate that Fas–FasL interactions can regulate apoptosis caused by external stimuli. In other systems, up-regulated expression of both Fas (11) and FasL (9) have correlated with the sensitivity of cells to Fas-induced apoptosis. If autocrine signaling via the Fas receptor downstream of DNA damage mediates apoptosis in TS− and Thy4, then up-regulated expression of one or both parameters should correlate with acute or delayed apoptosis in these models and should be independent of the proliferation state of the cell. Such a correlation was demonstrated for the expression of FasL, which was up-regulated only in those cells either committing to or undergoing apoptosis.
Subsequently, the expression of FasL was examined in parental GC3/cl cells that were induced to undergo apoptosis via thymineless stress following treatment with FUra and LV. Data were similar to those derived in TS− cells following dThd withdrawal, and levels of FasL were induced as cells committed to apoptosis.
Following the induction of DNA damage and functional wild-type p53 activity by thymineless stress in TS−, a signal must be generated that is linked to the up-regulated expression of FasL and autocrine signaling via Fas to induce apoptosis. However, regulation of FasL expression is clearly delayed in Thy4 and may be associated with up-regulated expression of Bcl-2. It is evident that Bcl-2 can protect TS-deficient cells from both the direct induction of Fas-mediated apoptosis and apoptosis induced by thymineless stress, an event occurring downstream of p53 and also downstream of Fas. Whether FasL may be directly or indirectly regulated by p53 in these models is, at present, unknown. However, the emerging literature continues to identify genes involved in the modulation of DNA damage responses. Thus, activation of genes that enhance survival responses in cells treated with DNA-damaging agents have been identified, and these include the transcription factor NF-κB (24), the Abl protein tyrosine kinase (25), which is also a negative regulator of Fas-mediated cell death (26), and Raf (27). The recently cloned ATM gene, deficient in humans with ataxia telangiectasia, may be important for the repair of DNA double-strand breaks and the regulation of cell cycle progression following DNA damage (28). However, how these events are directly linked initially to DNA damage and, subsequently, to the apoptotic machinery of the cell remain to be determined. In our study, we have identified Fas-mediated apoptosis as a critical component of thymineless death in colon carcinoma cells, and we have demonstrated that this is dependent upon functional p53. Potentially, defects in Fas signaling may represent a new mechanism of resistance to the majority of agents that target thymidylate synthase, agents that are, at present, the most effective in the palliative treatment of colon carcinoma. Defects in the Fas signaling pathway that may be relevant to colon carcinoma include reduced expression of Fas (11) and up-regulated expression of Bcl-2 (15), which may reduce the ability of cells of this type to undergo apoptosis. If an intact and functional Fas pathway exists at the level of and downstream of the membrane receptor, then up-regulated expression of FasL may correlate with sensitivity to FUra/LV combinations. However, this remains to be tested in an expanded panel of colon carcinoma cell lines and also in a clinical setting.
Acknowledgments
This work was supported by National Cancer Institute Awards R37 CA 32613 and Cancer Center Support Grant CA 21765 and by the American Lebanese Syrian Associated Charities.
ABBREVIATIONS
- FUra
5-fluorouracil
- LV
leucovorin
- moi
multiplicity of infection
- RT-PCR
reverse transcriptase–PCR
References
- 1.Watanabe-Fukunaga R, Brannan C I, Copeland N G, Jenkins N A, Nagata S. Nature (London) 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 2.Debatin K M, Suss D, Krammer P H. Eur J Immunol. 1994;24:753–758. doi: 10.1002/eji.1830240339. [DOI] [PubMed] [Google Scholar]
- 3.Rozzo S J, Eisenberg R A, Cohen P L, Kotzin B L. Semin Immunol. 1994;6:19–26. doi: 10.1006/smim.1994.1004. [DOI] [PubMed] [Google Scholar]
- 4.Alderson M R, Tough T W, Davissmith T, Braddy S, Falk B, Schooley K A, Goodwin R G, Smith C A, Ramsdell F, Lynch D A. J Exp Med. 1995;181:71–77. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunner T, Mogil R J, LaFace D, Yoo N J, Mahboudi A, Echeverri V, Martin S, Force W S, Lynch D H, Ware C V, Green D. Nature (London) 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 6.Rouvier E, Luciani M L, Golstein P. J Exp Med. 1993;177:195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Itoh N, Nagata S. J Biol Chem. 1993;268:10932–10937. [PubMed] [Google Scholar]
- 8.Smith C A, Farrah T, Goodwin R G. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 9.Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S I, Sameshima M, Hase A, Sato Y, Nagata S. Cell. 1991;66:233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 10.French L E, Hahne M, Viard I, Radlgruber G, Zanone R, Becker K, Muller C, Tschopp J. J Cell Biol. 1996;133:335–343. doi: 10.1083/jcb.133.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moller P, Koretz K, Leithauser F, Bruderlein S, Henne C, Quentmeier A, Krammer P H. Int J Cancer. 1994;57:371–377. doi: 10.1002/ijc.2910570314. [DOI] [PubMed] [Google Scholar]
- 12.Fearon E R, Vogelstein B. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 13.Bedi A, Pasricha P J, Akhtar A J, Barber J P, Bedi G C, Giardiello F M, Zehnbauer B A, Hamilton S R, Jones R J. Cancer Res. 1995;55:1811–1816. [PubMed] [Google Scholar]
- 14.Tamura T, Aoyama N, Saya H, Haga H, Futami S, Miyamoto M, Koh T, Ariyasu T, Tachi M, Kasuga M, Takahashi R. Oncogene. 1995;11:1939–1946. [PubMed] [Google Scholar]
- 15.Meterissian S, Kontogiannea M. Proc Am Assoc Cancer Res. 1996;37:15. [Google Scholar]
- 16.Houghton J A, Harwood F G, Houghton P J. Cancer Res. 1994;54:4967–4973. [PubMed] [Google Scholar]
- 17.Harwood F G, Frazier M W, Krakewski S, Reed J C, Houghton J A. Oncogene. 1996;12:2057–2067. [PubMed] [Google Scholar]
- 18.Houghton J A, Tillman D M, Harwood F G. Clin Cancer Res. 1995;1:723–730. [PubMed] [Google Scholar]
- 19.Houghton J A, Morton C L, Adkins D A, Rahman A. Cancer Res. 1993;53:4243–4250. [PubMed] [Google Scholar]
- 20.Seto M, Jaeger U, Hockett R D, Graninger W, Bennett S, Goldman P, Korsmeyer S J. EMBO J. 1988;7:123–131. doi: 10.1002/j.1460-2075.1988.tb02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becker T C, Noel R J, Coats W S, Gomez-Foix A M, Alam T, Gerard R D, Newgard C B. Methods Cell Biol. 1994;43:161–189. doi: 10.1016/s0091-679x(08)60603-2. [DOI] [PubMed] [Google Scholar]
- 22.Oshimi Y, Oda S, Honda Y, Nagata S, Miyazaki S. J Immunol. 1996;157:2909–2915. [PubMed] [Google Scholar]
- 23.O’Connell J, O’Sullivan G C, Collins J K, Shanahan F. J Exp Med. 1996;184:1075–1082. doi: 10.1084/jem.184.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang C-Y, Mayo M W, Baldwin A S. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 25.Yuan Z-M, Huang Y, Whang Y, Sawyers C, Reichselbaum R, Kharbanda S, Kufe D. Nature (London) 1996;382:272–274. doi: 10.1038/382272a0. [DOI] [PubMed] [Google Scholar]
- 26.McGahon A J, Nishioka W K, Martin S J, Mahboubi A, Cotter T J, Green D R. J Biol Chem. 1995;270:22625–22631. doi: 10.1074/jbc.270.38.22625. [DOI] [PubMed] [Google Scholar]
- 27.Kasid U, Suy S, Dent P, Ray S, Whiteside T L, Sturgill T W. Nature (London) 1996;382:813–816. doi: 10.1038/382813a0. [DOI] [PubMed] [Google Scholar]
- 28.Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley J N, Ried T, Tagle D, Wynshaw-Boris A. Cell. 1996;86:159–171. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]